Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review
Abstract
1. Introduction
2. Chitosan-Based Coatings/Films for Meat Preservation
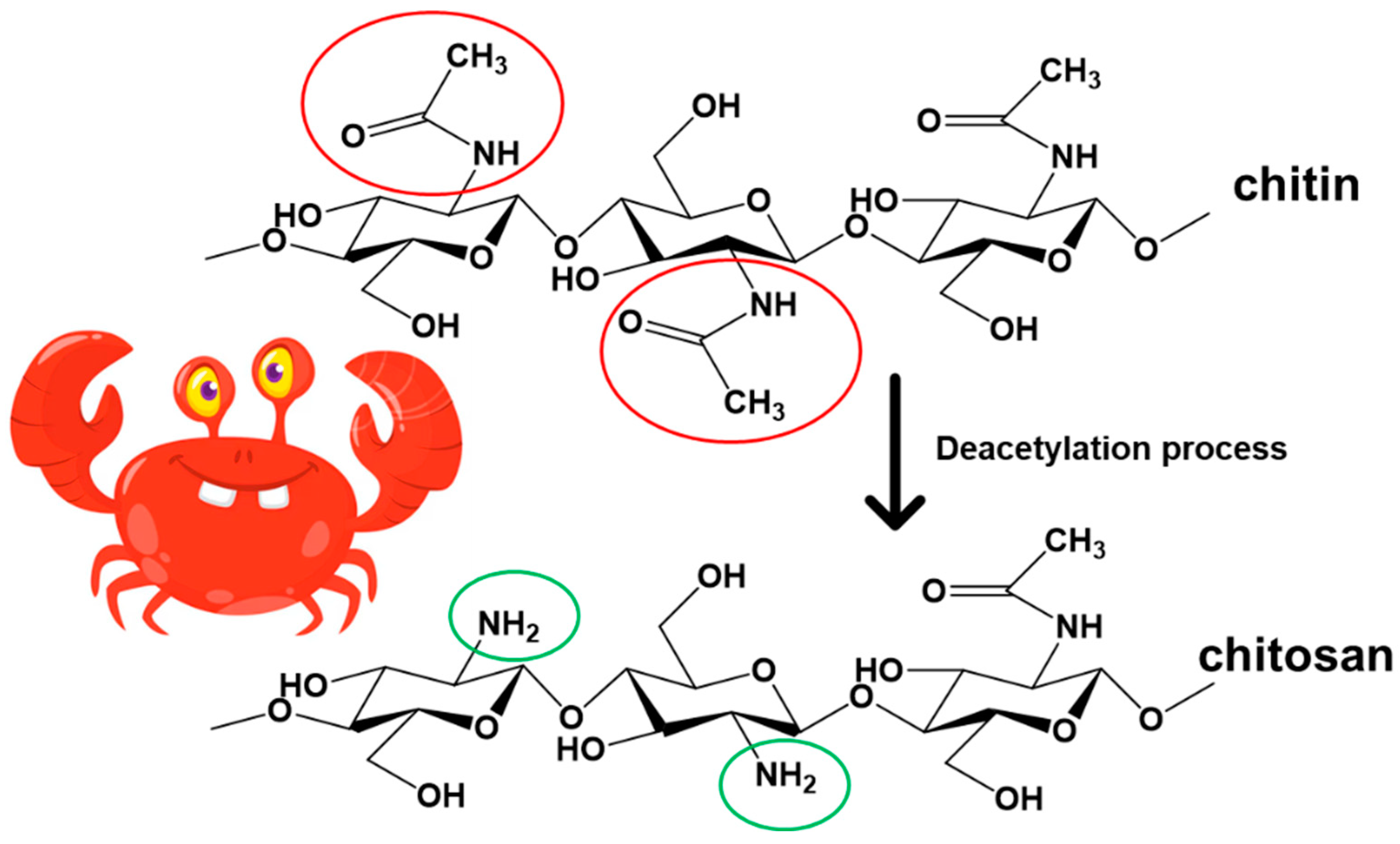
2.1. Brief Description of Chitosan
2.2. Application of Chitosan in Food Packaging
2.3. Antibacterial and Antifungal Mechanism of Chitosan in Food Preservation
3. Starch-Based Coatings/Films for Meat Preservation
3.1. Brief Description of Starch
3.2. Amylose–Amylopectin Ratios Influence the Structure and Properties of Starch-Based Films
3.3. Application of Starch-Based Films in Meat Preservation
4. Chitosan- and Starch-Based Biocomposite Coatings/Films for Meat Preservation
4.1. Brief Discussion on Chitosan–Starch-Based Composites
4.2. Antibacterial and Mechanical Attributes of Starch–Chitosan-Based Films
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Banda, L.J.; Tanganyika, J. Livestock provide more than food in smallholder production systems of developing countries. Anim. Front. 2021, 11, 7–14. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD: Paris, France, 2022. [Google Scholar]
- Mauricio, R.A.; Campos, J.A.D.B.; Nassu, R.T. Meat with edible coating: Acceptance, purchase intention and neophobia. Food Res. Int. 2022, 154, 111002. [Google Scholar] [CrossRef]
- Dai, L.; Wang, X.; Mao, X.; He, L.; Li, C.; Zhang, J.; Chen, Y. Recent advances in starch-based coatings for the postharvest preservation of fruits and vegetables. Carbohydr. Polym. 2024, 328, 121736. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Thakur, R.; Santhosh, R.; Kumar, Y.; Suryavanshi, V.R.; Singhi, H.; Madhubabu, D.; Wickramarachchi, S.; Pal, K.; Sarkar, P. Characteristics and application of animal byproduct-based films and coatings in the packaging of food products. Trends Food Sci. Technol. 2023, 140, 104143. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Hu, L. Recent Advances of Proteins, Polysaccharides and Lipids-Based Edible Films/Coatings for Food Packaging Applications: A Review. Food Biophys. 2024, 19, 29–45. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, E.-Q.; Xu, G.-F.; Naik, N.; Murugadoss, V.; Ma, M.-G.; Guo, Z.; Shi, Z.-J. Overview of renewable polysaccharide-based composites for biodegradable food packaging applications. Green Chem. 2022, 24, 480–492. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.S.; Hapiz, A.; Musa, S.A.; Alothman, Z.A.; Wilson, L.D.; Jawad, A.H. Biomagnetic chitosan-ethylene glycol diglycidyl ether/organo-nanoclay nanocomposite for azo dye removal: A statistical modeling by response surface methodology. Int. J. Biol. Macromol. 2024, 255, 128075. [Google Scholar] [CrossRef]
- Chen, X.; Chen, F.; Yang, Q.; Gong, W.; Wang, J.; Li, Y.; Wang, G. An environmental food packaging material part I: A case study of life-cycle assessment (LCA) for bamboo fiber environmental tableware. Ind. Crops Prod. 2023, 194, 116279. [Google Scholar] [CrossRef]
