Innovative Flexible Conductive Polymer Composites for Wearable Electrocardiogram Electrodes and Flexible Strain Sensors
Abstract
1. Introduction
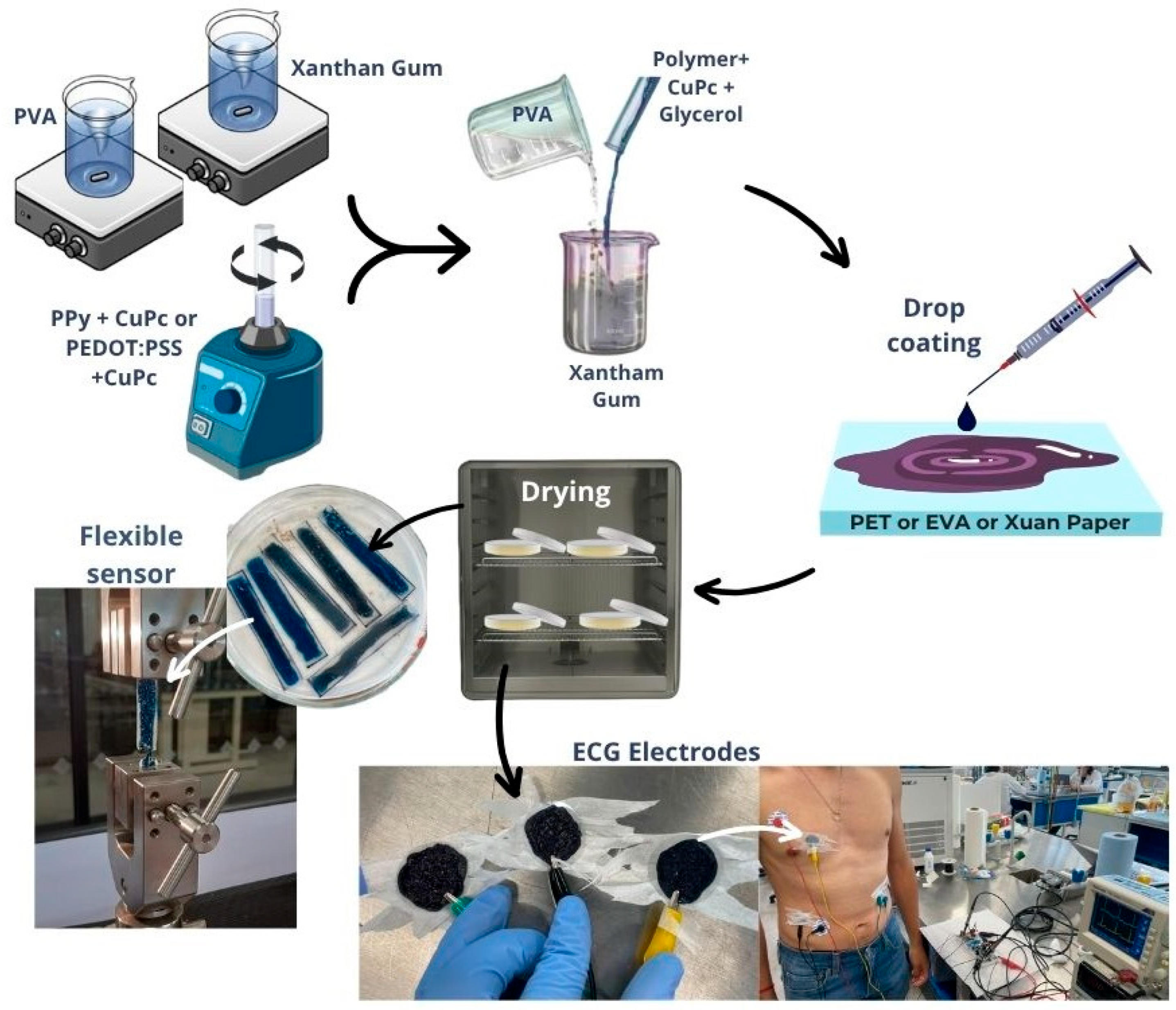
2. Materials and Methods
3. Results and Discussion
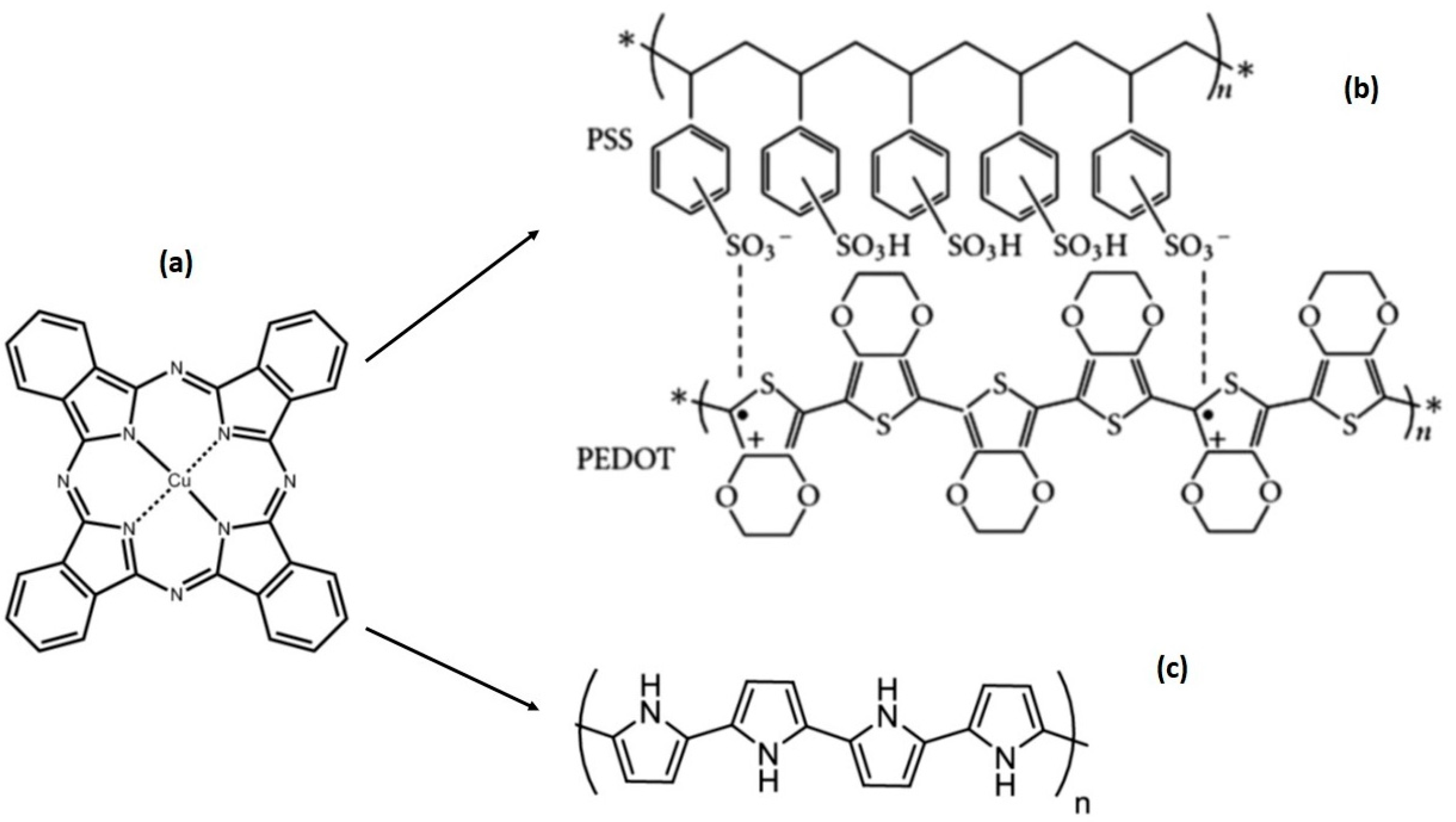
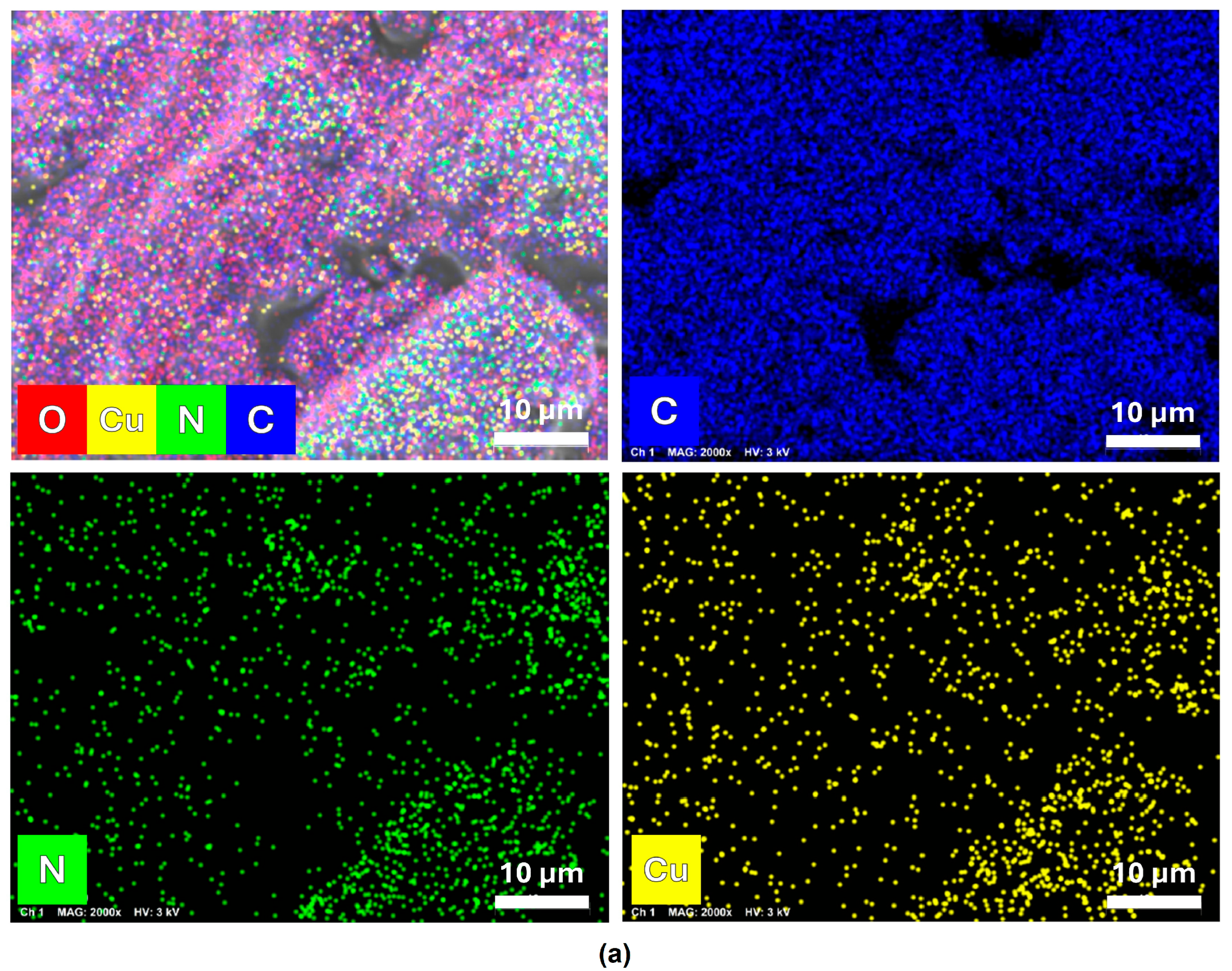
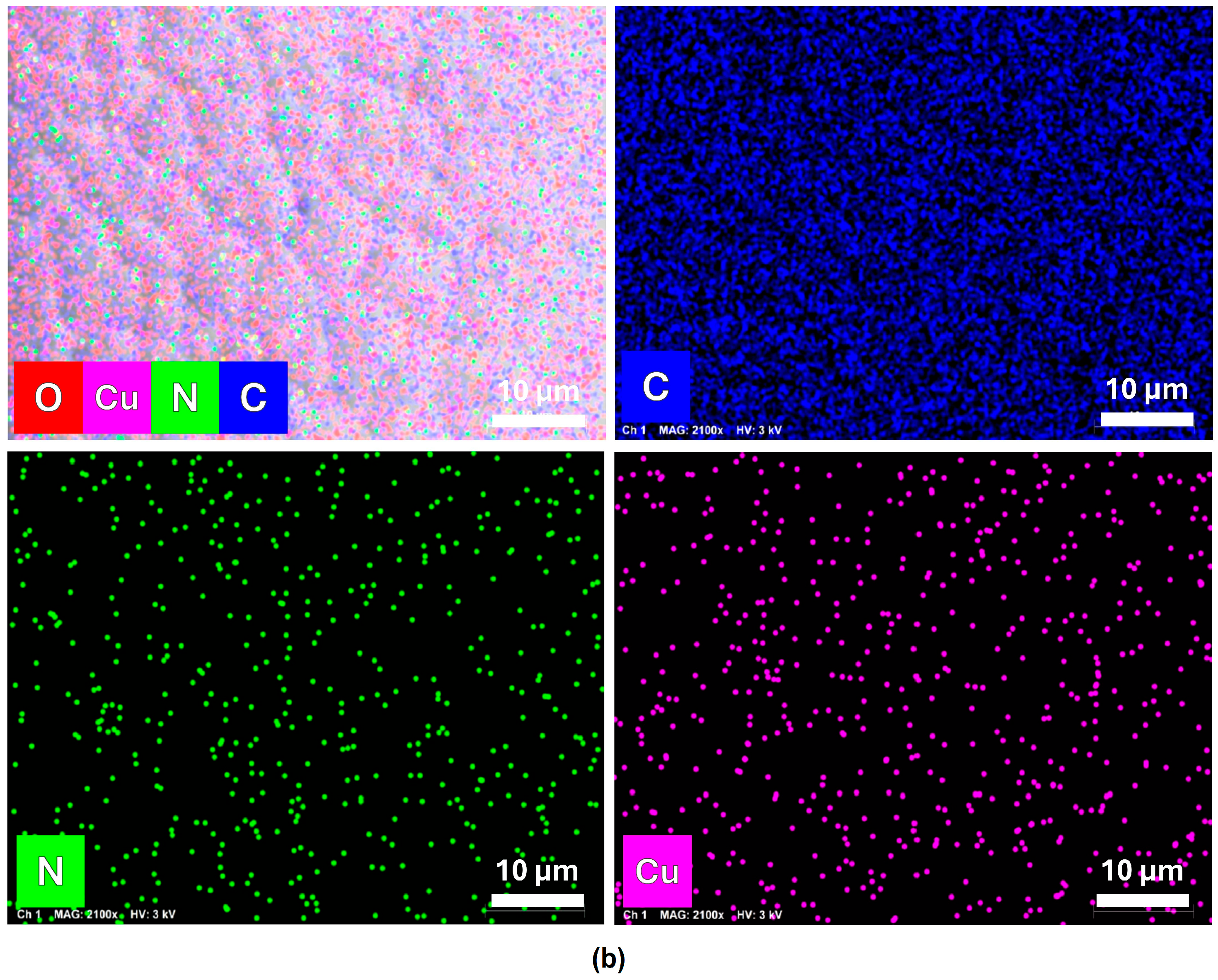
3.1. Morphological and Compositional Characterization of FCPCs
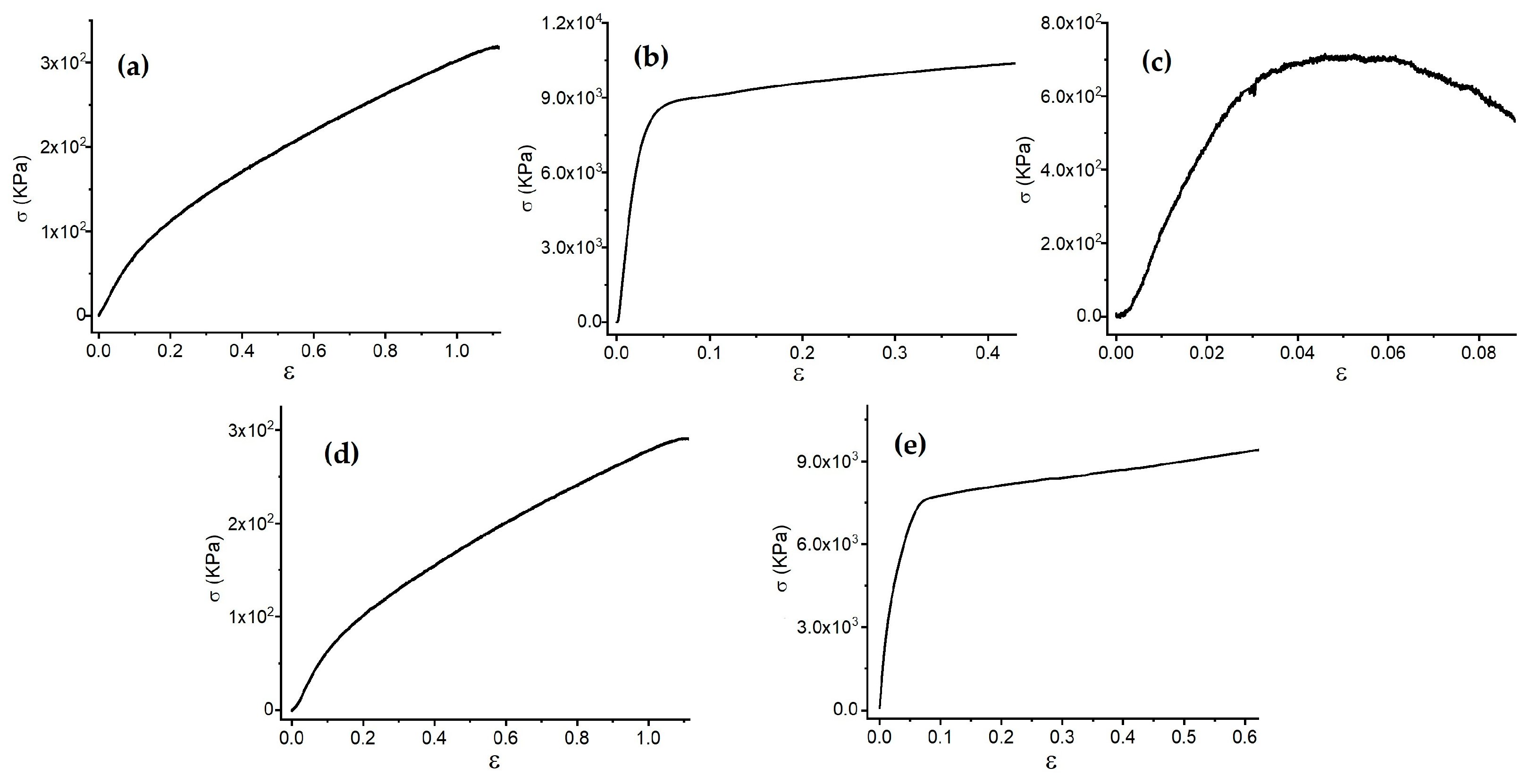
3.2. Stress–Strain Behavior of FCPCs
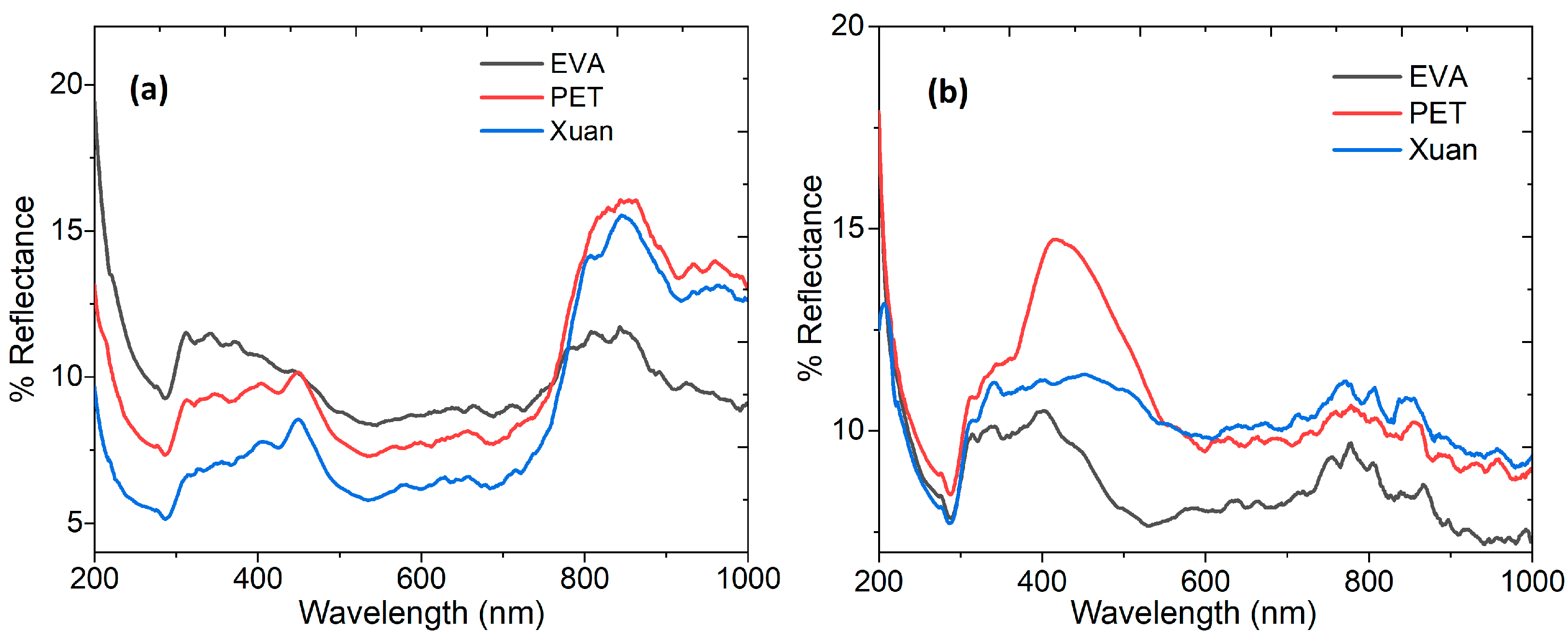
3.3. Evaluation of Optical Parameters in FCPCs
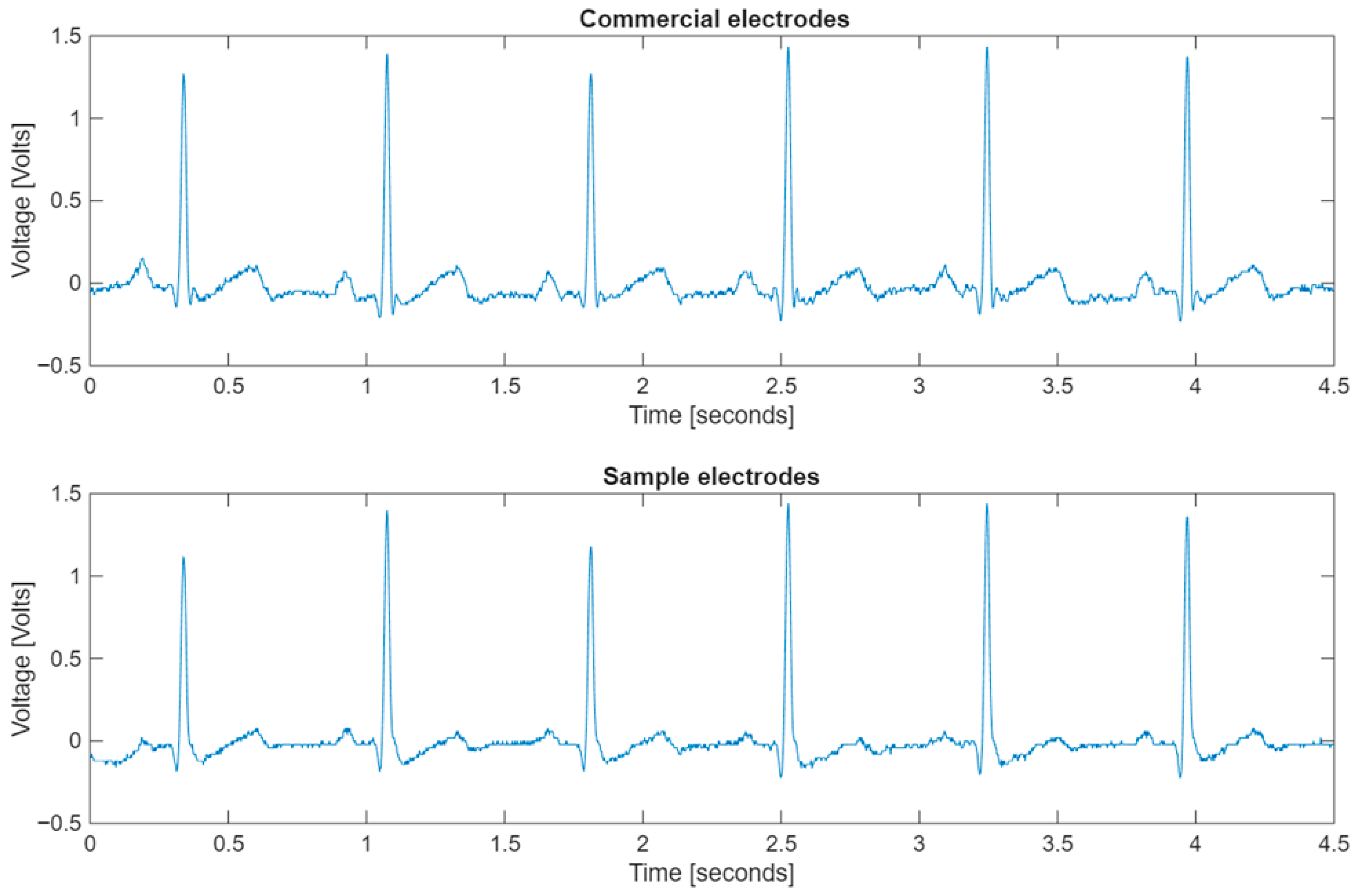
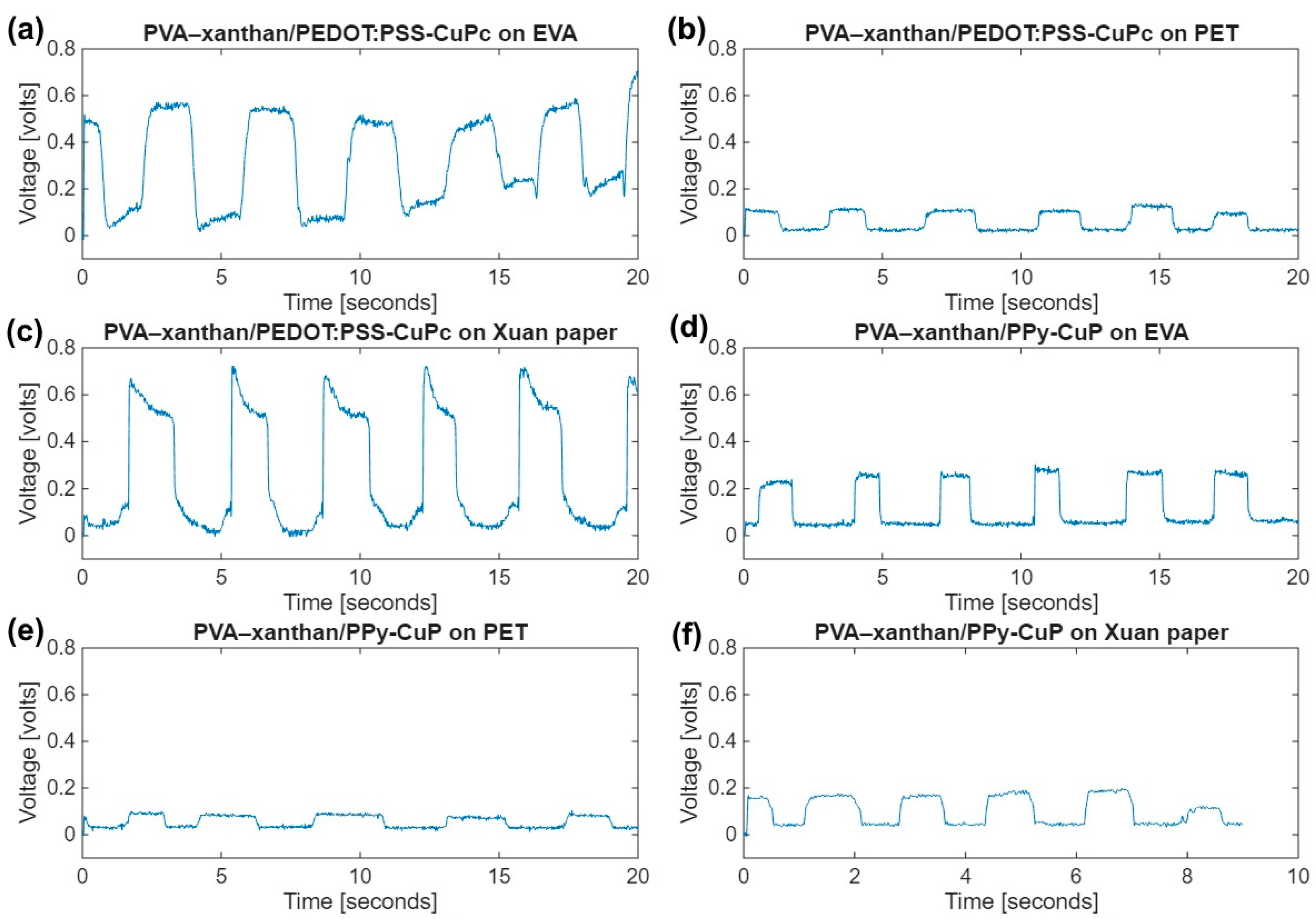
3.4. Electrical Evaluation of ECG Electrodes and Flexible Strain Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mijit, A.; Li, S.; Wang, Q.; Li, M.; Tai, Y. Silver Nanowire-Based Flexible Strain Sensor for Human Motion Detection. Sensors 2024, 24, 3329. [Google Scholar] [CrossRef]
- Nigusse, A.B.; Malengier, B.; Mengistie, D.A.; Tseghai, G.B.; Van Langenhove, L. Development of Washable Silver Printed Textile Electrodes for Long-Term ECG Monitoring. Sensors 2020, 20, 6233. [Google Scholar] [CrossRef]
- Le, K.; Servati, A.; Soltanian, S.; Servati, P.; Ko, F. Performance and Signal Quality Analysis of Electrocardiogram Textile Electrodes for Smart Apparel Applications. Front. Electron. 2019, 2, 685264. [Google Scholar] [CrossRef]
- Kolodziej, L.; Iwasińska-Kowalska, O.; Wróblewski, G.; Giżewski, T.; Jakubowska, M.; Lekawa-Raus, A. Hydrogels and Carbon Nanotubes: Composite Electrode Materials for Long-Term Electrocardiography Monitoring. J. Funct. Biomater. 2024, 15, 113. [Google Scholar] [CrossRef]
- Kumar, G.; Duggal, B.; Singh, J.P.; Shrivastava, Y. Efficacy of Various Dry Electrode-Based ECG Sensors: A Review. J. Biomed. Mater. Res. 2025, 113, e37845. [Google Scholar] [CrossRef]
- Vidhya, C.M.; Kumar, G.; Maithani, Y.; Duggal, B.; Singh, J.P. A performance evaluation of silver nanorods PDMS flexible dry electrodes for electrocardiogram monitoring. Sci. Rep. 2025, 15, 15799. [Google Scholar] [CrossRef]
