Multimetallic Layered Double Hydroxides as OER Catalysts for High-Performance Water Electrolysis
Abstract
1. Introduction
2. Material Synthesis
3. Results and Discussion
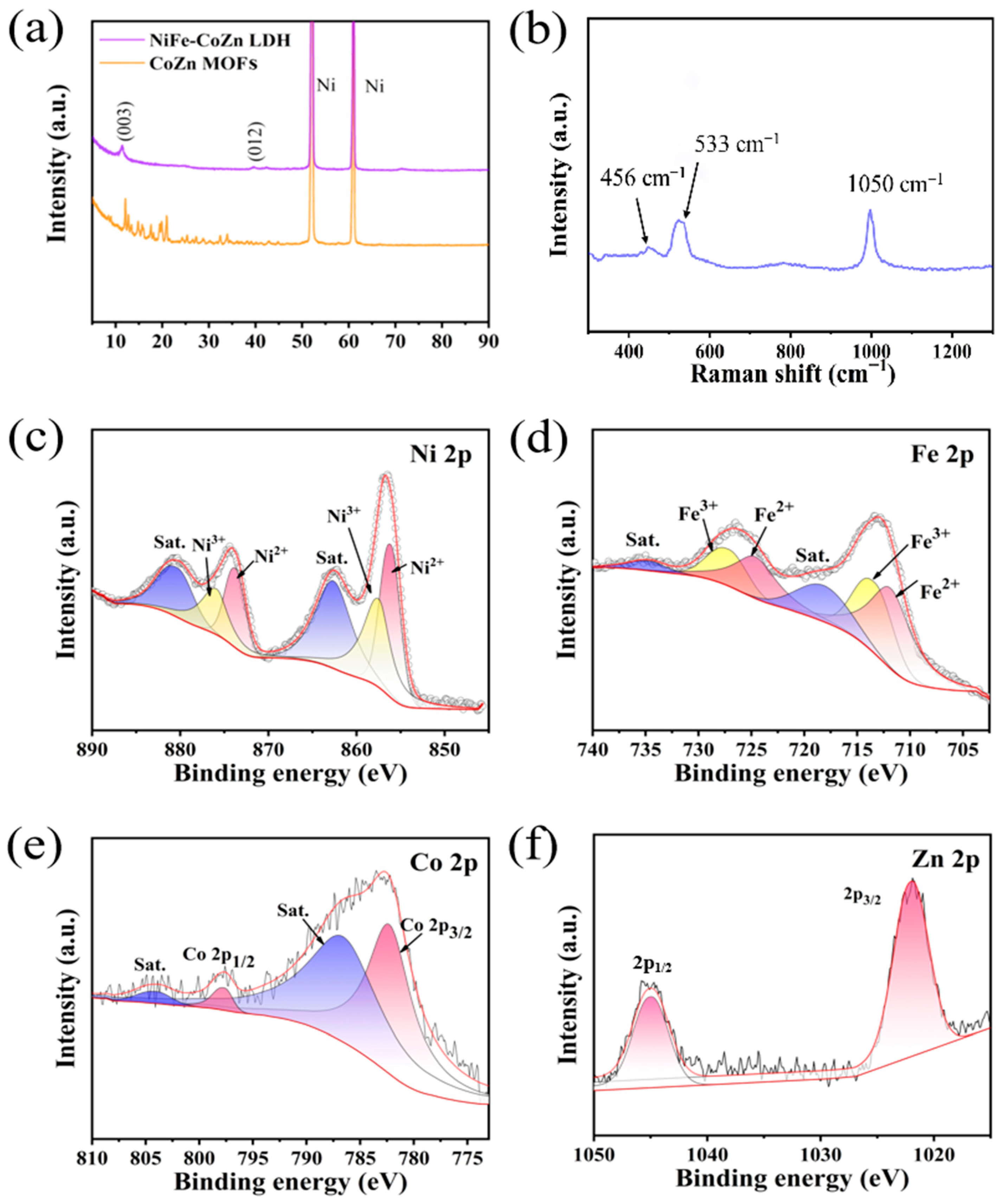
3.1. Structural and Morphological Characterisation
3.2. Electrochemical Properties of Alkaline Electrolyte Solutions
3.3. Investigation of Electrocatalytic Performance and Stability
3.4. Advantages over Conventional Electrodes
4. Conclusions
- (1)
- Long-term stability tests under industrial operating conditions (current density > 500 mA cm−2) are necessary to assess practical viability.
