Abstract
Hydrogen energy offers high energy density and carbon-free combustion, making it a promising fuel for next-generation propulsion and power generation systems. Hydrogen offers approximately three times more energy per unit mass than natural gas, and its combustion yields only water as a byproduct, making it an exceptionally clean and efficient energy source. Materials used in hydrogen-fueled combustion engines must exhibit high thermal stability as well as resistance to corrosion caused by high-temperature water vapor. This study introduces a novel ceramic matrix composite (CMC) designed for such harsh environments. The composite is made of yttria-stabilized zirconia (YSZ) fiber-reinforced silicoboron carbonitride [Si(B)CN]. CMCs were fabricated via the polymer infiltration and pyrolysis (PIP) method. Multiple PIP cycles, which help to reduce the porosity of the composite and enhance its properties, were utilized for CMC fabrication. The Si(B)CN precursor formed an amorphous ceramic matrix, where the presence of boron effectively suppressed crystallization and enhanced oxidation resistance, offering superior performance than their counter part. Thermogravimetric analysis (TGA) confirmed negligible mass loss (≤3%) after 30 min at 1350 °C. The real-time ablation performance of the CMC sample was assessed using a hydrogen torch test. The material withstood a constant heat flux of 185 W/cm2 for 10 min, resulting in a front-surface temperature of ~1400 °C and a rear-surface temperature near 700 °C. No delamination, burn-through, or erosion was observed. A temperature gradient of more than 700 °C between the front and back surfaces confirmed the material’s effective thermal insulation performance during the hydrogen torch test. Post-hydrogen torch test X-ray diffraction indicated enhanced crystallinity, suggesting a synergistic effect of the oxidation-resistant amorphous Si(B)CN matrix and the thermally stable crystalline YSZ fibers. These results highlight the potential of YSZ/Si(B)CN composites as high-performance materials for hydrogen combustion environments and aerospace thermal protection systems.
1. Introduction
The global transition towards sustainable energy and reduced carbon emissions has positioned hydrogen as a crucial fuel source for advanced aerospace and power generation systems [1,2]. Hydrogen offers a clean and efficient alternative to fossil fuels, boasting a gravimetric energy density approximately three times that of natural gas and producing only water vapor upon combustion. There is increasing research on improving combustion efficiency and performance in gas turbine chambers through the utilization of hydrogen as a fuel source [3,4]. Hydrogen energy has wide flammability limits, low required ignition energy, and high flame speeds [5,6]. Furthermore, hydrogen exhibits a higher diffusivity in air (0.6 cm2/s) compared to natural gas (0.3 cm2/s), which promotes faster and more uniform mixing with air, thereby enhancing combustion efficiency [7]. However, this same combustion process presents significant material science challenges, as the resulting high temperatures and corrosive water vapor environments can degrade conventional components in modern gas turbine engines. So, new materials are needed that can withstand hydrogen combustion environments. Ceramic matrix composites (CMCs) meet the rigorous requirements necessary to tackle the above challenge.
CMCs have emerged as promising candidates because of their high melting temperatures, excellent resistance to oxidation, corrosion, and ablation, low creep, and superior thermal cycling behavior [8]. CMCs are commonly fabricated by melt infiltration. However, this method often results in high porosity and brittle microstructures, limiting their ability to withstand severe mechanical and thermal stresses. As an alternative, polymer-derived ceramics (PDCs) are formed through the pyrolysis of preceramic polymers at lower temperatures, enabling the development of enhanced fracture toughness and improved performance at relatively reduced cost [9,10].
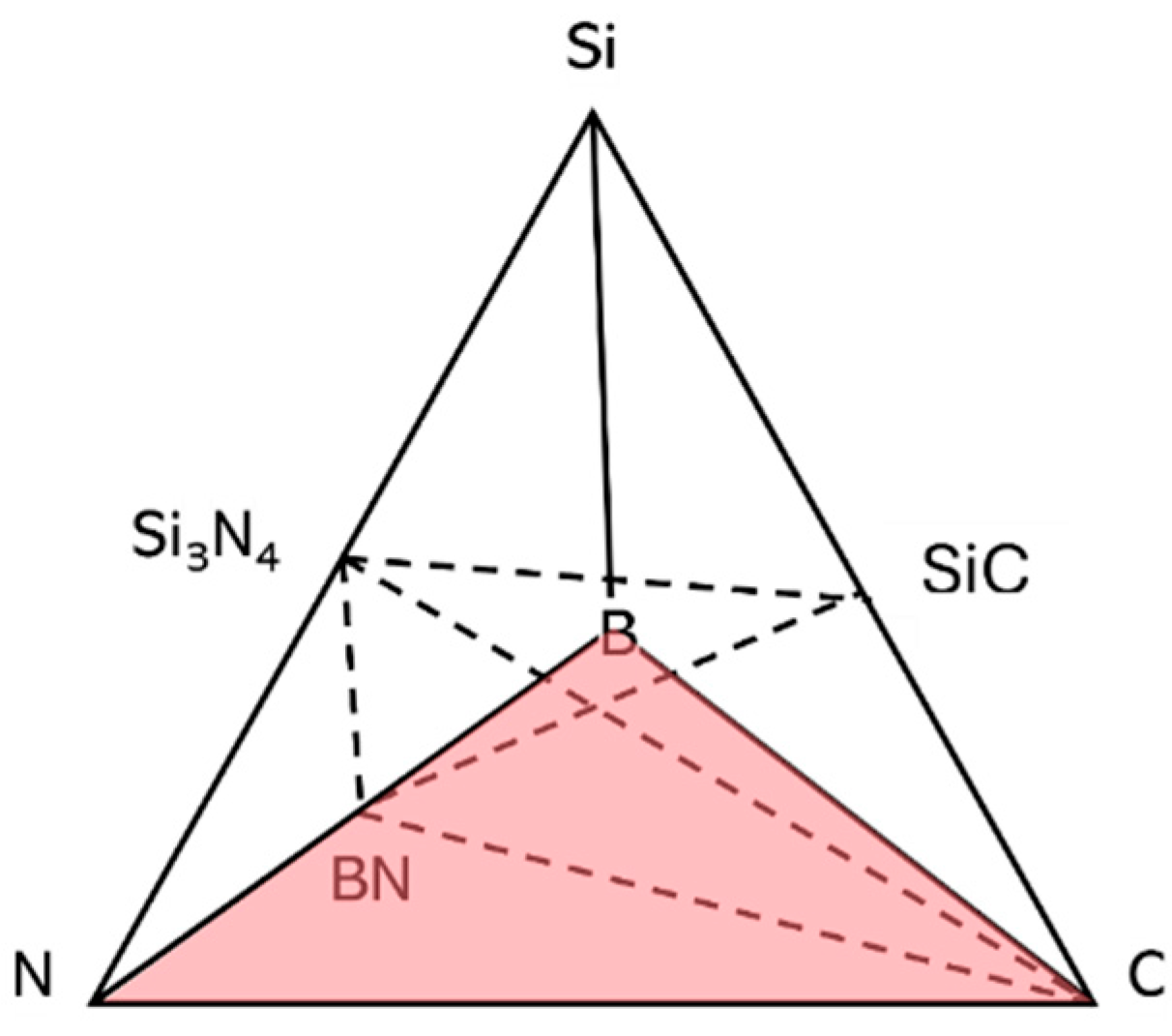
The development of Si(B)CN ceramics through the PDCs route has shown significant promise. These ceramics have demonstrated remarkable potential as high-temperature structural materials, owing to their superior mechanical strength at elevated temperatures, outstanding oxidation resistance, excellent thermal stability, and exceptional creep resistance. Such properties make Si(B)CN ceramics highly attractive for applications in extreme thermal and oxidative environments [11,12]. After pyrolysis at 1000 °C, the composition of the amorphous Si(B)CN ceramics is usually located within the four-phase field of the quaternary system, which is represented in Figure 1, SiC/Si3N4/BN/C. Si(B)CN ceramics exhibit structural singularities that allow their microstructure to remain amorphous under 1600 °C due to such possible structure. It is supposed that the BN(C) phase inhibits the growth of crystalline phases and subsequent decomposition reactions [13]. This is because in the Si(B)CN or Si(B)C system, boron plays a key role in the formation of the amorphous structure, retards the decomposition of silicon-nitride bonds, and reduces the diffusion of different species involved in the carbothermal reaction at high temperatures [14]. Therefore, to assess their resilience in the extreme thermal environment of hydrogen combustion engines, it is essential to determine the Si(B)CN ceramics performance through hydrogen combustion.