- Maithani, Y.; Choudhuri, B.; Mehta, B.R.; Singh, J.P. Self-adhesive, stretchable, and dry silver nanorods embedded polydimethylsiloxane biopotential electrodes for electrocardiography. Sens. Actuators A Phys. 2021, 332, 113068. [Google Scholar] [CrossRef]
- Le, K.; Servati, A.; Servati, P.; Ko, F. Signal Quality Analysis of Electrocardiogram Textile Electrodes for Smart Apparel Applications. In Proceedings of the 2019 IEEE International Flexible Electronics Technology Conference (IFETC), Vancouver, BC, Canada, 11–14 August 2019; pp. 1–3. [Google Scholar] [CrossRef]
- Duan, L.; D’hooge, D.R.; Cardon, L. Recent Progress on Flexible and Stretchable Piezoresistive Strain Sensors: From Design to Application. Progress Mater. Sci. 2020, 114, 100617. [Google Scholar] [CrossRef]
- Han, F.; Li, M.; Ye, H.; Zhang, G. Materials, Electrical Performance, Mechanisms, Applications, and Manufacturing Approaches for Flexible Strain Sensors. Nanomaterials 2021, 11, 1220. [Google Scholar] [CrossRef]
- Ha, H.; Lee, J.; Kim, S. Sensing Mechanism and Application of Mechanical Strain Sensor: A Mini-Review. Facta Univ. Ser. Mech. Eng. 2023, 21, 751–772. [Google Scholar] [CrossRef]
- Wu, S.; Kim, D.; Tang, X.; King, M.W.; Zhu, Y. Encapsulated Stretchable Amphibious Strain Sensors. Mater. Horiz. 2024, 11, 5070–5080. [Google Scholar] [CrossRef]
- Hakim, M.; Alfarros, Z.; Herianto, H.; Muflikhun, M. High Sensitivity Flexible Strain Sensor for Motion Monitoring Based on MWCNT@MXene and Silicone Rubber. Sci. Rep. 2025, 15, 3741. [Google Scholar] [CrossRef]
- Shen, G. Recent advances of flexible sensors for biomedical applications. Progress Nat. Sci. Mater. Int. 2021, 31, 872–882. [Google Scholar] [CrossRef]
- Ma, R.; Chou, S.; Xie, Y.; Pei, Q. Morphological/nanostructural control toward intrinsically stretchable organic electronics. Chem. Soc. Rev. 2019, 48, 1741–1786. [Google Scholar] [CrossRef]
- Castro, H.; Correia, V.; Pereira, N.; Costab, P.; Oliveiraa, J.; Lanceros-Méndez, S. Printed Wheatstone bridge with embedded polymer based piezoresistive sensors for strain sensing applications. Addit. Manuf. 2018, 20, 119–125. [Google Scholar] [CrossRef]
