Electrospun Aligned Gelatin/Chitosan Nanofibrous Membranes for a Better Culture of Mesothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Nanofibrous Membranes (NFMs)
2.3. Cross-Linking of Electrospun Nanofibrous Membranes (NFMs)
2.4. Characterization of Electrospun Nanofibrous Membranes (NFMs)
2.5. In Vitro Cell Culture
2.6. Cell Morphology
2.7. Cell Proliferation
2.8. Biocompatibility
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Cell Viability by Live/Dead Staining
2.11. Cytosketelon Staining
2.12. Immunofluorescence (IF) Staining
2.13. Statistical Analysis
3. Results and Discussion
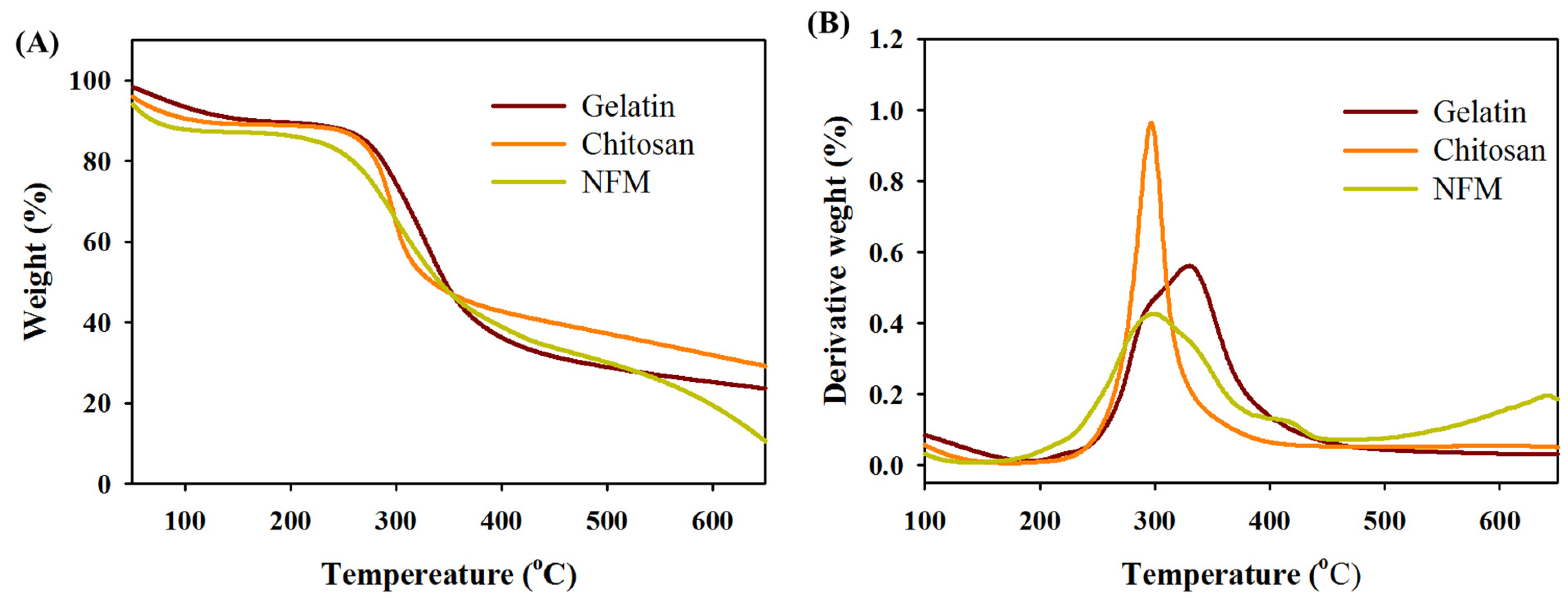
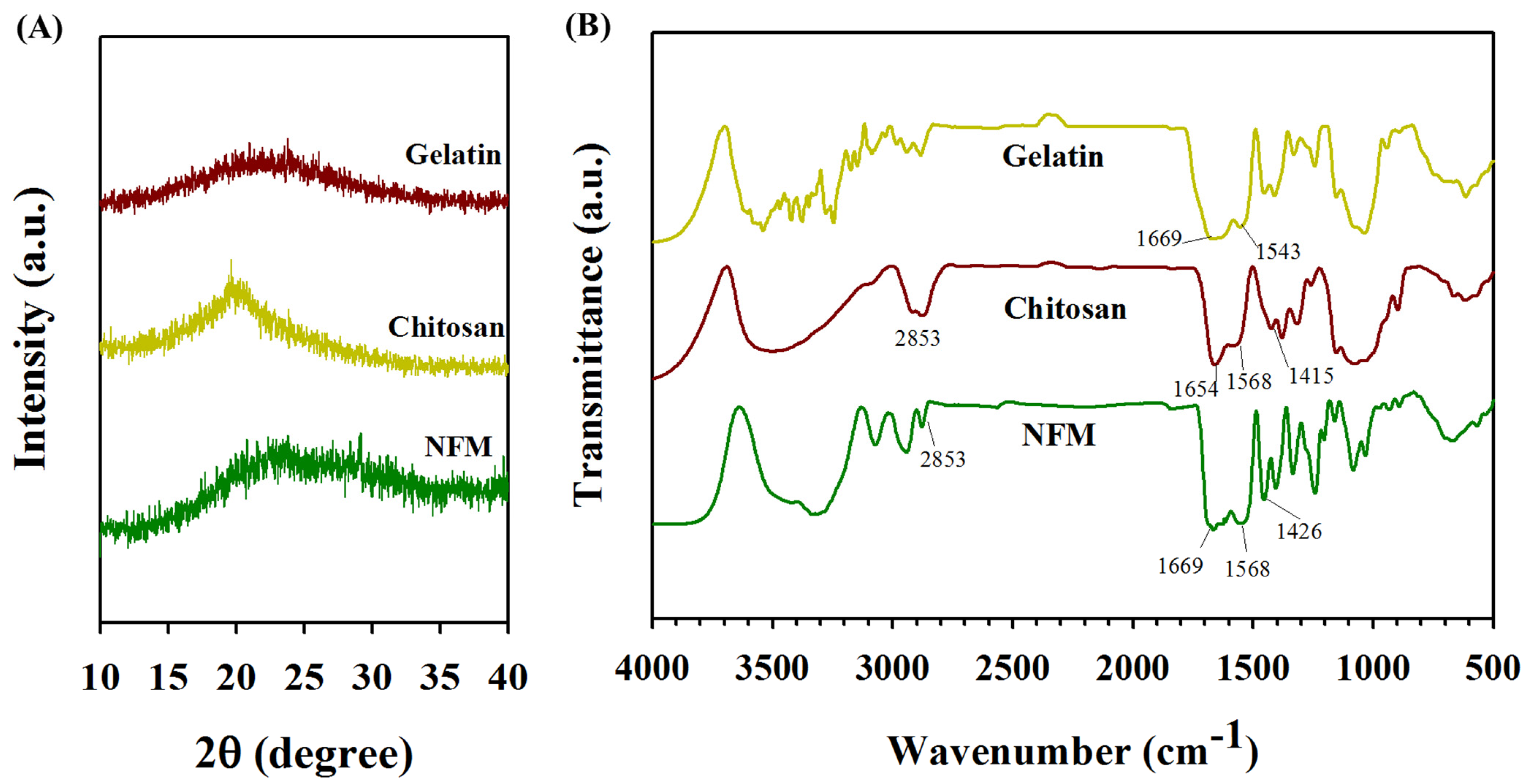
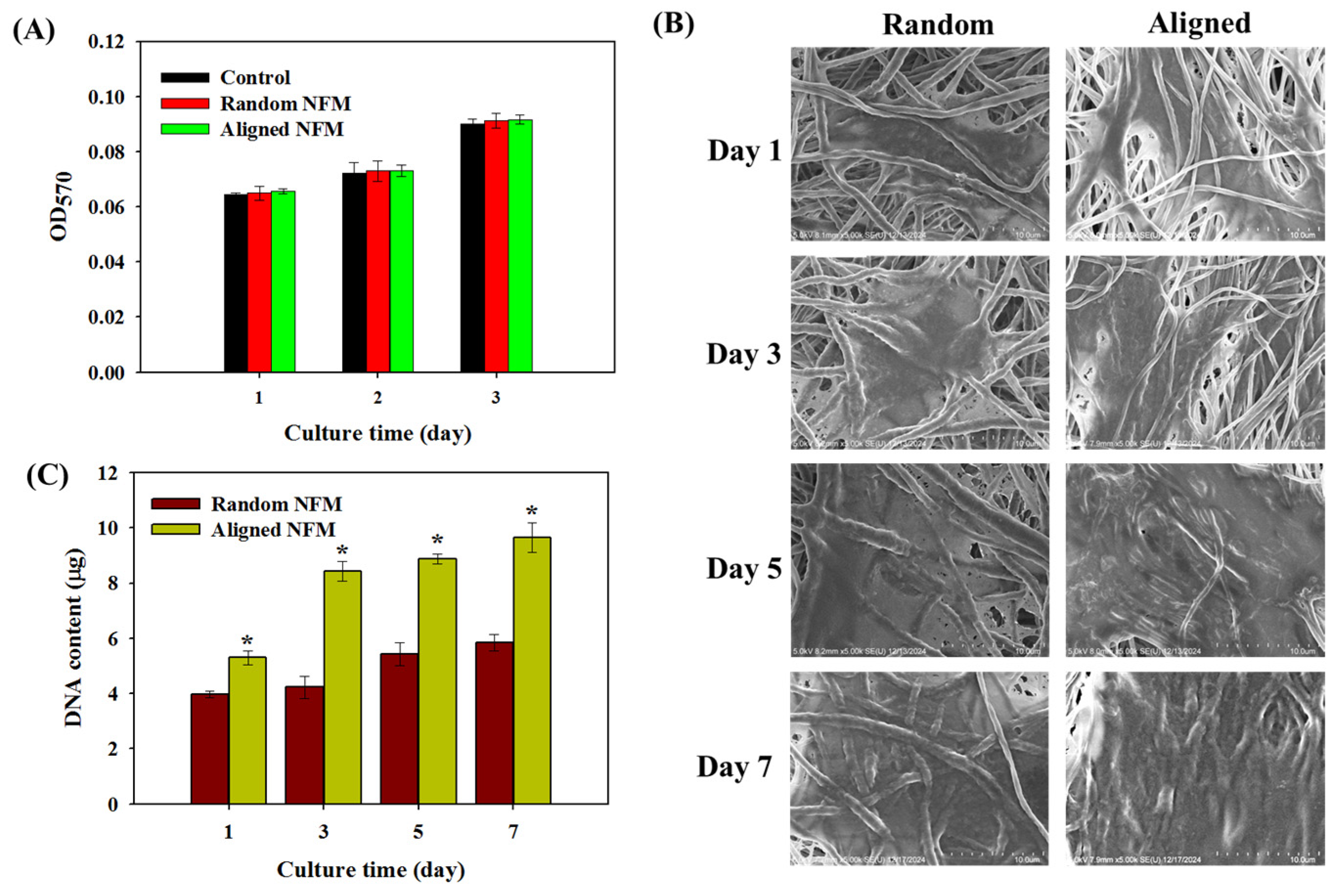
3.1. Characterization of Electrospun Nanofibrous Membranes (NFMs)
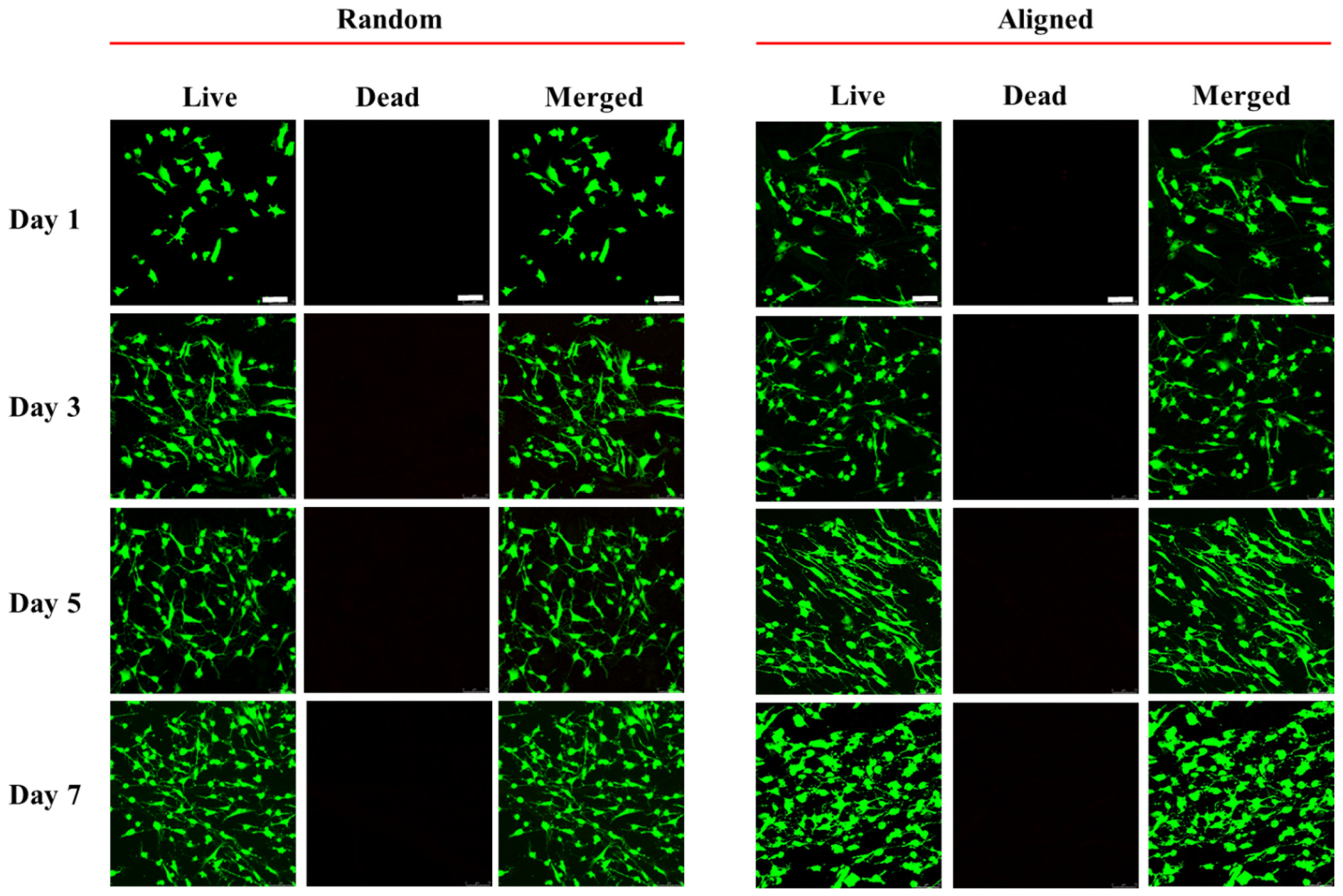
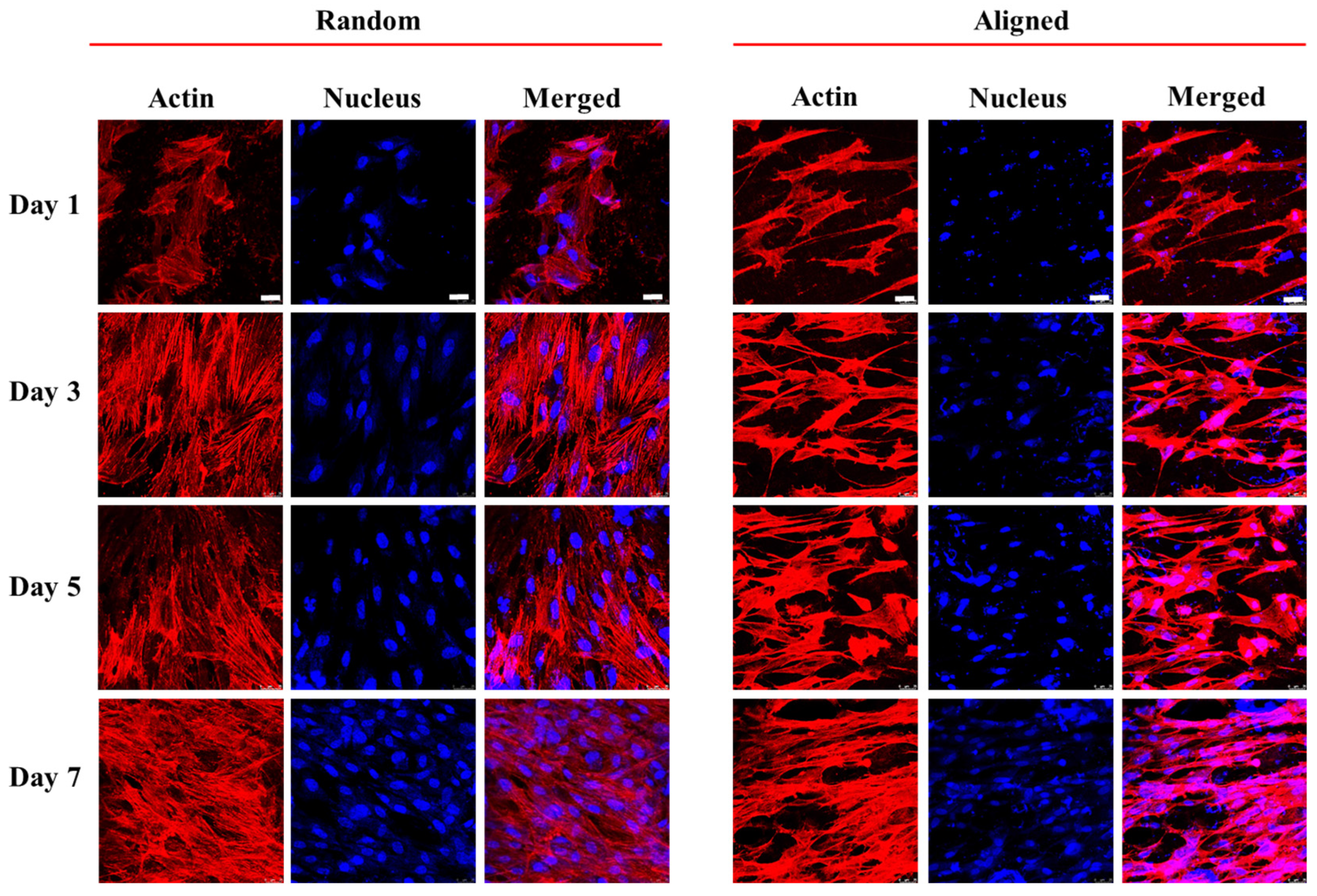
3.2. In Vitro Cell Culture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, J.; Li, L.; Yang, Z.; Chen, X. Phototherapy meets immunotherapy: A win–win strategy to fight against cancer. Nanophotonics 2021, 10, 3229–3245. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Prêle, C.M.; Lansley, S.M.; Herrick, S.E. The origin of regenerating mesothelium: A historical perspective. Int. J. Artif. Organs 2007, 30, 484–494. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef]
- Brunkhorst, R.; Mahiout, A. Pyruvate neutralizes peritoneal dialysate cytotoxicity: Maintained integrity and proliferation of cultured human mesothelial cells. Kidney Int. 1995, 48, 177–181. [Google Scholar] [CrossRef]