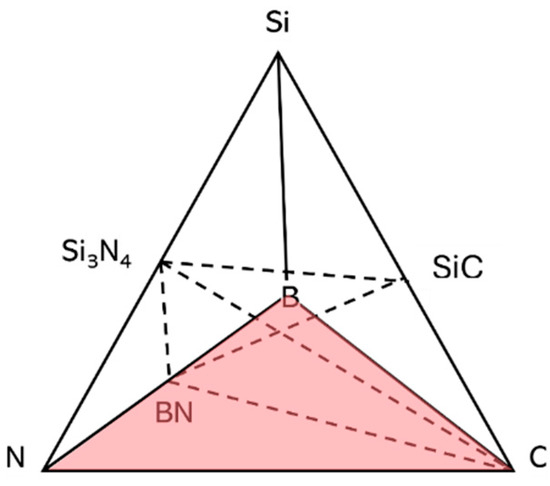
Figure 1.
Quaternary Si(B)CN diagram highlighting the four-phase field SiC/Si3N4/BN/C represented by Viard et al. [13].
Several fiber-reinforced Si(B)CN-based CMC systems have been investigated in the literature. For instance, Lee et al. developed a carbon fiber (Cf)-reinforced Si(B)CN composite, and thermal stability tests revealed that degradation of the Si(B)CN matrix initiated at approximately 1500 °C [15]. Jia et al. reported high-temperature properties and interface evolution of C/Si(B)CN composites prepared by polymer infiltration and pyrolysis (PIP). In this CMC, Si(B)CN matrix could remain amorphous up to 1500 °C and SiC grains appeared at 1600 °C but without Si3N4 [16]. Lu et al. fabricated SiC/Si(B)CN composites from polyborosilazane-derived Si(B)CN, and oxidation tests in air at 1200–1500 °C demonstrated excellent performance [17]. Although progress has been made in fabricating Si(B)CN-based CMCs with carbon fiber or SiC fiber, their performance in hydrogen combustion environments is limited by intrinsic material drawbacks. Carbon fibers are thermally conductive and suffer from rapid oxidative degradation when exposed to water vapor and oxygen at high temperatures. Similarly, SiC fibers demonstrate good high-temperature mechanical properties and oxidation resistance in air; however, they undergo active oxidation in water vapor environments typical of hydrogen combustion. In contrast, yttria-stabilized zirconia (YSZ) fibers offer distinct advantages that make them more suitable as reinforcements for Si(B)CN matrices in hydrogen combustion environments. YSZ exhibits exceptionally high thermal stability, withstanding temperatures up to ~2200 °C [18], and has long been employed in thermal barrier coatings (TBCs) for turbine blades due to its excellent oxidation resistance and low thermal conductivity [19,20].
Prior studies have primarily relied on oxy-acetylene torch tests or plasma flames and reaches higher flame temperatures, but do not fully replicate the chemical and thermal challenges posed by hydrogen fuel, especially the corrosive effect of high-temperature water vapor. For instance, Li et al. investigated the effect of graphene on the ablation properties and microstructures of graphene-reinforced Si(B)CN ceramics by subjecting a sample to a 10 s ablation test in a flowing oxyacetylene flame, during which the surface temperature reached a maximum of 2400 °C [21]. Liang et al. used the same method with a heat-flow rate reached about 420 W/m2 to investigate SiCf/Cf/Si(B)CN ceramic composites’ ablation properties under oxyacetylene combustion for 10 s [22]. In addition, Ding et al. investigated ablation behavior and thermal damage mechanisms of Cf/SiBCN composites in plasma ablation flame which used argon and hydrogen as plasma-forming gases under a heat flux of 4.02 MW/m2 for 60 s [23]. One exception is a study by Kubota et al., in which they tested oxidation by an oxygen–hydrogen torch where the samples were rapidly heated under oxygen–hydrogen combustion gas and exposed for 10 min [24]. The primary purpose of this work is to bridge this research gap by developing a new CMC material that combines a Si(B)CN matrix with YSZ fibers and to evaluate its high-temperature stability and ablation performance specifically under a hydrogen torch test. In this work, we develop a YSZ fiber-reinforced Si(B)CN CMCs and evaluate its high-temperature oxidation resistance under a hydrogen flame with a constant heat flux of 185 W/cm2 for 10 min. Si(B)CN was synthesized through the PDCs route and remains amorphous with no significant weight loss up to ~1350 °C—indicating excellent thermal stability of the matrix itself. Meanwhile, the YSZ fiber performance was rigidized and integrated to impart both structural reinforcement and thermal insulation properties. This combination is explicitly chosen to tackle the high-temperature, corrosive hydrogen combustion environment. During the hydrogen torch exposure, the YSZ/Si(B)CN composite survived without delamination, burn-through, or significant erosion. In fact, it maintained a large thermal gradient, e.g., >700 °C, difference between the flame-facing surface and back face due to improved thermal insulating effect. These findings demonstrate a viable materials solution for hydrogen-fueled engines, offering both high thermal stability and corrosion resistance in extreme combustion environments.
2. Materials and Methods
2.1. Materials
YSZ twill-weave fiber mats (phase-stabilized with 10 wt% Yttria) were obtained from Zircar Zirconia, Inc. [18] (Florida, NY, USA), along with a YSZ rigidizing agent made of sub-micron YSZ particles in an aqueous acetate solution. Rigidizing agent was required to improve the structural robustness of the porous preform due to the intrinsic low tensile strength of the raw ceramic fiber. Table 1 represents the properties of YSZ twill-weave fiber mats (ZYW-30A). Si(B)CN matrix was synthesized by using methylvinyldichlorosilane (MVDCS), methyldichlorosilane (MDCS), hexamethyldisilazane (HMDZ), boron trichloride (BTC (1 M in hexane) and dicumyl peroxide (DCP) which were supplied by Millipore Sigma Co., Ltd. (St. Louis, MO, USA).

Table 1.
Properties and characteristics of the YSZ twill-weave fiber mats.
2.2. Synthesis of Liquid Polymer Precursor for Si(B)CN
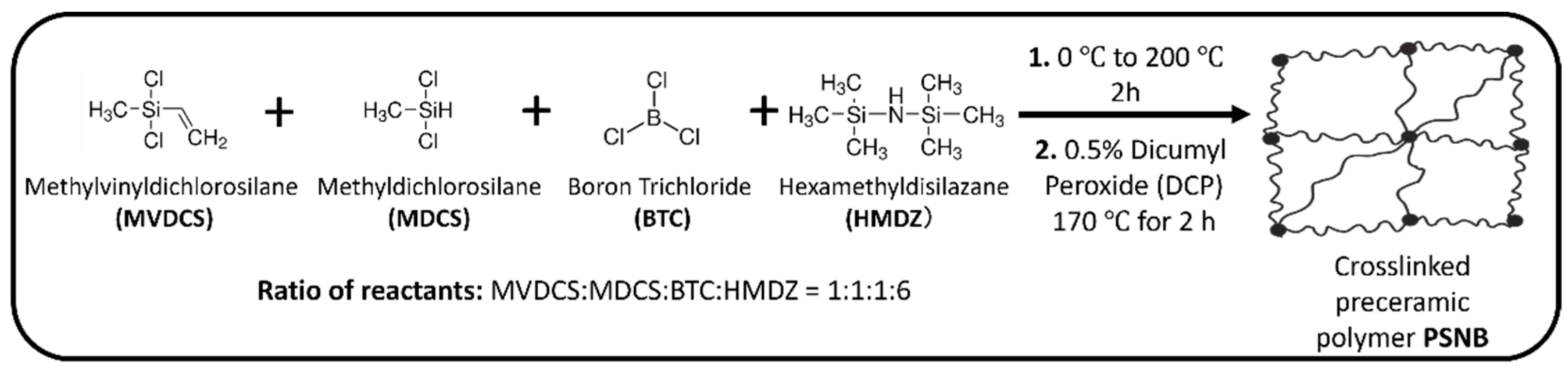
The liquid polyborosilazane precursor for Si(B)CN ceramic is synthesized by co-condensation reaction of boron trichloride, organodichlorosilanes, and hexamethyldisilazane [11]. Figure 2 illustrates the synthesis of preceramic Si(B)CN resin, carried out with a reactant molar ratio of 1:1:1:6 (MVDCS:MDCS:BTC:HMDZ), using a Schlenk line (obtained from Chemglass Life Sciences (Vineland, NJ, USA)) maintaining nitrogen (N2) atmosphere. Each batch contained 8 mL of MVDCS and other reactants, added in amounts calculated from the specified molar ratios. During the synthesis process, HMDZ was added dropwise to the reaction contents (a mixture of MVDCS, MDCS, and BTC solution), which was kept in an ice-water bath for 30 min. Then the reaction mixture was heated to 200 °C slowly and maintained the temperature for 2 h to remove by-product of Me3SiCl, solvent, and unreacted starting materials. After cooling down the reaction mixture to room temperature, 15 mL light-yellow viscous liquid preceramic polymer precursor (PSNB) was obtained from a single batch. PSNB stands for a polymeric single-source precursor that contains Si, B, C, and N in its backbone. Curing is an effective way to increase the ceramic yield by formation of crosslinking framework of the polymeric precursors. Before curing, 0.5 wt% DCP was added to PSNB and stirred the mixture thoroughly at room temperature.