- Díez-Pascual, A.; Rahdar, A. Graphene-Based Polymer Composites for Flexible Electronic Applications. Micromachines 2022, 13, 1123. [Google Scholar] [CrossRef]
- Tran, H.; Nguyen, T.H.; Vu, V. Fabrication of Zinc phthalocyanines: Characterisation and optical properties. Vietnam J. Sci. Technol. 2024, 66, 7–11. [Google Scholar] [CrossRef]
- Günsel, A.; Bilgiçli, A.T.; Pişkin, H.; Çaylak Delibaş, N.; Yarasir, M.N.; Gündüz, B. Synthesis, characterization, and optical and surface properties of (4-(trifluoromethylthio)phenoxy) copper(II) phthalocyanine. New J. Chem. 2018, 42, 6013–6022. [Google Scholar] [CrossRef]
- Evangelista, F.; Carravetta, V.; Stefani, G.; Jansik, B.; Alagia, M.; Stranges, S.; Ruocco, A. Electronic structure of copper phthalocyanine: An experimental and theoretical study of occupied and unoccupied levels. J. Chem. Phys. 2007, 126, 124709. [Google Scholar] [CrossRef]
- Hassan, A.; Gould, R. Structural Studies of Thermally Evaporated Thin Films of Copper Phthalocyanine. Phys. Status Solidi A 1992, 132, 91–101. [Google Scholar] [CrossRef]
- Christie, R.; Abel, A. Phthalocyanine pigments: General principles. Phys. Sci. Rev. 2021, 6, 671–677. [Google Scholar] [CrossRef]
- Elgazzar, E. Improvement the efficacy of Al/CuPc/n-Si/Al Schottky diode based on strong light absorption and high photocarriers response. Mater. Res. Express 2020, 7, 095102. [Google Scholar] [CrossRef]
- Demir, S.; Akbay, S.; Canımkurbey, B.; Yuksel, F. Novel Copper(II) Phthalocyanines as Semiconductor Layer in Organic Field Effect Transistors: Synthesis and Investigation of Electrical Properties. J. Mol. Struct. 2024, 1308, 137981. [Google Scholar] [CrossRef]
- Huseynova, G.; Hyun Kim, Y.; Lee, J.-H.; Lee, J. Rising advancements in the application of PEDOT:PSS as a prosperous transparent and flexible electrode material for solution-processed organic electronics. J. Inf. Disp. 2019, 21, 71–91. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Progress Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, X.; Ma, R.; Zhu, W.; Li, Y.; Chen, Z.; Zhou, J.; Li, W.; Liu, T.; He, Z.; et al. Dopamine Semiquinone Radical Doped PEDOT:PSS: Enhanced Conductivity, Work Function and Performance in Organic Solar Cells. Adv. Energy Mater. 2020, 10, 2000743. [Google Scholar] [CrossRef]