- Uiterwijk, H.; Franssen, C.F.M.; Kuipers, J.; Westerhuis, R.; Nauta, F.L. Glucose Exposure in Peritoneal Dialysis Is a Significant Factor Predicting Peritonitis. Am. J. Nephrol. 2020, 51, 237–243. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prêle, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Prele, C.M.; Pengelly, S.; Herrick, S.E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 2016, 106, 1018–1024. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Wilkosz, S. Structure and function of mesothelial cells. Cancer Treat. Res. 2007, 134, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, C.C.; Rodriguez-Campins, B.; Hmadcha, A.; Soria, B. Use of Mesothelial Cells and Biological Matrices for Tissue Engineering of Simple Epithelium Surrogates. Front. Bioeng. Biotechnol. 2015, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Hiriart, E.; Deepe, R.; Wessels, A. Mesothelium and Malignant Mesothelioma. J. Dev. Biol. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.E.; Mutsaers, S.E. Mesothelial progenitor cells and their potential in tissue engineering. Int. J. Biochem. Cell Biol. 2004, 36, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Nishimura, J.; Hiraki, M.; Takahashi, H.; Haraguchi, N.; Hata, T.; Ikenaga, M.; Murata, K.; Yamamoto, H.; Mizushima, T.; et al. Regeneration of peritoneal mesothelial cells after placement of hyaluronate carboxymethyl-cellulose (Seprafilm®). Surg. Today 2017, 47, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of natural origin chitosan-gelatin-alginate composite scaffold by foaming method without using surfactant. J. Appl. Polym. Sci. 2013, 127, 3228–3241. [Google Scholar] [CrossRef]