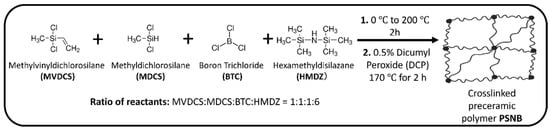
Figure 2.
Schematic representation of synthesis of crosslinked preceramic polymer PSNB.
2.3. Preparation of YSZ/Si(B)CN Ceramics Matrix Composites
YSZ twill-weave fiber mats were selected as the ceramic fibers for CMCs because of their superior thermal and chemical resistivity. The YSZ laminates were strengthened mechanically and dimensionally by using zirconium oxide rigidizer, which was composed of sub-micron YSZ particles in an aqueous solution of zirconium acetate. By increasing the YSZ concentration of the composites, this integration enhanced their thermal performance even further. Eight layers of YSZ fiber were fabricated using the hand layup process in a stacked sequence. The YSZ twill-weave fiber mats were subsequently impregnated with a YSZ rigidizer solution to form the base structure (preform). The autoclave (ASC Process Systems, Valencia, CA, USA) was used for curing the preform at 150 °C for 2h while maintaining constant pressure of 20 inch-Hg.
For curing, the preform YSZ fiber was vacuum soaked at 20 inch-Hg pressure in preceramic Si(B)CN resin to ensure complete penetration of the polymer. Then, the fiber with matrix was heated to 170 °C at a heating rate of 10 °C/min, with a dwell time of 2 h under N2. After cooling down, a hard bulk crosslinked YSZ/Si(B)CN preceramic composite was obtained. The polymer infiltration and pyrolysis (PIP) procedure was used in this study for the fabrication of YSZ/Si(B)CN ceramic matrix composites. The cured fiber with matrix was then pyrolyzed in a high-temperature sintering furnace from room temperature to the 850 °C for an additional 2 h in a flowing argon atmosphere with a heating rate of 5 °C/min. After that, the material cooled down to room temperature at the same rate. The final YSZ/Si(B)CN ceramic matrix composite was fabricated by two PIP cycles.
2.4. Hydrogen Torch Test
A hydrogen torch testing rig was designed and built to subject the YSZ/Si(B)CN samples to a constant heat flux H2 flame for a minimum of 10 min [25]. This setup used a circular foil heat flux gauge (TG 1000-1, Vatell Corporation (Christiansburg, VA, USA)) to map the heat flux output of the torch setup at various distances from the torch tip. The CMC samples were loaded onto the sample holder and held in place by a stainless-steel faceplate. A spring-loaded K-type thermocouple (OMEGA Engineering, Michigan City, IN, USA) was positioned on the center of the back side of the sample to measure back-face temperature, while an infrared radiation thermometer (IR-HAQNE, CHINO Corporation, Torrance, CA, USA) was used to record the temperature of the flame-exposed front face of the sample. Then the CMC samples were exposed to the hydrogen flame torch for 10 min continuously before the test ended.
2.5. Material Characterization
The YSZ/Si(B)CN ceramic matrix composites were characterized by a series of tests such as density determination, nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), and thermogravimetric analysis (TGA). Density was tested by a Cole-Parmer Density Determination Kit (128 × 128 mm Weighing Pan). 1H-NMR spectra were recorded in CDCl3 solution with a Bruker AVANCE 400 spectrometer. SEM measurements were carried out with Cu Ka radiation on a Zeiss ULTRA-55 FEG scanning electron microscope. Energy Dispersive Spectroscopy (EDS) was performed by the Noran System 7 EDS with a Silicon Drift Detector x-ray detector. XRD were carried out on a Basic Diffraction Analytical Empyrean, with a basic X-Ray diffractometer crystal structure and lattice parameters of the materials. And TGA-MS was carried out on a Diamond TGA-MS unit (PerkinElmer Shelton, CT, USA). TGA-MS was carried out under nitrogen at temperatures between 20 and 1350 °C with a heating rate of 10 °C/min. The oxidation resistance of the ceramics in a compressed air environment was also recorded with this instrument.
3. Results and Discussion
3.1. Microstructural Analysis
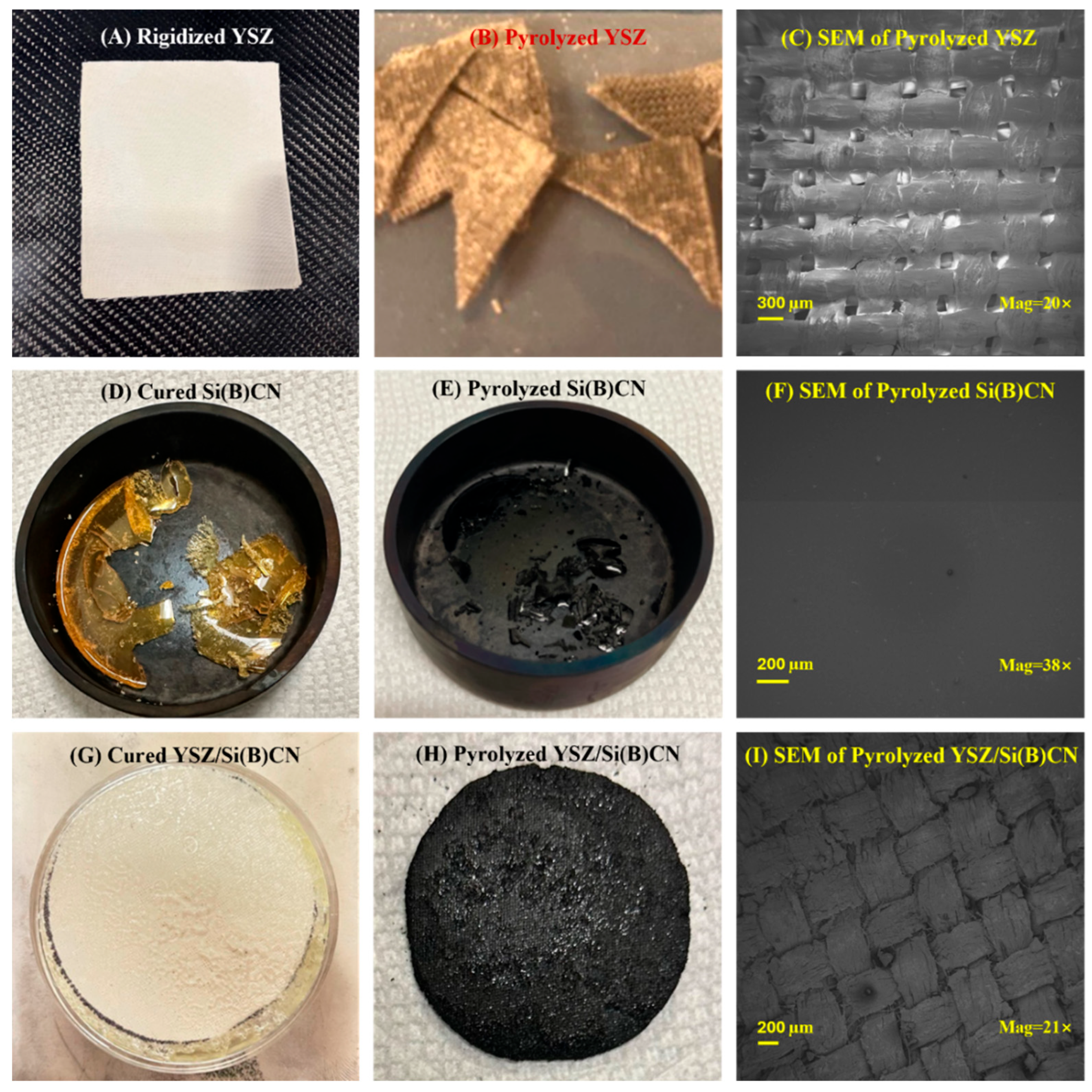
As demonstrated in Figure 3, from left to right there are YSZ twill-weave fiber mats with rigidizer, Si(B)CN matrix, and YSZ/Si(B)CN CMC, respectively. The YSZ fibers were white in Figure 3A. After pyrolysis at 850 °C, the color was changed to gray in Figure 3B. And there was some powder residue because of the addition of the rigidizer. In Figure 3C, the surface of eight layers of YSZ fiber mats show clear pores where the fibers interweave with each other. From Figure 3D, the Si(B)CN ceramic was transformed from a yellow liquid to a complete yellow transparent solid after curing. As Figure 3E, this material changed color from yellow to black and shrunk to 1/3 of its original size after pyrolysis. From the SEM in Figure 3F, the surface of Si(B)CN became dense and free of pores.