- (2)
- The as-prepared catalyst will be integrated into an anion exchange membrane water electrolyzer (AEMWE) for industrial-scale overall water splitting tests under high current densities.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.-H.; Yuan, C.-Z.; Huang, X.; Zhao, H.; Wu, F.; Xin, L.; Zhang, X.; Ye, S.; Chen, Y. Tailoring the electron redistribution of RuO2 by constructing a Ru-O-La asymmetric configuration for efficient acidic oxygen evolution. eScience 2025, 5, 100307. [Google Scholar] [CrossRef]
- Guo, B.-R.; Chen, M.-X.; Li, S.-W.; Gao, R.-H.; Sang, B.-H.; Ren, X.-Q.; Liu, Z.; Cao, X.; Liu, J.; Ding, Y.-N.; et al. Construction of iron oxyhydroxide/nickel sulfate hydroxide hybrid electrocatalyst for efficient oxygen evolution. Rare Metals 2024, 43, 6394–6404. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.; Wang, L.; Yang, T.; Wang, K.; Wang, E.; Yu, X.; Wang, H.; Chou, K.-C.; Hou, X. Synthesis of various morphologies of CoFe bimetallic hydroxides for enhanced OER performance. Int. J. Miner. Metall. Mater. 2025, 32, 2024–2033. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Lu, X.F.; Xi, S.; Du, Y.; Lou, X.W.D. Metal Atom-Doped Co3O4 Hierarchical Nanoplates for Electrocatalytic Oxygen Evolution. Adv. Mater. 2020, 32, e2002235. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Yuan, Y.; Guo, Z.; Chen, G.; Yang, J.; Bao, Q.; Guo, L.; Chen, C. Transition metal-based layered double hydroxides and their derivatives for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 63, 918–936. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning Surface Electronic Configuration of NiFe LDHs Nanosheets by Introducing Cation Vacancies (Fe or Ni) as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Small 2018, 14, e1800136. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Zhang, B.; Lv, S.; Wang, T.; Liu, X. Tuning electronic structure of CoNi LDHs via surface Fe doping for achieving effective oxygen evolution reaction. Appl. Surf. Sci. 2021, 565, 150506. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Xiao, X.; Zhang, F.; Song, S.; Chen, S.; Ren, Z. Recent Advances in Self-Supported Layered Double Hydroxides for Oxygen Evolution Reaction. Research 2020, 2020, 3976278. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent advance of layered double hydroxides materials: Structure, properties, synthesis, modification and applications of wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 111191. [Google Scholar] [CrossRef]
- Deka, S.; Jaiswal, M.K.; Rajput, P.; Choudhury, B. Mechanistic insights into electrocatalytically reduced OER performance in marigold-like trimetallic NiFe-based LDH: Charge localisation and d-band orbital filling. J. Mater. Chem. A 2024, 12, 9532–9545. [Google Scholar] [CrossRef]
- Kulkarni, R.; Lingamdinne, L.P.; Karri, R.R.; Momin, Z.H.; Koduru, J.R.; Chang, Y.-Y. Catalytic efficiency of LDH@carbonaceous hybrid nanocomposites towards water splitting mechanism: Impact of plasma and its significance on HER and OER activity. Coord. Chem. Rev. 2023, 497, 215460. [Google Scholar] [CrossRef]