- Krithica, N.; Natarajan, V.; Madhan, B.; Sehgal, P.K.; Mandal, A.B. Type I Collagen Immobilized Poly(caprolactone) Nanofibers: Characterization of Surface Modification and Growth of Fibroblasts. Adv. Eng. Mater. 2012, 14, B149–B154. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, W.; Sun, B.; Li, H.; Wu, T.; Ke, Q.; Huang, C.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. A comparison of nanoscale and multiscale PCL/gelatin scaffolds prepared by disc-electrospinning. Colloids Surf. B Biointerfaces 2016, 146, 632–641. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Govindaraju, D.T.; Kao, H.-H.; Chien, Y.-M.; Chen, J.-P. Composite Polycaprolactone/Gelatin Nanofiber Membrane Scaffolds for Mesothelial Cell Culture and Delivery in Mesothelium Repair. Int. J. Mol. Sci. 2024, 25, 9803. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-J.; Lee, Y.-H.; Wu, M.-H.; Yang, M.-C.; Chien, C.-T. Electrospun anti-adhesion barrier made of chitosan alginate for reducing peritoneal adhesions. Carbohydr. Polym. 2012, 88, 1304–1312. [Google Scholar] [CrossRef]
- Govindaraju, D.T.; Chen, C.H.; Kuo, C.Y.; Shalumon, K.T.; Chien, Y.M.; Kao, H.H.; Chen, J.P. Development of high resilience spiral wound suture-embedded gelatin/PCL/heparin nanofiber membrane scaffolds for tendon tissue engineering. Int. J. Biol. Macromol. 2022, 221, 314–333. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kuo, C.-Y.; Govindaraju, D.T.; Chen, K.-S.; Chen, J.-P. Polycaprolactone/Chitosan Composite Nanofiber Membrane as a Preferred Scaffold for the Culture of Mesothelial Cells and the Repair of Damaged Mesothelium. Int. J. Mol. Sci. 2022, 23, 9517. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Potential of electrospun core-shell structured gelatin-chitosan nanofibers for biomedical applications. Carbohydr. Polym. 2016, 136, 1098–1107. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Zhang, K.-H.; Chen, F.; Ke, Q.-F.; Mo, X.-M. Cross-linking of gelatin and chitosan complex nanofibers for tissue-engineering scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Casper, C.L.; Yamaguchi, N.; Kiick, K.L.; Rabolt, J.F. Functionalizing Electrospun Fibers with Biologically Relevant Macromolecules. Biomacromolecules 2005, 6, 1998–2007. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Chen, Y.; Yang, G.; Huang, J.; Xiong, L. Acupoint catgut from electrospun tri-layer core–shell fibers loaded with adenosine for a long-term analgesia effect. Eur. Polym. J. 2024, 220, 113447. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath Nanofibers with a Cellulose Acetate Coating for the Synergistic Release of Zinc Ion and Drugs. Mol. Pharm. 2024, 21, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, C.; Chen, Y.; Ji, Y.; Yu, D.-G.; Bligh, S.W.A. Medicated tri-layer fibers based on cellulose acetate and polyvinylpyrrolidone for enhanced antibacterial and wound healing properties. Carbohydr. Polym. 2025, 348, 122856. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, F.; Li, J.; Zhang, S.; Shen, M.; Huang, M.; Shi, X. Design of electrospun nanofibrous mats for osteogenic differentiation of mesenchymal stem cells. Nanomedicine 2018, 14, 2505–2520. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Li, D.L.; Chuang, A.D.; Dash, B.S.; Chen, J.P. Tension Stimulation of Tenocytes in Aligned Hyaluronic Acid/Platelet-Rich Plasma-Polycaprolactone Core-Sheath Nanofiber Membrane Scaffold for Tendon Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 11215. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; MacEwan, M.R.; Bridgman, P.C.; Xia, Y. Neurite Outgrowth on Electrospun Nanofibers with Uniaxial Alignment: The Effects of Fiber Density, Surface Coating, and Supporting Substrate. ACS Nano 2014, 8, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, H.; Yuan, G.; Lin, K.; Su, J. The effects of alignment and diameter of electrospun fibers on the cellular behaviors and osteogenesis of BMSCs. Mater. Sci. Eng. C 2021, 120, 111787. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khosravi, F.; Neisiany, R.E.; Shakiba, M.; Zare, M.; Lakshminarayanan, R.; Chellappan, V.; Abdouss, M.; Ramakrishna, S. Advances in electrospinning of aligned nanofiber scaffolds used for wound dressings. Curr. Opin. Biomed. Eng. 2022, 22, 100393. [Google Scholar] [CrossRef]
- Koukorava, C.; Ward, K.; Ahmed, K.; Almaghrabi, S.; Dauleh, S.; Pereira, S.M.; Taylor, A.; Haddrick, M.; Cross, M.J.; Wilm, B. Mesothelial Cells Exhibit Characteristics of Perivascular Cells in an In Vitro Angiogenesis Assay. Cells 2023, 12, 2436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, N.; Kreeger, P.K.; Notbohm, J. Topological defects in the mesothelium suppress ovarian cancer cell clearance. APL Bioeng. 2021, 5, 036103. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 183–188. [Google Scholar] [CrossRef]