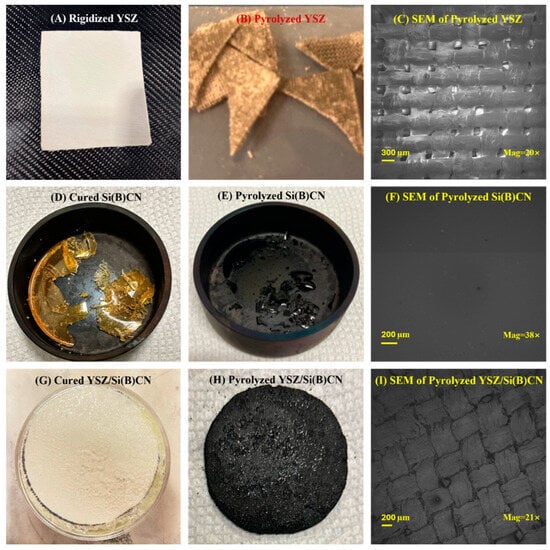
Figure 3.
(A) Rigidized YSZ, (B) pyrolyzed YSZ, (C) SEM image of pyrolyzed YSZ, (D) cured Si(B)CN, (E) pyrolyzed Si(B)CN, (F) SEM image of pyrolyzed Si(B)CN, (G) cured YSZ/Si(B)CN, (H) pyrolyzed YSZ/Si(B)CN, and (I) SEM image of pyrolyzed YSZ/Si(B)CN.
Figure 3G shows the cured YSZ fiber mats which were soaked in preceramic Si(B)CN resin. The white YSZ fibers were surrounded by yellow Si(B)CN matrix. Figure 3H demonstrates the YSZ/Si(B)CN CMC with a black color after two PIP cycles. And the Si(B)CN after pyrolysis can be seen embedded in the surface of the CMC. The SEM of YSZ/Si(B)CN in Figure 3I shows a dense and uniform distribution of the Si(B)CN matrix within the CMC. The YSZ fibers and Si(B)CN matrix is homogeneously dispersed, indicating effective infiltration and dispersion during the PIP cycles. Compared with Figure 3C, the pores in the fiber mats were filled with the matrix, which supported the fabrication of YSZ/Si(B)CN. The elemental mapping presented in the Supporting Information (Figure S1) also confirms a uniform distribution of all constituent elements throughout the composite. No significant clustering was detected. This indicates homogeneous and effective integration of phases throughout. Thus, microstructural analysis signifies strong interfacial bonding, which is essential for high-temperature applications.
EDS analysis revealed the existence of silicon (Si), boron (B), carbon (C), nitrogen (N), and oxygen (O) in the resulting ceramics after pyrolysis at 850 °C. This confirms the successful synthesis of Si(B)CN, while also demonstrating that YSZ reinforcement through the PIP method is an effective fabrication approach.
According to Table 2, YSZ consisted of 76.4% zirconia (Zr), 7.2% yttria (Y), 15.3% O, and 1.1% C. Zr provided the largest weight of YSZ, and the weight ratio of Zr and Y was around 10:1. After combining with Si(B)CN matrix and YSZ fibers, the B, C, N, O, Si, and Zr were detected by EDS in this CMC material. But the percentage of different elements has changed based on their mole weights and original portion. The Y element was not found, which had a small percentage even in fibers.

Table 2.
Elemental composition of Si(B)CN, YSZ, and YSZ/Si(B)CN from EDS analysis.
EDS confirmed the presence of the key elements: Zr, Si, B, C, N, and O. The mapping shows (Figure S1) a relatively even spatial distribution of the constituents, further verifying the composite homogeneity. Boron was consistently detected in the matrix, implying successful incorporation of the Si(B)CN network, which is known to enhance thermal stability and oxidation resistance.
3.2. Density of YSZ/Si(B)CN
After the measurement and calculation, the density of Si(B)CN is around 1.55 g/cm3, and YSZ is 3.42 g/cm3, while YSZ/Si(B)CN is around 3.15 g/cm3. The density of YSZ fiber was the highest, while YSZ/Si(B)CN was slightly lower. This is because the Si(B)CN resin with lowest density filled the voids of the YSZ twill-weave fiber mats, and the mass increases while the volume also increases. And it showed that the porosity of the CMC was lower than YSZ fibers. Under the combined influence of mass and volume, the density result is tabulated in Table 3.

Table 3.
Density of YSZ, Si(B)CN, YSZ/Si(B)CN.
According to the tested densities of the YSZ fibers, Si(B)CN matrix, and CMC, neglecting the presence of the air volume, the volume percentages of fibers and matrix in the CMC could be calculated as the following equations [26]:
where is the density of CMC specimens. is the density of fibers, and is the density of matrix. and are the volume of fibers and matrix, respectively, in the CMC.
After calculating, the fiber volume was about 85.6%, and the matrix volume was 14.4% in the YSZ/Si(B)CN CMC. The volume of fibers in the CMC is an important factor for their mechanical properties and performance.
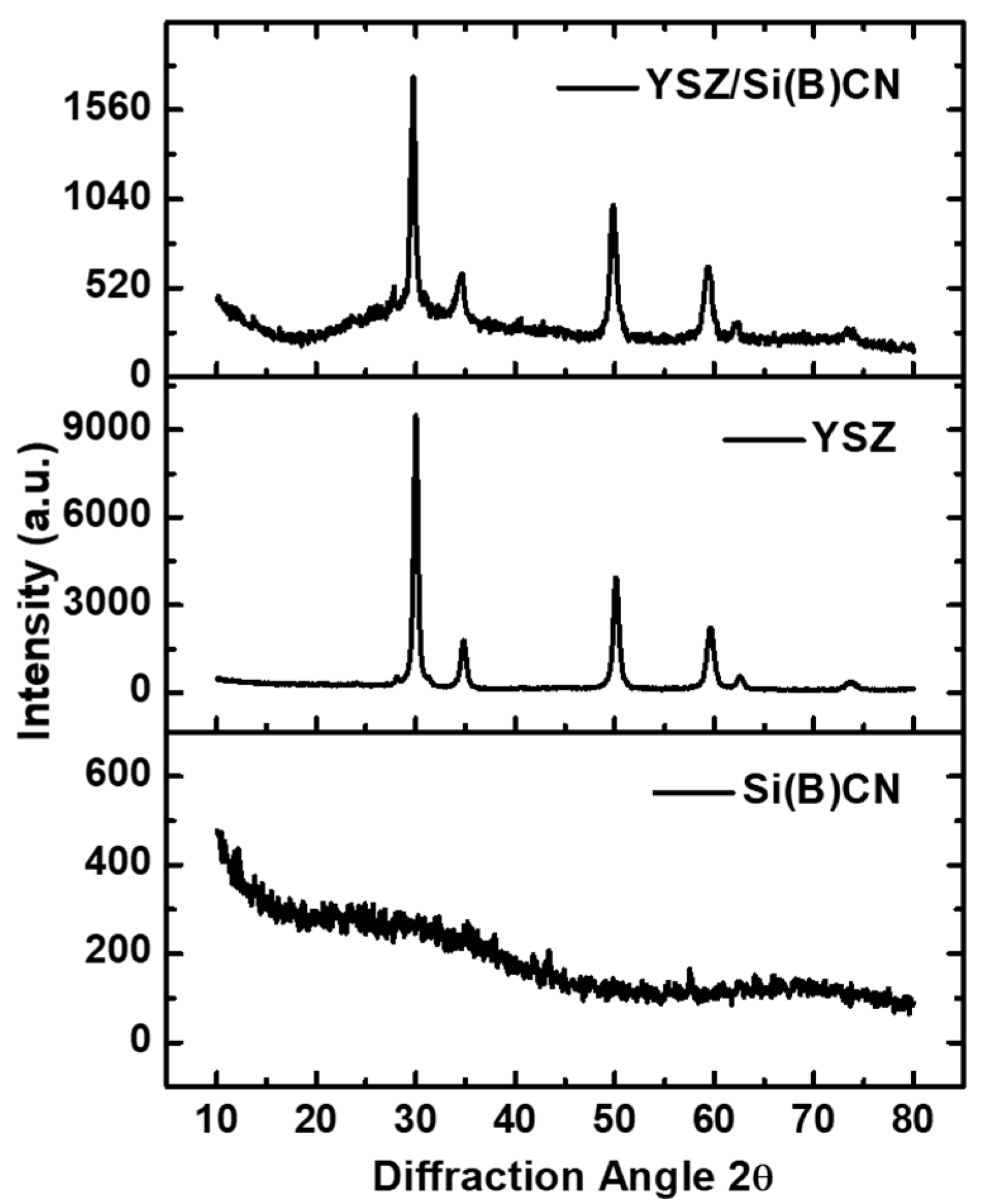
3.3. XRD Analysis of YSZ/Si(B)CN
Figure 4 illustrates the XRD characterization of Si(B)CN ceramics, YSZ fibers, and the YSZ/Si(B)CN CMC, respectively, after a constant pyrolysis at 850 °C. The results clearly indicate that the Si(B)CN ceramics retained their amorphous structure following pyrolysis. The presence of boron in Si(B)CN delays the onset of crystallization because of originating with other atoms from strong covalent bonds, enabling the material to maintain its amorphous structure at higher temperatures. The XRD diagram demonstrates there were six characteristic peaks for YSZ before or after the PIP cycles. XRD patterns of the YSZ/Si(B)CN displayed characteristic peaks corresponding to crystalline phases of YSZ, along with a broad hump attributed to the amorphous Si(B)CN phase. Rietveld refinement analysis suggest that the diffraction pattern of YSZ/Si(B)CN is dominated by tetragonal/cubic YSZ, with a minor monoclinic contribution (~2–3 wt%), evidenced by the weak shoulder/peak observed at ~27–28.5° 2θ.