- Yu, J.; Le, T.A.; Tran, N.Q.; Lee, H. Earth-Abundant Transition-Metal-Based Bifunctional Electrocatalysts for Overall Water Splitting in Alkaline Media. Chemistry 2020, 26, 6423–6436. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.K.; Lee, S.Y.; Kim, H.G.; Lee, G.B.; Lee, J.H.; Kim, M.; Joo, Y.C. Electrochemical oxidation of boron-doped nickel-iron layered double hydroxide for facile charge transfer in oxygen evolution electrocatalysts. RSC Adv. 2021, 11, 8198–8206. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yang, K.; Huang, D.; Cheng, G.; Ai, X.; Liu, Y.; Fang, J.; Li, H.; Yu, L.; Zhai, T. Schottky Heterojunction Nanosheet Array Achieving High-Current-Density Oxygen Evolution for Industrial Water Splitting Electrolyzers. Adv. Energy Mater. 2021, 11, 2102353. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Lu, Q.; Xing, Z.; Yang, X. Atomic and electronic modulation of self-supported nickel-vanadium layered double hydroxide to accelerate water splitting kinetics. Nat. Commun. 2019, 10, 3899. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, L.; Jiang, W.; Wu, Y.; Liu, B.; Sun, Y.; Chu, X.; Liu, C. Rich oxygen vacancy and amorphous/crystalline ruthenium-doped CoCu -layered double hydroxide electrocatalysts for enhanced oxygen evolution reactions. J. Colloid Interface Sci. 2024, 671, 283–293. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Xu, J.; Sun, H.; Shi, Z. Constructing surface concave defects on NiFe layered double hydroxides by electrochemical reduction for efficient oxygen evolution reaction. Chem. Eng. J. 2024, 482, 148858. [Google Scholar] [CrossRef]
- Cao, W.; Wu, J.; Zhou, C.; Gao, X.; Hu, E.; Zhang, J.; Chen, Z. Reinforcement of Electrocatalytic Oxygen Evolution Activity Enabled by Constructing Silver-Incorporated NiCo-PBA@NiFe-LDH Hierarchical Nanoboxes. Small 2024, 20, e2309769. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; Wang, D.; Luo, D.; Song, S.; Yuan, C.; Karim, A.; Chen, S.; Ren, Z. Facile synthesis of nanoparticle-stacked tungsten-doped nickel iron layered double hydroxide nanosheets for boosting oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 8096–8103. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Ma, X.; Zhu, C.; Wang, Y.; Xie, Y.; Chou, S.L.; Huang, Y.; Chen, Y. Regulation of Morphology and Electronic Structure of FeCoNi Layered Double Hydroxides for Highly Active and Stable Water Oxidization Catalysts. Adv. Energy Mater. 2021, 11, 2102141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Hu, J.; Li, S.; Du, Y.; Han, X.; Xu, P. Metal–Organic Frameworks Derived Interconnected Bimetallic Metaphosphate Nanoarrays for Efficient Electrocatalytic Oxygen Evolution. Adv. Funct. Mater. 2020, 30, 1910498. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent Development of Zeolitic Imidazolate Frameworks (ZIFs) Derived Porous Carbon Based Materials as Electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, Z.P.; Luan, D.; Zang, S.Q.; Lou, X.W.D. Synergetic Cobalt-Copper-Based Bimetal-Organic Framework Nanoboxes toward Efficient Electrochemical Oxygen Evolution. Angew. Chem. 2021, 60, 26397–26402. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiao, J.; Huang, C.; Zhou, J.; Qiu, M.; Yu, Y.; Ren, Z.; Chu, C.W.; Yu, J.C. High-performance seawater oxidation by a homogeneous multimetallic layered double hydroxide electrocatalyst. Proc. Natl. Acad. Sci. USA 2022, 119, e2202382119. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, Z.; Zhang, Y.; Niu, S.; Zhao, J.; Li, S.; Xu, P. Understanding the Effect of Second Metal on CoM (M = Ni, Cu, Zn) Metal-Organic Frameworks for Electrocatalytic Oxygen Evolution Reaction. Small 2021, 17, e2105150. [Google Scholar] [CrossRef]
- Ning, M.; Wu, L.; Zhang, F.; Wang, D.; Song, S.; Tong, T.; Bao, J.; Chen, S.; Yu, L.; Ren, Z. One-step spontaneous growth of NiFe layered double hydroxide at room temperature for seawater oxygen evolution. Mater. Today Phys. 2021, 19, 100419. [Google Scholar] [CrossRef]
- Li, S.; Xi, C.; Jin, Y.-Z.; Wu, D.; Wang, J.-Q.; Liu, T.; Wang, H.-B.; Dong, C.-K.; Liu, H.; Kulinich, S.A.; et al. Ir–O–V Catalytic Group in Ir-Doped NiV(OH)2 for Overall Water Splitting. ACS Energy Lett. 2019, 4, 1823–1829. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.J.; Tang, T.; Yuan, L.P.; Luo, H.; Hu, J.S. Autogenous Growth of Hierarchical NiFe(OH)x/FeS Nanosheet-On-Microsheet Arrays for Synergistically Enhanced High-Output Water Oxidation. Adv. Funct. Mater. 2019, 29, 1902180. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Yang, T.; Wang, K.; Wang, H.; Wang, E.; Chou, K.-C.; Hou, X. Ultra-stable electrocatalysts for overall urea degradation by NiFeCoZn–S modified N-doped carbon. Ceram. Int. 2024, 50, 44440–44446. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Jiang, K.; Xu, L.; Guan, M.; Bao, J.; Ji, H.; Li, H. Constructing a CeO2−x@CoFe-layered double hydroxide heterostructure as an improved electrocatalyst for highly efficient water oxidation. Inorg. Chem. Front. 2020, 7, 4461–4468. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Dong, J.; Lu, X.F.; Lou, X.W. Intramolecular electronic coupling in porous iron cobalt (oxy)phosphide nanoboxes enhances the electrocatalytic activity for oxygen evolution. Energy Environ. Sci. 2019, 12, 3348–3355. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, J.; Pan, H.; Li, J.; Guo, K.; Zhao, Y.; Xu, C. Enhanced oxygen evolution reaction of defective CoP/MOF-integrated electrocatalyst by partial phosphating. J. Mater. Chem. A 2020, 8, 14099–14105. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel–Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Liu, S.; Wan, R.; Lin, Z.; Liu, Z.; Liu, Y.; Tian, Y.; Qin, D.-D.; Tang, Z. Probing the Co role in promoting the OER and Zn–air battery performance of NiFe-LDH: A combined experimental and theoretical study. J. Mater. Chem. A 2022, 10, 5244–5254. [Google Scholar] [CrossRef]
- Meng, L.; Xuan, H.; Wang, J.; Liang, X.; Li, Y.; Yang, J.; Han, P. Flower-like Co3O4@NiFe-LDH nanosheets enable high-performance bifunctionality towards both electrocatalytic HER and OER in alkaline solution. J. Alloys Compd. 2022, 919, 165877. [Google Scholar] [CrossRef]