- Lo, C.; Wu, Y.; Awuyah, E.; Meli, D.; Nguyen, D.M.; Wu, R.; Xu, B.; Strzalka, J.; Rivnay, J.; Martin, D.C.; et al. Influence of the molecular weight and size distribution of PSS on mixed ionic-electronic transport in PEDOT:PSS. Polym. Chem. 2022, 13, 2764–2775. [Google Scholar] [CrossRef]
- Mahmood, J.; Arsalani, N.; Naghash-Hamed, S.; Hanif, Z.; Geckeler, K. Preparation and characterization of hybrid polypyrrole nanoparticles as a conducting polymer with controllable size. Sci. Rep. 2024, 14, 11653. [Google Scholar] [CrossRef]
- Hao, L.; Dong, C.; Yu, D. Polypyrrole Derivatives: Preparation, Properties and Application. Polymers 2024, 16, 2233. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Pu, A.; Wang, L.; Li, W.; Zhou, X.; Tang, C.Y.; Ban, K.; Yang, M.; Xu, L. Strong and high-conductivity hydrogels with all-polymer nanofibrous networks for applications as high-capacitance flexible electrodes. npj Flex. Electron. 2024, 8, 56. [Google Scholar] [CrossRef]
- Kaminski, W.; Rozsíval, V.; Jelínek, P. Theoretical study of electronic and transport properties of PPy–Pt(111) and PPy–C(111):H interfaces. J. Phys. Condens. Matter 2010, 22, 045003. [Google Scholar] [CrossRef]
- Lee, M.-H.; Teng, K.-H.; Liang, Y.-Y.; Ding, C.-F.; Chen, Y.-C. Flexible biodegradable wearables based on conductive leaf networks. Sustain. Mater. Technol. 2025, 43, e01263. [Google Scholar] [CrossRef]
- Teng, K.-H.; Lee, M.-H. PEDOT:PSS treated Xuan paper as a green electronics material for wearable dry electrocardiogram electrodes and flexible strain sensors. J. Environ. Chem. Eng. 2025, 13, 115716. [Google Scholar] [CrossRef]
- Rahman, S.; Karmakar, C.; Natgunanathan, I.; Yearwood, J.; Palaniswami, M. Robustness of electrocardiogram signal quality indices. J. R. Soc. Interface 2022, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mark, G.R.; Clifford, G.D. Robust heart rate estimation from multiple asynchronous noisy sources using signal quality indices and a Kalman filter. Physiol. Meas. 2008, 29, 15. [Google Scholar] [CrossRef]