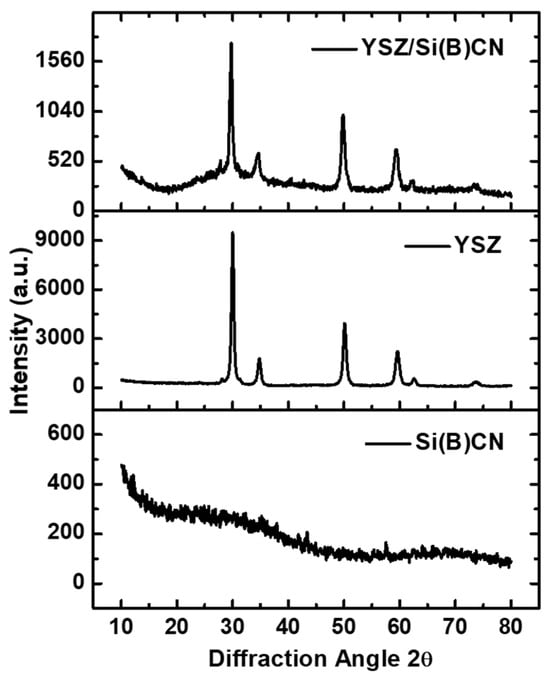
Figure 4.
XRD patterns of the Si(B)CN matrix, YSZ fiber, and YSZ/Si(B)CN CMC materials after pyrolysis at 850 °C.
The tetragonal phase of YSZ is particularly advantageous due to its favorable thermal and mechanical stability under high-temperature oxidative conditions. Moreover, this process was accomplished at a relatively low temperature. This is a main advantage of the amorphous ceramics that can be obtained at low temperatures around 800–1000 °C or even lower, which is a clear advantage compared to crystalline ones (obtained at higher temperatures ≥1500 °C) in terms of cost [13]. In addition, the amorphous matrix may have a protective effect by encapsulating into the YSZ fibers and maintaining the structural integrity of the YSZ/Si(B)CN ceramics during high-temperature tests.
3.4. NMR Characterization of YSZ/Si(B)CN
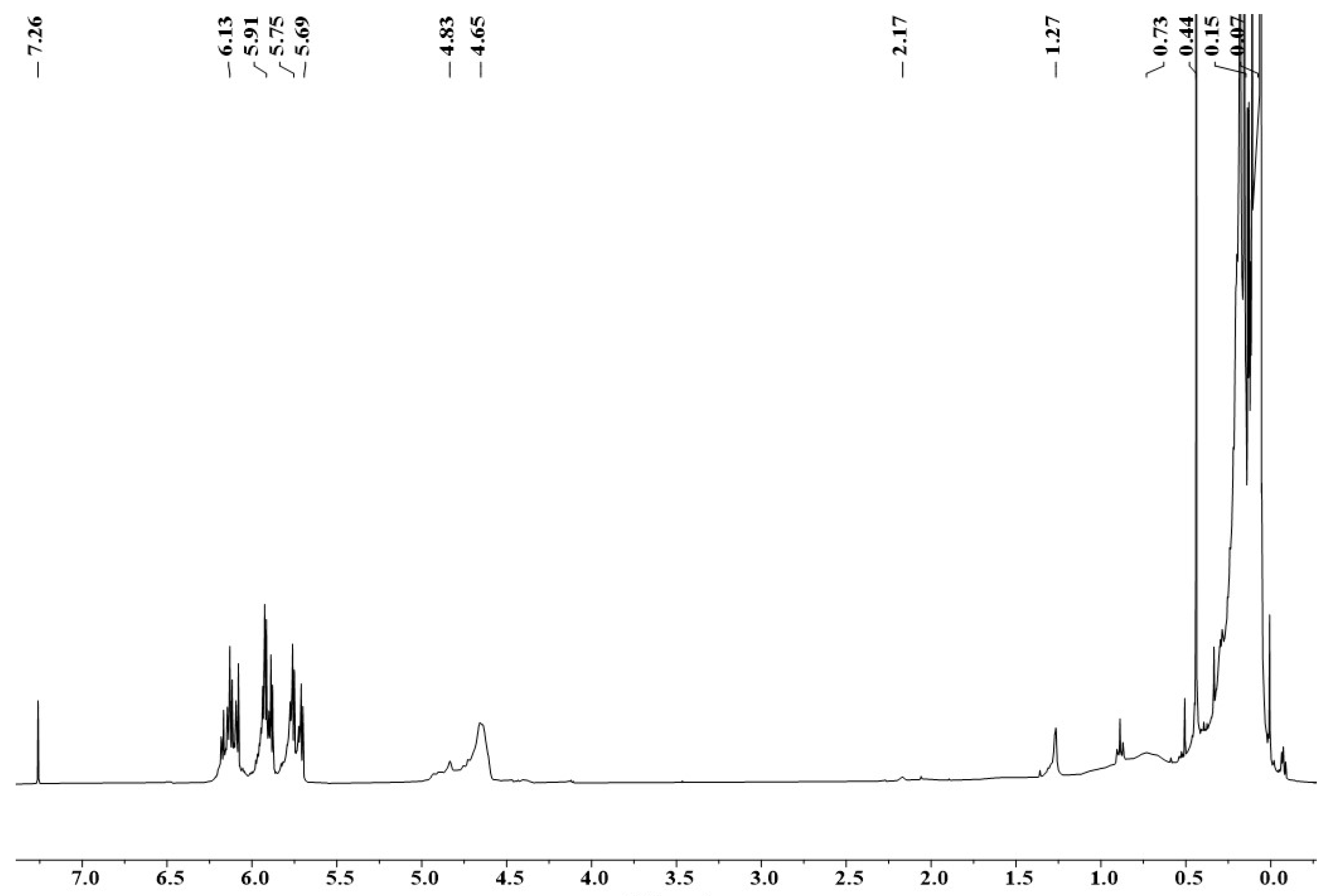
It was confirmed by 1H-NMR spectroscopy (Figure 5) that polymer precursors PSNB containing both Si-H and Si-Vi groups can be obtained using MDVCS and MDCS as starting chemicals. From Figure 5, the broad resonances at 4.65–4.83 and 5.69–6.13 ppm which are characteristic resonance peak of Si-H and Si-(CH=CH2). The multiple peaks at 0.07 ppm are attributed to Si-CH3, and a peak at 0.44 ppm can be attributed to Si-NH. The broad peak at 0.73 and 1.27 ppm is assigned to B-NH-Si, and 2.17 ppm belongs to B2NH in borazine ring, respectively. 1H-NMR spectra clearly revealed successful synthesis of polymeric precursor preceramic Si(B)CN resin, similarly to the synthesis results in Zhang et al. [11].
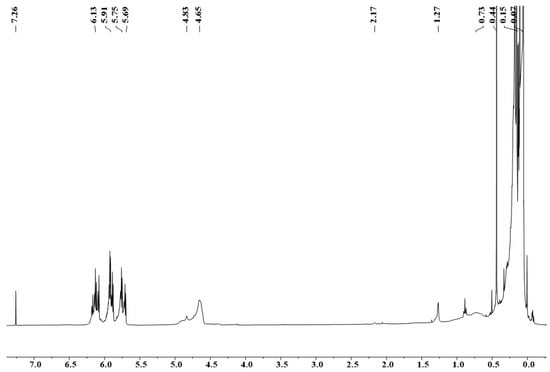
Figure 5.
1H-NMR spectrum of PSNB.