- Kao, H.H.; Kuo, C.Y.; Chen, K.S.; Chen, J.P. Preparation of Gelatin and Gelatin/Hyaluronic Acid Cryogel Scaffolds for the 3D Culture of Mesothelial Cells and Mesothelium Tissue Regeneration. Int. J. Mol. Sci. 2019, 20, 4527. [Google Scholar] [CrossRef]
- Jafari, J.; Emami, S.H.; Samadikuchaksaraei, A.; Bahar, M.A.; Gorjipour, F. Electrospun chitosan-gelatin nanofiberous scaffold: Fabrication and in vitro evaluation. Biomed. Mater. Eng. 2011, 21, 99–112. [Google Scholar] [CrossRef]
- Zhan, J.; Morsi, Y.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. In vitro evaluation of electrospun gelatin–glutaraldehyde nanofibers. Front. Mater. Sci. 2016, 10, 90–100. [Google Scholar] [CrossRef]
- Peng, N.B.; He, J.H. Insight into the Wetting Property of a Nanofiber Membrane by the Geometrical Potential. Recent Pat. Nanotechnol. 2020, 14, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Fare, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Bharadwaj, T.; Chrungoo, S.; Verma, D. Self-assembled chitosan/gelatin nanofibrous aggregates incorporated thermosensitive nanocomposite bioink for bone tissue engineering. Carbohydr. Polym. 2024, 324, 121544. [Google Scholar] [CrossRef]
- Dizdarević, A.; Marić, M.; Shahzadi, I.; Ari Efiana, N.; Matuszczak, B.; Bernkop-Schnürch, A. Imine bond formation as a tool for incorporation of amikacin in self-emulsifying drug delivery systems (SEDDS). Eur. J. Pharm. Biopharm. 2021, 162, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, D.T.; Chen, C.H.; Shalumon, K.T.; Kao, H.H.; Chen, J.P. Bioactive Nanostructured Scaffold-Based Approach for Tendon and Ligament Tissue Engineering. Nanomaterials 2023, 13, 1847. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kafi, M.; El-Said, W.A.; Kim, T.H.; Choi, J.W. Cell adhesion, spreading, and proliferation on surface functionalized with RGD nanopillar arrays. Biomaterials 2012, 33, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Hu, J.; Han, Y. Random and aligned electrospun gelatin nanofiber mats for human mesenchymal stem cells. Mater. Res. Innov. 2018, 23, 208–215. [Google Scholar] [CrossRef]
- Doglioni, C.; Dei Tos, A.P.; Laurino, L.; Iuzzolino, P.; Chiarelli, C.; Celio, M.R.; Viale, G. Calretinin: A novel immunocytochemical marker for mesothelioma. Am. J. Surg. Pathol. 1996, 20, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Gotzos, V.; Vogt, P.; Celio, M.R. The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol. Res. Pract. 1996, 192, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Nagel, H.; Hemmerlein, B.; Ruschenburg, I.; Hüppe, K.; Droese, M. The value of anti-calretinin antibody in the differential diagnosis of normal and reactive mesothelia versus metastatic tumors in effusion cytology. Pathol. Res. Pract. 1998, 194, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995, 7, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Suassuna, J.H.; Das Neves, F.C.; Hartley, R.B.; Ogg, C.S.; Cameron, J.S. Immunohistochemical studies of the peritoneal membrane and infiltrating cells in normal subjects and in patients on CAPD. Kidney Int. 1994, 46, 443–454. [Google Scholar] [CrossRef][Green Version]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Yáñez-Mo, M.; Selgas, R.; Sánchez-Madrid, F.; López-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 2005, 6, 262–268. [Google Scholar]