- Behar, J.; Johnson, A.; Clifford, G.D. A Comparison of Single Channel Fetal ECG Extraction Methods. Ann. Biomed. Eng. 2014, 42, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Behar, J.; Andreotti, F.; Clifford, G.D.; Oster, J. R-peak estimation using multimodal lead switching. In Proceedings of the Computing in Cardiology 2014, Cambridge, MA, USA, 7–10 September 2014; pp. 281–284. Available online: https://ieeexplore.ieee.org/abstract/document/7043034 (accessed on 28 July 2025).
- Zong, W.; Moody, G.B.; Jiang, D. A robust open-source algorithm to detect onset and duration of QRS complexes. In Proceedings of the Computers in Cardiology, 2003, Thessaloniki, Greece, 21–24 September 2003; pp. 737–740. [Google Scholar] [CrossRef]
- He, T.; Clifford, G.; Tarassenko, L. Application of independent component analysis in removing artefacts from the electrocardiogram. Neural Comput. Appl. 2006, 15, 105–116. [Google Scholar] [CrossRef]
- Murthy, V.K.; Grove, T.M.; Harvey, G.A.; Haywood, L.J. Clinical Usefulness of ECG Frequency Spectrum Analysis. In Proceedings of the Second Annual Symposium on Computer Application in Medical Care; Washington, DC, USA, 5–9 November 1978, pp. 610–612. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC2231746/ (accessed on 5 August 2025).
- Cosme, I.; Carrillo-Sendejas, J.C.; Guerra-Hernandez, S.F.; Sánchez Vergara, M.E.; Netzahualcoyotl, C.; Moreno, M. Xanthan gum as a green, non-toxic, surfactant-free modifier for enhanced deposition of PEDOT: PSS in crystalline silicon hybrid solar cells. Surf. Interfaces 2025, 72, 107232. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Fan, Q.; Zhang, W.; Zuo, X.; Tian, M.; Zhu, S.; Zhang, X.; Qu, L. Recent Progress on Smart Fiber and Textile Based Wearable Strain Sensors: Materials, Fabrications and Applications. Adv. Fiber Mater. 2022, 4, 361–389. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, X.; Li, C.; Ruan, R.; You, H. Self-Healing and Self-Adhesive Substrate-Free Tattoo Electrode. Materials 2023, 16, 3499. [Google Scholar] [CrossRef]
- Zhang, L.; Senthil Kumar, K.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef]