3.5. High-Temperature Stability
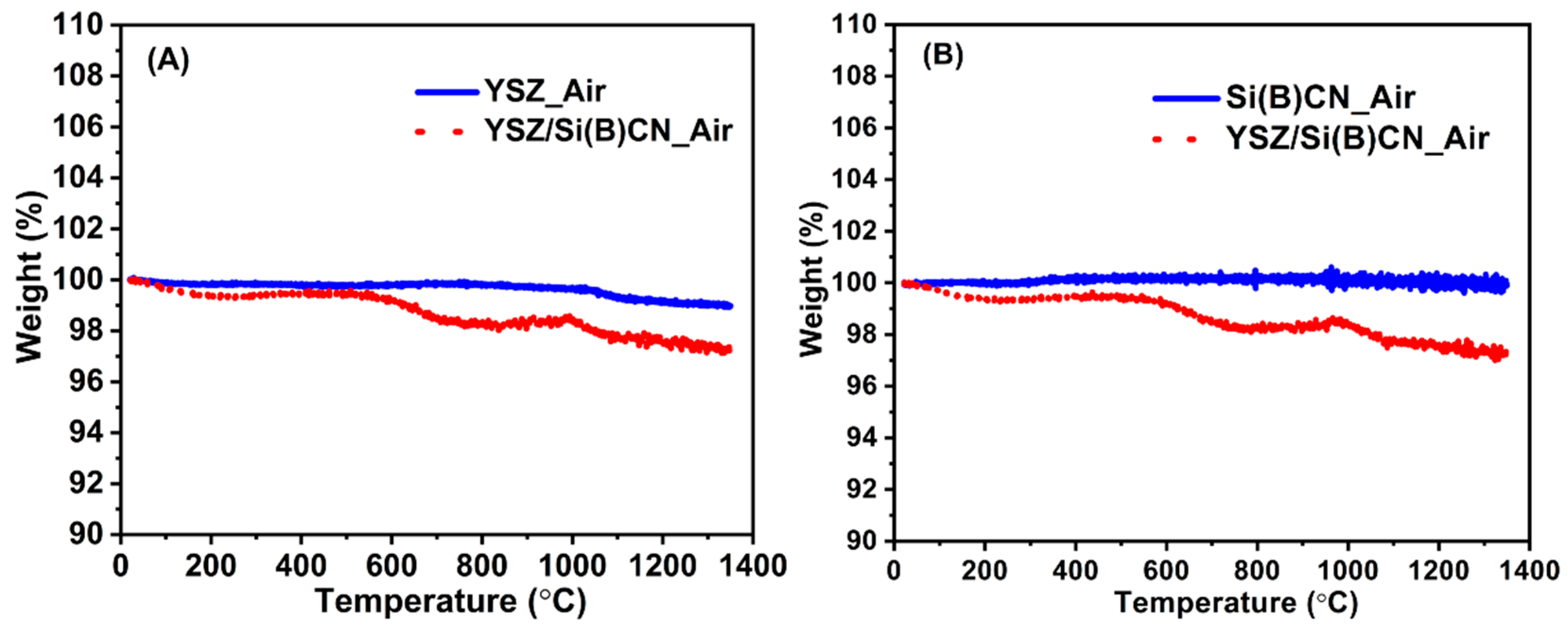
As shown in Figure 6, TGA demonstrates that almost no weight loss was observed for Si(B)CN ceramics at 1350 °C after 30 min. YSZ fibers exhibited about 2% weight loss, while around 3% weight loss was observed on the YSZ/Si(B)CN ceramics matrix composite. Therefore, the weight loss from YSZ was dominant. From Figure 3B, the twill-weave YSZ fiber mats with rigidizer showed some powder residue after pyrolysis, so the weight might change due to the rigidizer.
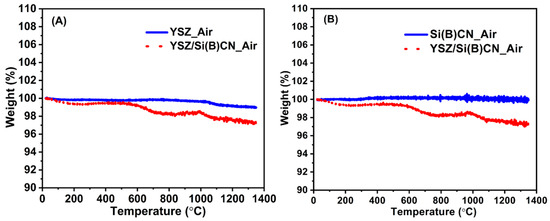
Figure 6.
Thermal stability analysis and anti-oxidation property analysis of YSZ, Si(B)CN, and YSZ/Si(B)CN ceramic matrix composites. (A) The weight loss of YSZ and YSZ/Si(B)CN samples at 1350 °C for 30 min. (B) The weight loss of Si(B)CN and YSZ/Si(B)CN samples at 1350 °C for 30 min.
From this result, Si(B)CN ceramics exhibit excellent thermal properties that allow their macrostructure to maintain a good structural integrity at elevated temperatures. Boron in Si(B)CN played a key role in improving oxidation resistance, preventing further degradation of the underlying material. The existence of boron, which can prevent silicon nitride decomposition and crystallization of ceramic, therefore further stabilizes the amorphous state in Si(B)CN ceramics [13]. This property is particularly important for applications in extreme environments where maintaining CMC structural integrity is critical.
After TGA, the YSZ/Si(B)CN sample was analyzed in EDS. According to Table 4, the weight percentage of N decreased from 1.5% to 1%, and Zr decreased from 72.7% to 62.2%. Under 1350 °C for 30 min, the reduction in Zr altered the relative elemental ratios. The percentage of O increased from 13.1% to 22%, which was improved by 68%. Other elements increased slightly as B increased to 2.3%, C increased to 6.2%, and Si increased to 6.4%. The overall ~10% mass loss of Zr in YSZ fiber after hydrogen torch test may be attributed to probable surface reaction due to the presence of Si in the Si(B)CN matrix and also the silica-based rigidizer. At temperatures ≥1300 °C, Zr does not volatilize but instead reacts with SiO2 to form zircon (ZrSiO4). Such interfacial reactions consume Zr locally, explaining the decrease in Zr % after ablation while the bulk YSZ structure remains largely intact [27,28].

Table 4.
EDS elemental analysis of YSZ/Si(B)CN after TGA measurement.
The minimal weight loss in macro structure and relatively intact microstructure of YSZ/Si(B)CN in TGA lasting 30 min at 1350 °C, which showed this high thermal stability under oxidative conditions reinforces the material’s suitability for extreme environments, including hydrogen-rich combustion atmospheres.
3.6. Results of Hydrogen Torch Test
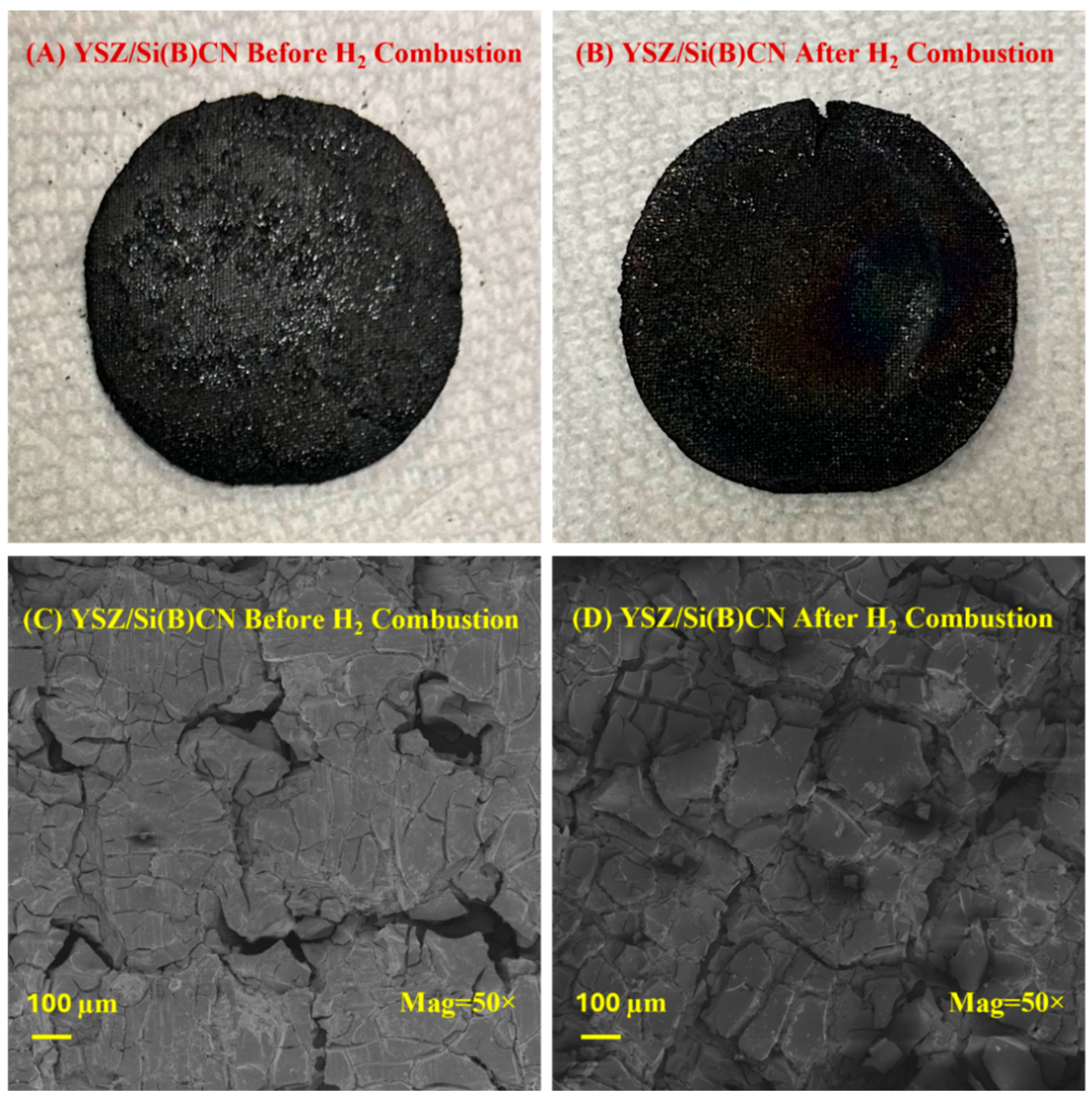
The high-temperature performance of YSZ/Si(B)CN was tested using a hydrogen torch, with the samples exposed to a hydrogen flame at a constant heat flux (Supporting Video). An infrared radiation thermometer was used to record the temperature of the front side of the sample exposed to the flame, and a spring-loaded K-type thermocouple in contact with the back side of the sample was used to record the back side temperature throughout the test. As shown in Figure 7A,B, the material exhibits minimal erosion or degradation even after 10 min of continuous high heat flux torch testing. The SEM images presented in Figure 7C,D effectively capture the key microstructural features relevant to our study. Prior to hydrogen torch testing, the composite surface exhibited comparatively higher porosity. Upon exposure to the hydrogen flame at ~1400 °C, a glassy oxide layer formed and infiltrated these pores, thereby shielding the underlying material from direct erosion by high-temperature water vapor and oxygen. This protective oxide layer contributed to the observed stability of the composite during testing. Upon cooling, surface cracks were generated because of thermal stress, which is consistent with thermal expansion mismatch and rapid cooling effects. Importantly, no evidence of delamination, burn-through, or thickness reduction was observed after hydrogen combustion.