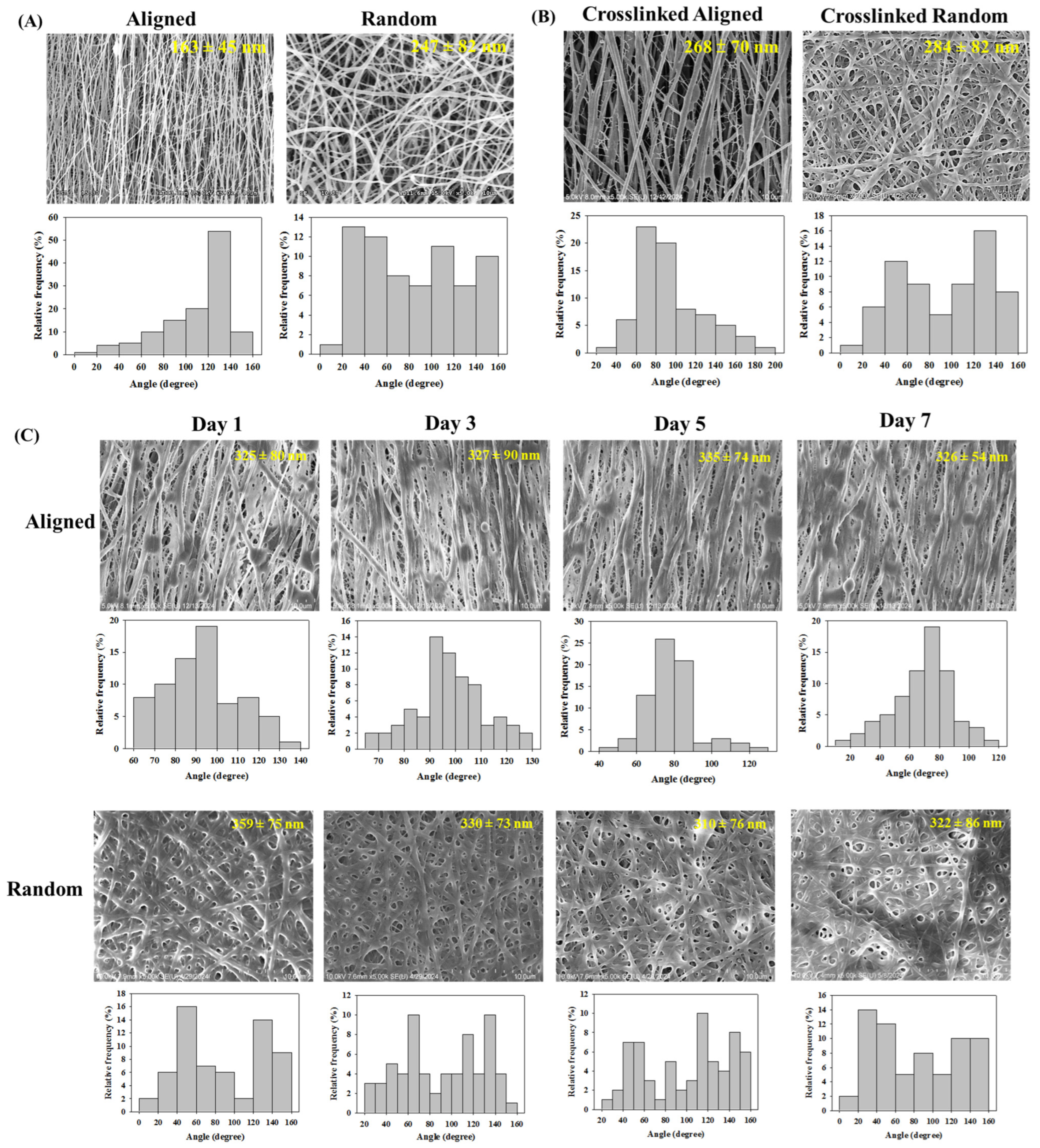

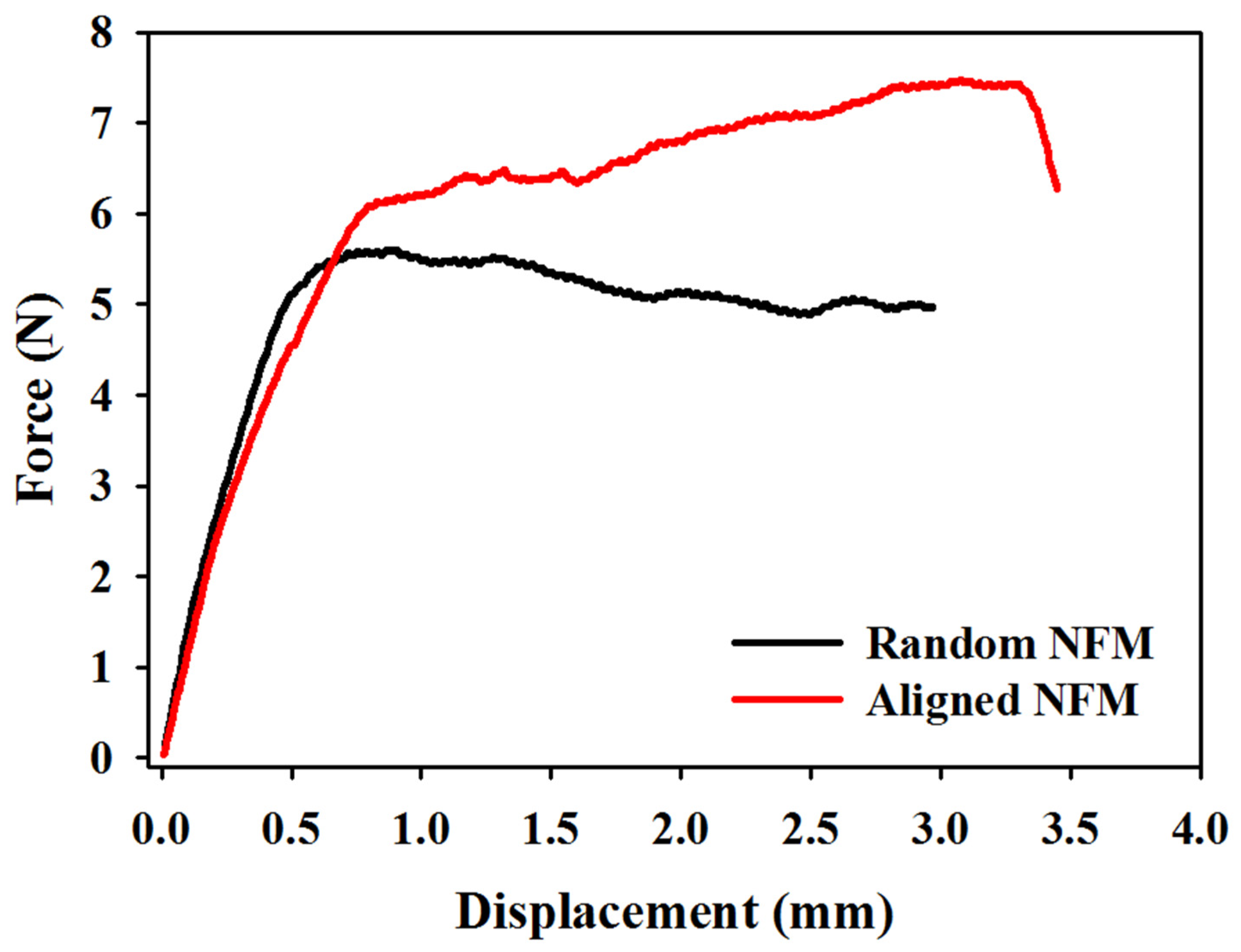



| Membranes | Maximum Load (N) | Maximum Displacement (mm) | Stiffness (N/mm) |
|---|---|---|---|
| Random NFM | 6.24 ± 0.60 | 3.54 ± 0.55 | 9.88 ± 0.53 |
| Aligned NFM | 6.68 ± 0.69 | 4.03 ±0.54 | 12.28 ± 0.38 * |
| Culture Time (Days) | Calretinin (%) | E-Cadherin (%) | ||
|---|---|---|---|---|
| Random | Aligned | Random | Aligned | |
| 3 | 0.25 ± 0.02 | 0.35 ± 0.01 * | 0.23 ± 0.02 | 0.29 ± 0.01 * |
| 7 | 0.43 ± 0.01 | 0.50 ± 0.02 * | 0.32 ± 0.02 | 0.43 ± 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, H.-H.; Govindaraju, D.T.; Dash, B.S.; Chen, J.-P. Electrospun Aligned Gelatin/Chitosan Nanofibrous Membranes for a Better Culture of Mesothelial Cells. J. Compos. Sci. 2025, 9, 31. https://doi.org/10.3390/jcs9010031
Kao H-H, Govindaraju DT, Dash BS, Chen J-P. Electrospun Aligned Gelatin/Chitosan Nanofibrous Membranes for a Better Culture of Mesothelial Cells. Journal of Composites Science. 2025; 9(1):31. https://doi.org/10.3390/jcs9010031
Chicago/Turabian StyleKao, Hao-Hsi, Darshan Tagadur Govindaraju, Banendu Sunder Dash, and Jyh-Ping Chen. 2025. "Electrospun Aligned Gelatin/Chitosan Nanofibrous Membranes for a Better Culture of Mesothelial Cells" Journal of Composites Science 9, no. 1: 31. https://doi.org/10.3390/jcs9010031
APA StyleKao, H.-H., Govindaraju, D. T., Dash, B. S., & Chen, J.-P. (2025). Electrospun Aligned Gelatin/Chitosan Nanofibrous Membranes for a Better Culture of Mesothelial Cells. Journal of Composites Science, 9(1), 31. https://doi.org/10.3390/jcs9010031