- Zhou, K.; Dai, K.; Liu, C.; Shen, C. Flexible conductive polymer composites for smart wearable strain sensors. Smart Mat. 2020, 1, e1010. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, P.; Liang, Y.; Zhang, J.; Huang, Y.; Wu, S.; Kuo, S.-W.; Ten, C. Network cracks-based wearable strain sensors for subtle and large strain detection of human motions. J. Mater. Chem. C 2018, 6, 5140–5147. [Google Scholar] [CrossRef]
- Dong, L.-Y.; Zhu, I.-J. Fire-Resistant Inorganic Analogous Xuan Paper with Thousands of Years’ Super-Durability. ACS Sustain. Chem. Eng. 2018, 6, 17239–17251. [Google Scholar] [CrossRef]
- Dong, L.-Y.; Zhu, Y.-J.; Wu, J.; Yu, H.-P. Comparison of Aging Performances and Mechanisms: Super-Durable Fire-Resistant “Xuan Paper” Versus Chinese Traditional Xuan Paper. Molecules 2025, 30, 263. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Li, Y.; Fu, P. Research on Strengthening Fragile Paper with Polyvinylamine. Polymers 2024, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Lee, J.; Kwon, Y.; Lee, Y.J.; Yeo, W. Recent Progress in the Development of Flexible Wearable Electrodes for Electrocardiogram Monitoring During Exercise. Adv. NanoBiomed Res. 2024, 4, 2300169. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, M.; Chen, T.; Mu, X.; Sun, Q.; Zhou, J. Advances in graphene-based flexible wearable piezoresistive strain sensors. J. Mater. Sci: Mater. Electron. 2025, 36, 1211. [Google Scholar] [CrossRef]
- Hu, X.; Huang, T.; Liu, Z.; Wang, G.; Chen, D.; Guo, Q.; Yang, S.; Jin, Z.; Lee, J.-M.; Ding, G. Conductive graphene-based e-textiles for highly sensitive, breathable, and water-resistant multimodal gesture-distinguishable sensors. J. Mater. Chem. A 2020, 8, 14778–14787. [Google Scholar] [CrossRef]
- Cranston, R.; Lessard, B. Metal phthalocyanines: Thin-film formation, microstructure, and physical properties. RSC Adv. 2021, 11, 21716–21737. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An Article on Optics of Paint Layers. Fuer Tekn. Phys. 1931, 12, 593–609. Available online: https://www.graphics.cornell.edu/~westin/pubs/kubelka.pdf (accessed on 29 July 2025).