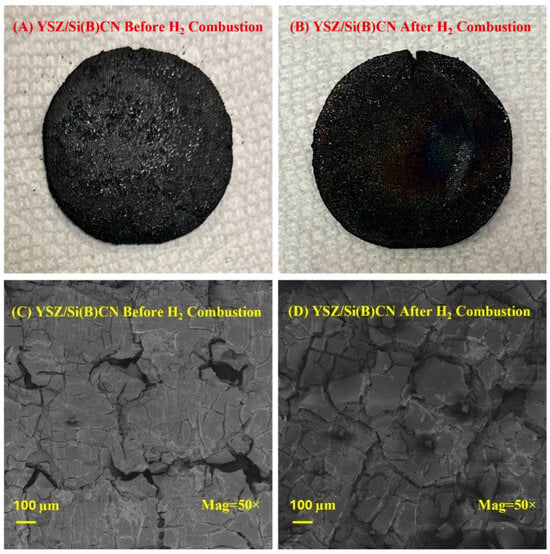
Figure 7.
(A,B) were photos of YSZ/Si(B)CN CMC before and after hydrogen torch test, respectively. (C,D) were SEM of YSZ/Si(B)CN before and after hydrogen torch test, respectively.
As shown in Table 5, the main composition of the interface layer was B, C, N, Si, O, and Zr. After the hydrogen torch test, the relative proportions of B, C, Si, and O increased, whereas N and Zr decreased, indicating possible reactions involving N and Zr. These compositional changes confirm that the CMC underwent chemical reactions under hydrogen combustion. Consistent with the TGA results, the weight loss (~47%) was primarily attributed to Zr depletion, likely due to reactions with high-temperature flame and water vapor. The reduction in Zr altered the relative elemental ratios, leading to an O content increase to 26.2% (approximately double the initial value), B to 4%, C to 9.1% (both nearly twice their initial levels), and Si to 21.5% (about 2.5 times higher than before the H2 torch test).

Table 5.
Elemental analysis of YSZ/Si(B)CN after the torch test.
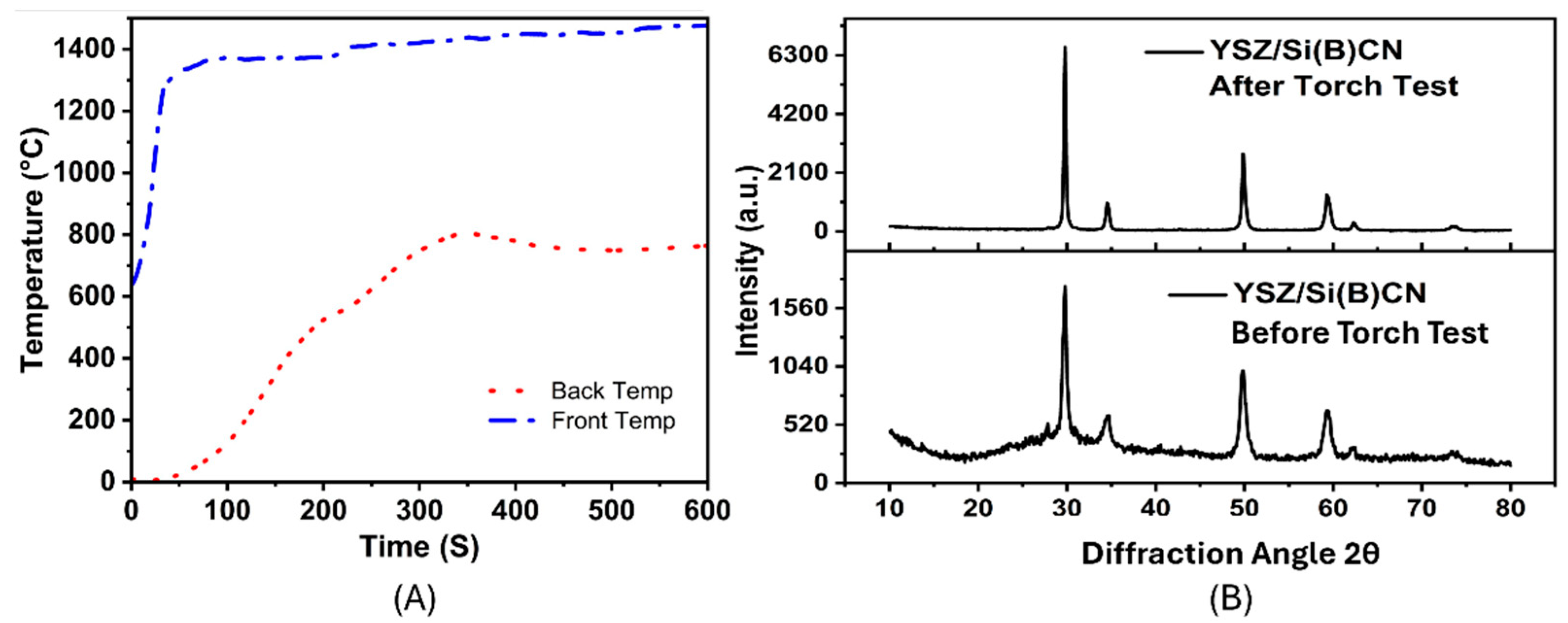
Additionally, the CMC samples demonstrated reliable and positive results when tested with a hydrogen torch. Throughout the torch exposure, the temperature at the front of the sample remained stable at around 1400 °C, while the temperature at the rear stayed significantly lower, consistently hovering around 700 °C, as shown in Figure 8A. From start to finish, the difference in temperature between the front and the back sides suggests that YSZ/Si(B)CN has some insulating properties. After 6 min, the temperature of the front and back stabilized, and the oxidation of the surface acted as a protection mechanism for the material.
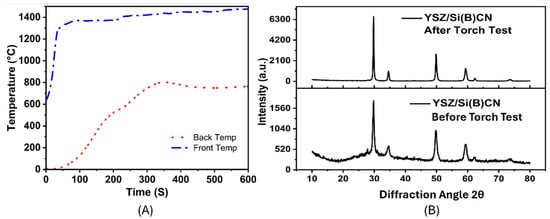
Figure 8.
(A) Front and back temperature plots of YSZ/Si(B)CN samples during 10 min of hydrogen flame exposure. (B) XRD of YSZ/Si(B)CN samples before and after the hydrogen torch test.
The exceptional thermal stability of silicoboron carbonitride (SiBCN) extends the applicability of Si3N4-based ceramics beyond 1400 °C, a temperature traditionally regarded as the upper limit for Si3N4. Following hydrogen combustion, the characteristic peaks of Si3N4 disappeared, indicating its decomposition [13]. As shown in Figure 8B, the as-fabricated composite contains crystalline YSZ peaks along with a broad amorphous hump from the Si(B)CN matrix, which is typical since boron-rich Si(B)CN resists crystallization up to ~1600 °C. Minor secondary phases cannot be excluded at this stage because residual rigidizer (submicron YSZ particles) and partially crystallized Si–N or Si–C bonds may contribute to weak reflections. After hydrogen torch exposure (~1400 °C), the increased peak intensity suggests partial crystallization of the amorphous Si(B)CN and enhanced ordering at the YSZ fiber interface. The YSZ reinforcement remains stable and can also promote crystallization under thermal flux. Thus, the observed enhancement in crystallinity arises from synergistic effects of thermal activation of the amorphous Si(B)CN and the stability of the YSZ phase.