- Mo, J.; Chen, Y.; Guo, J.; Huang, H.; Chen, B.-M.; Xu, R.; Guo, Z. Microstructure and oxygen vacancy modulation realizing high OER performance in Co-NiFe LDH. Mater. Today Commun. 2025, 42, 111162. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Wu, X. Realizing efficient electrochemical overall water electrolysis through hierarchical CoP@NiCo-LDH nanohybrids. Nano Energy 2023, 114, 165877. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Xu, Y.; Li, H.; Tu, W.; Zhou, J. Heterogeneous ultra-thin FeCo-LDH@Co(OH)2 nanosheets facilitated electrons transfer for oxygen evolution reaction. Chem. Eng. J. 2023, 472, 145076. [Google Scholar] [CrossRef]
- Dao, H.T.; Hoa, V.H.; Sidra, S.; Mai, M.; Zharnikov, M.; Kim, D.H. Dual efficiency enhancement in overall water splitting with defect-rich and Ru atom-doped NiFe LDH nanosheets on NiCo2O4 nanowires. Chem. Eng. J. 2024, 485, 150054. [Google Scholar] [CrossRef]
- Sreenivasulu, M.; Hadrihalli, A.; Alshehri, M.A.; Shetti, N.P. Rational Designing of Nickel–Iron Containing Layered Double Hydroxide [NiFe@LDH] Electrocatalysts for Effective Water Splitting. Energy Fuels 2024, 38, 12888–12899. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Zhang, S.; Liu, Z.; Xiao, J. Cr doping and heterostructure-accelerated NiFe LDH reaction kinetics assist the MoS2 oxygen evolution reaction. Nanoscale 2024, 16, 3650–3658. [Google Scholar] [CrossRef]

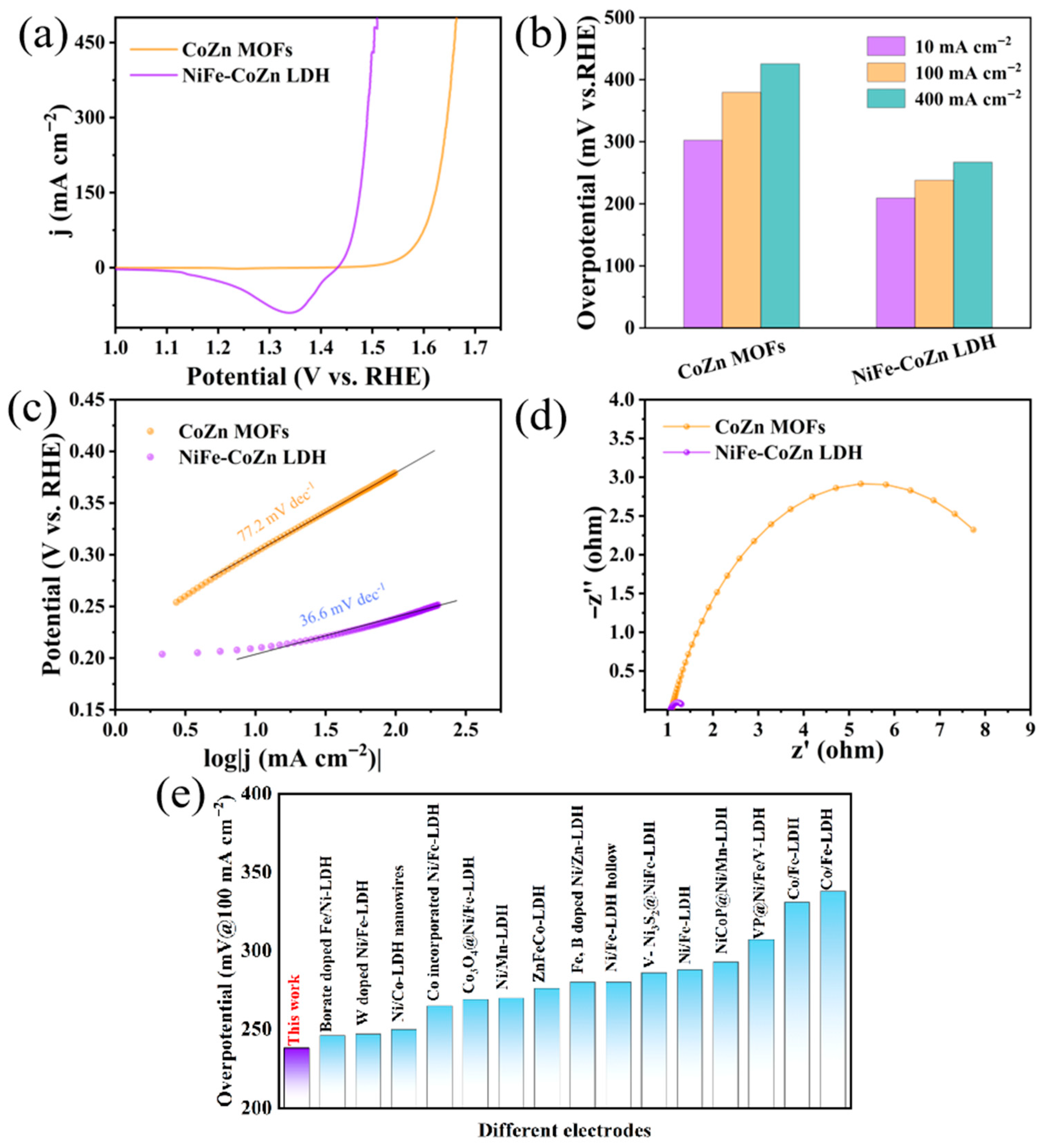




| Electrocatalyst | Tafel Slope | Overpotential at 10 mA cm−2 | References |
|---|---|---|---|
| Co@NiFe-LDH | 44 mV dec−1 | 253 mV | [36] |
| Co3O4@NiFe-LDH/NF | 40.4 mV dec−1 | 215 mV | [37] |
| Co-NiFe LDH | 38.4 mV dec−1 | 254 mV | [38] |
| CoP@NiCo-LDH | 46.6 mV dec−1 | 225 mV | [39] |
| FeCo-LDH@Co(OH)2 | 75.8 mV dec−1 | 230 mV | [40] |
| def-Ru-NiFe LDH/NCO | 52 mV dec−1 | 225 mV | [41] |
| NiFe@LDH | 76 mV dec−1 | 260 mV | [42] |
| MoS2/NiFeCr LDH | 61 mV dec−1 | 224 mV | [43] |
| This work | 36.8 mV dec−1 | 209 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Wang, L.; Yang, T.; Liu, S.; Yang, L.; Wang, E.; Yu, X.; Wang, H.; Chou, K.-C.; Hou, X. Multimetallic Layered Double Hydroxides as OER Catalysts for High-Performance Water Electrolysis. J. Compos. Sci. 2025, 9, 540. https://doi.org/10.3390/jcs9100540
Zhan Y, Wang L, Yang T, Liu S, Yang L, Wang E, Yu X, Wang H, Chou K-C, Hou X. Multimetallic Layered Double Hydroxides as OER Catalysts for High-Performance Water Electrolysis. Journal of Composites Science. 2025; 9(10):540. https://doi.org/10.3390/jcs9100540
Chicago/Turabian StyleZhan, Yiqin, Linsong Wang, Tao Yang, Shuang Liu, Liming Yang, Enhui Wang, Xiangtao Yu, Hongyang Wang, Kuo-Chih Chou, and Xinmei Hou. 2025. "Multimetallic Layered Double Hydroxides as OER Catalysts for High-Performance Water Electrolysis" Journal of Composites Science 9, no. 10: 540. https://doi.org/10.3390/jcs9100540
APA StyleZhan, Y., Wang, L., Yang, T., Liu, S., Yang, L., Wang, E., Yu, X., Wang, H., Chou, K.-C., & Hou, X. (2025). Multimetallic Layered Double Hydroxides as OER Catalysts for High-Performance Water Electrolysis. Journal of Composites Science, 9(10), 540. https://doi.org/10.3390/jcs9100540