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Stat. Sol. 1966, 15, 627–637. [Google Scholar] [CrossRef]
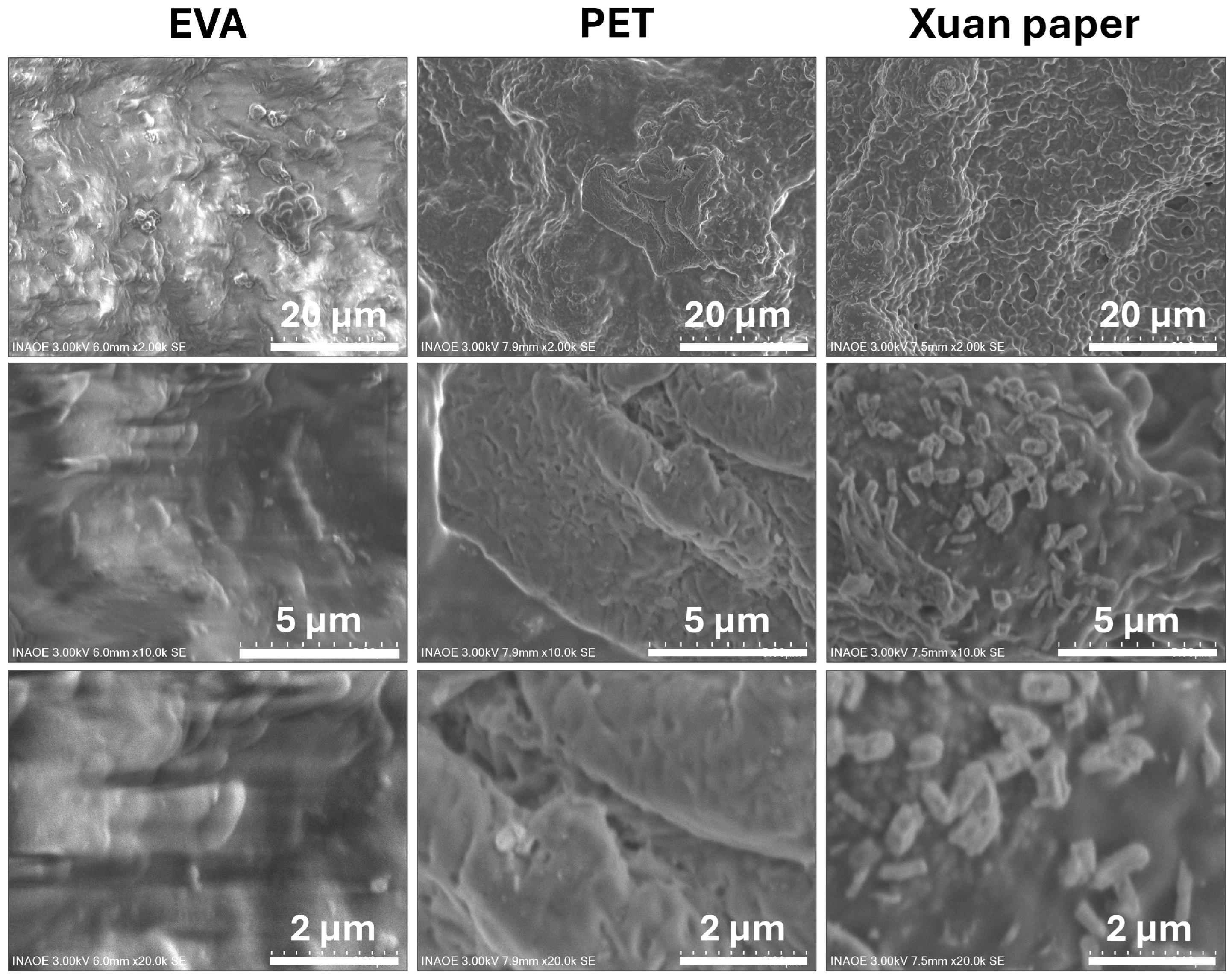
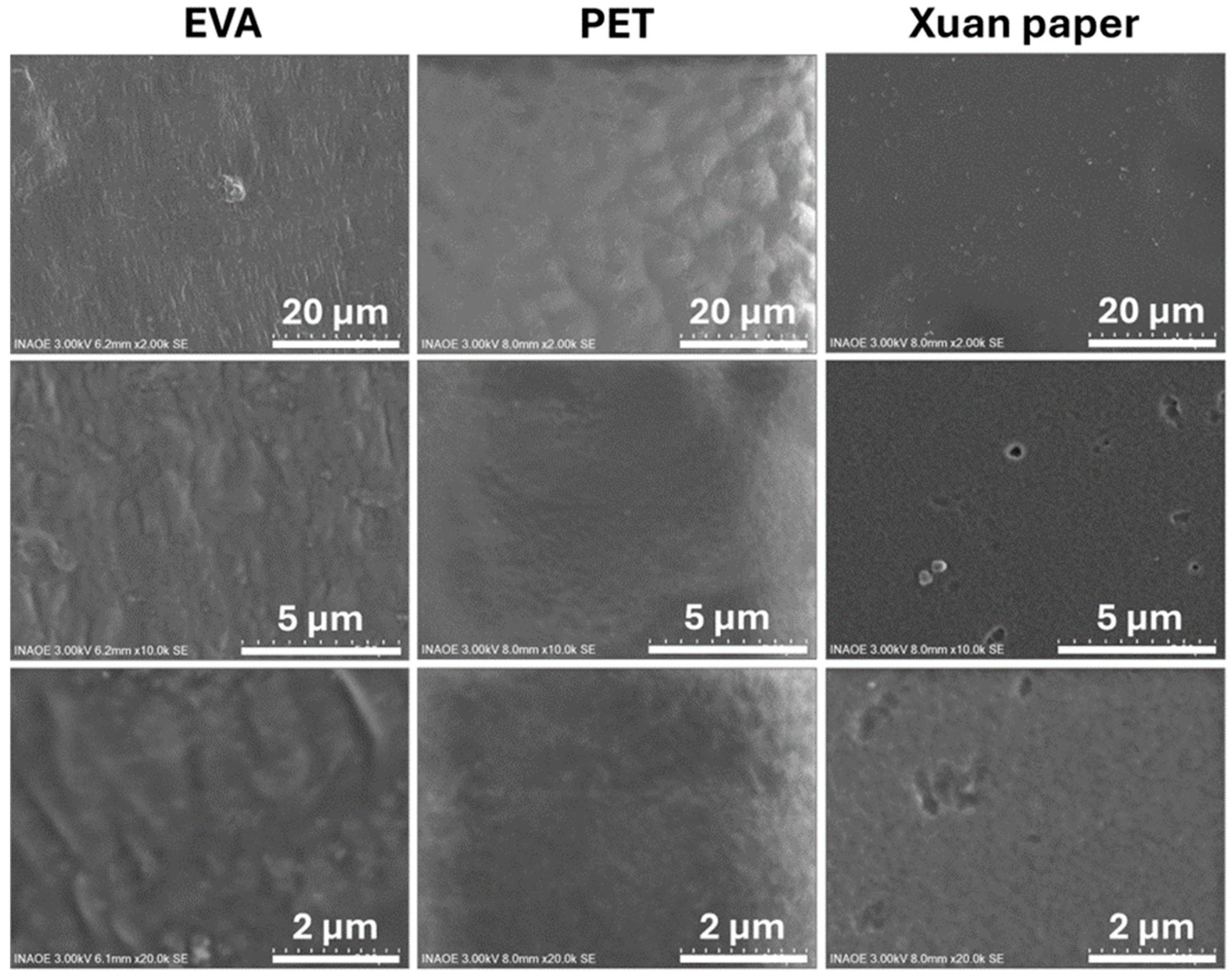
| Sample | Thickness (mm) |
|---|---|
| EVA | 2.00 |
| PET | 0.64 |
| Xuan paper | 0.12 |
| PVA-xanthan/PPy-CuPc on EVA | 0.12 |
| PVA-xanthan/PPy-CuPc on PET | 0.23 |
| PVA-xanthan/PPy-CuPc on Xuan paper | 0.049 |
| PVA-xanthan/PEDOT:PSS-CuPc on EVA | 0.31 |
| PVA-xanthan/PEDOT:PSS-CuPc on PET | 0.24 |
| PVA-xanthan/PEDOT:PSS-CuPc on Xuan paper | 0.053 |
| Element | PVA–Xanthan/PPy-CuPc | PVA-Xanthan/PEDOT:PSS-CuPc | ||||
|---|---|---|---|---|---|---|
| EVA | PET | Xuan Paper | EVA | PET | Xuan Paper | |
| C | 62.92% | 64.69% | 71.40% | 66.48% | 72.69% | 62.90% |
| N | 2.27% | 3.15% | 0.58% | 0.81% | 0.33% | 0.83% |
| O | 32.65% | 30.45% | 27.84% | 32.70% | 26.71% | 36.27% |
| Cu | 0.28% | 1.70% | 0.18% | - | 0.27% | - |
| Na | 1.88% | - | - | - | - | - |
| Sample | Young’s Modulus (KPa) | Limit of Proportionality (KPa) | Rupture Strength (KPa) | Strain Range (%) |
|---|---|---|---|---|
| EVA | 917 | 40 | 320 | 6.6 |
| PET | 331,982 | 7792 | 12,344 | 2.4 |
| Xuan paper | 648,849 | 584 | 431 | 0.02 |
| PVA-xanthan/PPy-CuPc on EVA | 796 | 46 | 320 | 9.9 |
| PVA-xanthan/PPy-CuPc on PET | 310,744 | 6240 | 10,400 | 42 |
| PVA-xanthan/PPy-CuPc on Xuan paper | 28,832 | 446 | 447 | 1.5 |
| PVA-xanthan/PEDOT:PSS-CuPc on EVA | 640 | 44 | 290 | 8.0 |
| PVA-xanthan/PEDOT:PSS-CuPc on PET | 159,648 | 4074 | 9300 | 34 |
| PVA-xanthan/PEDOT:PSS-CuPc on Xuan paper | - | - | - | - |
| Sample | Optical Band Gap (eV) |
|---|---|
| PVA-xanthan/PPy-CuPc on EVA | 3.4 |
| PVA-xanthan/PPy-CuPc on PET | 3.4 |
| PVA-xanthan/PPy-CuPc on Xuan paper | 3.1 |
| PVA-xanthan/PEDOT:PSS-CuPc on EVA | 3.2 |
| PVA-xanthan/PEDOT:PSS-CuPc on PET | 3.0 |
| PVA-xanthan/PEDOT:PSS-CuPc on Xuan paper | 3.2 |
| Commercial Electrode | Electrode from Sample | |||||