This YSZ/Si(B)CN CMC, with only two PIP cycles, has already shown promising high-temperature performance under a hydrogen torch test. So, more thermally efficient CMCs can be produced through repeated infiltration processes, resulting in higher ceramic yields and densities. The material’s thick structure enhances its ability to control and dissipate heat, facilitating faster thermal stabilization.
4. Conclusions
In this work, a low-viscosity polymer precursor for Si(B)CN ceramics was synthesized via the co-ammonolysis of BCl3 and dichlorosilanes, followed by hexamethyldisilazane. The derived Si(B)CN ceramics exhibited good resistance to crystallization, thermal degradation, and oxidation up to 1350 °C. YSZ/Si(B)CN ceramic matrix composites were subsequently fabricated using the polymer infiltration and pyrolysis (PIP) process. Under 10 min of hydrogen torch exposure, the composites maintained structural integrity with a front-surface temperature of ~1400 °C and a rear-surface temperature of ~700 °C, showing no macroscopic failure such as delamination or burn-through.
The observed performance can be attributed to two main factors: (i) the infiltration of the reactive Si(B)CN precursor reduced residual porosity and improved the densification of the YSZ/Si(B)CN interface, and (ii) the inherent thermal and oxidation resistance of the Si(B)CN matrix contributed to suppressing excessive degradation under high-temperature hydrogen combustion. While these findings highlight the potential of YSZ/Si(B)CN composites for hydrogen-fueled combustors and aerospace thermal protection systems, further work is needed to establish detailed phase evolution, interfacial reactions, and mechanical property retention under hydrogen combustion environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9100537/s1, Figure S1: EDS elemental mapping analysis of YSZ/Si(B)CN. Video S1: Hydrogen torch test at a constant heat flux.
Author Contributions
Conceptualization, J.G.; Data curation, Y.W., C.M. and F.F.; Formal analysis, Y.W., C.M. and J.G.; Funding acquisition, J.G.; Investigation, Y.W. and C.M.; Methodology, J.G.; Project administration, J.G.; Supervision, J.G.; Validation, Y.W., C.M. and J.B.D.; Writing—original draft, Y.W. and C.M.; Writing—review and editing, C.M. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This material is based upon work supported by the Department of Energy [National Nuclear Security Administration] Fossil Energy and Carbon Management under Award Number DE-FE0032228.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The meaning of net zero and how to get it right. Nat. Clim. Change 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Acar, C.; Bicer, Y.; Demir, M.E.; Dincer, I. Transition to a new era with light-based hydrogen production for a carbon-free society: An overview. Int. J. Hydrogen Energy 2019, 44, 25347–25364. [Google Scholar] [CrossRef]
- Yu, X.; LeBlanc, S.; Sandhu, N.; Wang, L.; Wang, M.; Zheng, M. Decarbonization potential of future sustainable propulsion—A review of road transportation. Energy Sci. Eng. 2024, 12, 438–455. [Google Scholar] [CrossRef]
- Blanco, D.; Rivera, Y.; Berna-Escriche, C.; Muñoz-Cobo, J.L. Economy decarbonization using green hydrogen and electricity, forecasts and sensitivity analysis for the Canarian Islands in 2040. J. Energy Storage 2024, 80, 110232. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Fosudo, T.; Kar, T.; Windom, B.; Olsen, D. Low-carbon fuels for spark-ignited engines: A comparative study of compressed natural gas and liquefied petroleum gas on a CFR engine with exhaust gas recirculation. Fuel 2024, 360, 130456. [Google Scholar] [CrossRef]
- Schwarz, S.; Daurer, G.; Gaber, C.; Demuth, M.; Prieler, R.; Hochenauer, C. Experimental investigation of the combustion characteristics in oxy-fuel combustion of hydrogen-enriched natural gas on a semi-industrial scale. Int. J. Hydrogen Energy 2024, 49, 323–337. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Zaykoski, J.A. Oxidation-based materials selection for 2000 °C + hypersonic aerosurfaces: Theoretical considerations and historical experience. J. Mater. Sci. 2004, 39, 5887–5904. [Google Scholar] [CrossRef]
- Sun, J.; Ye, D.; Zou, J.; Chen, X.; Wang, Y.; Yuan, J.; Liang, H.; Qu, H.; Binner, J.; Bai, J. A review on additive manufacturing of ceramic matrix composites. J. Mater. Sci. Technol. 2023, 138, 1–16. [Google Scholar] [CrossRef]
- Alvi, S.A.; Akhtar, F. High temperature tribology of polymer derived ceramic composite coatings. Sci. Rep. 2018, 8, 15105. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, F.; Han, J.; Luo, Y.; Xu, C. Synthesis and characterization of a new liquid polymer precursor for Si–B–C–N ceramics. J. Mater. Sci. 2011, 46, 5940–5947. [Google Scholar] [CrossRef]
- Xu, H.; Peng, Y.; Wei, Z.; Wang, M.; Lia, A.; Shuaia, S.; Luan, X. Oxidation behavior of 3D SiCf/SiBCN composites at 800–1200 °C. J. Eur. Ceram. Soc. 2021, 41, 148–157. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si–B–C–N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20, 1800360. [Google Scholar] [CrossRef]
- Kong, J.; Wang, M.; Zou, J.; An, L. Soluble and Meltable Hyperbranched Polyborosilazanes toward High-Temperature Stable SiBCN Ceramics. ACS Appl. Mater. Interfaces 2015, 7, 6733–6744. [Google Scholar] [CrossRef]
- Lee, S.-H.; Weinmann, M. Cfiber/SiCfiller/Si–B–C–Nmatrix composites with extremely high thermal stability. Acta Mater. 2009, 57, 4374–4381. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, X.; Chen, S.; Gou, Y.; Li, Y.; Hu, H. High-temperature properties and interface evolution of C/SiBCN composites prepared by precursor infiltration and pyrolysis. J. Eur. Ceram. Soc. 2019, 39, 4645–4653. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, Y. Oxidation behavior of SiC–SiBCN ceramics. Ceram. Int. 2015, 41, 1023–1030. [Google Scholar] [CrossRef]
- Datasheet of YSZ Product; Zircar Zirconia, Inc.: Florida, NY, USA; Available online: https://www.zircarzirconia.com/applications (accessed on 1 September 2025).
- Saremia, M.; Afrasiabi, A.; Kobayashi, A. Microstructural analysis of YSZ and YSZ/Al2O3 plasma sprayed thermal barrier coatings after high temperature oxidation. Surf. Coat. Technol. 2008, 202, 3233–3238. [Google Scholar] [CrossRef]
- Keyvani, A.; Saremi, M.; Sohi, M.H. Oxidation resistance of YSZ-alumina composites compared to normal YSZ TBC coatings at 1100 °C. J. Alloys Compd. 2011, 509, 8370–8377. [Google Scholar] [CrossRef]
- Li, D.; Yang, Z.; Jia, D.; Duan, X.; He, P.; Zhou, Y. Ablation behavior of graphene reinforced SiBCN ceramics in an oxyacetylene combustion flame. Corros. Sci. 2015, 100, 85–100. [Google Scholar] [CrossRef]
- Liang, B.; Yang, Z.; Li, Y.; Yuan, J.; Jia, D.; Zhou, Y. Ablation behavior and mechanism of SiCf/Cf/SiBCN ceramic composites with improved thermal shock resistance under oxyacetylene combustion flow. Ceram. Int. 2015, 41, 8868–8877. [Google Scholar] [CrossRef]
- Ding, Q.; Ni, N.; Ni, D.; Ni, Z.; Jiang, Y.; Chen, B.; Chen, X.; Lu, J.; Niu, Y.; Zhou, H.S. Thermal damage and microstructure evolution mechanisms of Cf/SiBCN composites during plasma ablation. Corros. Sci. 2020, 169, 108621. [Google Scholar] [CrossRef]
- Kubota, Y.; Yano, M.; Inoue, R.; Kogo, Y.; Goto, K. Oxidation behavior of ZrB2-SiC-ZrC in oxygen-hydrogen torch environment. J. Eur. Ceram. Soc. 2018, 38, 1095–1102. [Google Scholar] [CrossRef]
- Deb, J.B.; Varela, C.; Faysal, F.; Maiti, C.; Gou, J. Deep Artificial Neural Network Modeling of the Ablation Performance of Ceramic Matrix Composites in the Hydrogen Torch Test. J. Compos. Sci. 2025, 9, 239. [Google Scholar] [CrossRef]
- Chawla, K.K. Ceramic Matrix Composites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Fua, L.; Wang, B.; Xia, W. New insights into the formation mechanism of zircon in a ZrO2-SiO2 nanocrystalline glass-ceramic: A TEM study. Ceram. Int. 2022, 48, 27097–27105. [Google Scholar] [CrossRef]
- Dillon, R.P.; Mecartney, M.L. Dynamic formation of zircon during high temperature deformation of zirconia-silica composites with alumina additions. J. Mater. Sci. 2007, 42, 3537–3543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).