|---|---|---|---|---|---|---|
| kSQI | bSQI | sSQI | kSQI | bSQI | sSQI | |
| PVA-xanthan/PPy-CuPc on EVA | 27.023 | 1 | 0.539 | 23.425 | 1 | 0.653 |
| PVA-xanthan/PPy-CuPc on PET | 30.411 | 1 | 0.521 | 32.202 | 1 | 0.557 |
| PVA-xanthan/PPy-CuPc on Xuan paper | 26.722 | 1 | 0.577 | 11.099 | 1 | 0.629 |
| PVA-xanthan/PEDOT:PSS- CuPc on EVA | 9.063 | 1 | 0.576 | 26.545 | 1 | 0. 651 |
| PVA-xanthan/PEDOT:PSS- CuPc on PET | 27.329 | 1 | 0.564 | 22.426 | 1 | 0.638 |
| PVA-xanthan/PEDOT:PSS- CuPc on Xuan paper | 30.399 | 1 | 0.596 | 28.296 | 1 | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Vergara, M.E.; Hernández Méndez, J.A.; Herrera Navarro, C.I.; Martínez-Alanís, M.; Guerra Hernández, S.F.; Cosme, I. Innovative Flexible Conductive Polymer Composites for Wearable Electrocardiogram Electrodes and Flexible Strain Sensors. J. Compos. Sci. 2025, 9, 512. https://doi.org/10.3390/jcs9100512
Sánchez Vergara ME, Hernández Méndez JA, Herrera Navarro CI, Martínez-Alanís M, Guerra Hernández SF, Cosme I. Innovative Flexible Conductive Polymer Composites for Wearable Electrocardiogram Electrodes and Flexible Strain Sensors. Journal of Composites Science. 2025; 9(10):512. https://doi.org/10.3390/jcs9100512
Chicago/Turabian StyleSánchez Vergara, María Elena, Joaquín André Hernández Méndez, Carlos Ian Herrera Navarro, Marisol Martínez-Alanís, Selma Flor Guerra Hernández, and Ismael Cosme. 2025. "Innovative Flexible Conductive Polymer Composites for Wearable Electrocardiogram Electrodes and Flexible Strain Sensors" Journal of Composites Science 9, no. 10: 512. https://doi.org/10.3390/jcs9100512
APA StyleSánchez Vergara, M. E., Hernández Méndez, J. A., Herrera Navarro, C. I., Martínez-Alanís, M., Guerra Hernández, S. F., & Cosme, I. (2025). Innovative Flexible Conductive Polymer Composites for Wearable Electrocardiogram Electrodes and Flexible Strain Sensors. Journal of Composites Science, 9(10), 512. https://doi.org/10.3390/jcs9100512