- Heras, M.; Huang, C.-C.; Chang, C.-W.; Lu, K.-H. Trends in chitosan-based films and coatings: A systematic review of the incorporated biopreservatives, biological properties, and nanotechnology applications in meat preservation. Food Packag. Shelf Life 2024, 42, 101259. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Wilson, L.D. Flax fiber-chitosan biocomposites with tailored structure and switchable physicochemical properties. Carbohydr. Polym. Technol. Appl. 2023, 6, 100397. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Cao, S.; Li, D.; Wu, Y.; Duan, P.; Liu, S.; Li, X.; Zhang, X.; Chen, Y. Fabrication and characterization of chitosan/anthocyanin intelligent packaging film fortified by cellulose nanocrystal for shrimp preservation and visual freshness monitoring. Int. J. Biol. Macromol. 2024, 264, 130692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and UV Blocking Properties of Composite Chitosan Films with Curcumin Grafted Cellulose Nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Moalla, S.; Ammar, I.; Fauconnier, M.-L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lan, W.; Xie, J. Phenolic acid-chitosan derivatives: An effective strategy to cope with food preservation problems. Int. J. Biol. Macromol. 2024, 254, 127917. [Google Scholar] [CrossRef]
- Su, C.-Y.; Li, D.; Wang, L.-J.; Wang, Y. Biodegradation behavior and digestive properties of starch-based film for food packaging—A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 6923–6945. [Google Scholar] [CrossRef]
- FPelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-Friendly Alternative for Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Rahmasari, Y.; Yemiş, G.P. Characterization of ginger starch-based edible films incorporated with coconut shell liquid smoke by ultrasound treatment and application for ground beef. Meat Sci. 2022, 188, 108799. [Google Scholar] [CrossRef]
- Lipatova, I.; Yusova, A.; Makarova, L. Fabrication and characterization of starch films containing chitosan nanoparticles using in situ precipitation and mechanoactivation techniques. J. Food Eng. 2021, 304, 110593. [Google Scholar] [CrossRef]
- Othman, S.H.; Othman, N.F.L.; Shapi’i, R.A.; Ariffin, S.H.; Yunos, K.F.M. Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers 2021, 13, 390. [Google Scholar] [CrossRef]
- Othman, S.H.; Shapi’I, R.A.; Ronzi, N.D.A. Starch biopolymer films containing chitosan nanoparticles: A review. Carbohydr. Polym. 2024, 329, 121735. [Google Scholar] [CrossRef] [PubMed]
- Shapi’i, R.A.; Othman, S.H.; Basha, R.K.; Naim, M.N. Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles. Nanotechnol. Rev. 2022, 11, 1464–1477. [Google Scholar] [CrossRef]
- Bajer, D. Hypophosphite cross-linked starch succinate/chitosan membranes as alternative for packaging and pharmaceutical application. Int. J. Biol. Macromol. 2023, 249, 126103. [Google Scholar] [CrossRef]
- Yong, H.; Xu, F.; Yun, D.; Hu, H.; Liu, J. Antioxidant packaging films developed by in-situ cross-linking chitosan with dialdehyde starch-catechin conjugates. Int. J. Biol. Macromol. 2022, 222, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Lipatova, I.; Losev, N.; Makarova, L.; Rodicheva, J.; Burmistrov, V. Effect of composition and mechanoactivation on the properties of films based on starch and chitosans with high and low deacetylation. Carbohydr. Polym. 2020, 239, 116245. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Chen, J.; Li, S.; Liu, J.; Zhou, Z.; Qin, Z.; Wang, H.; Su, M.; Li, L.; Bai, Z. An antibacterial packaging film based on amylose starch with quaternary ammonium salt chitosan and its application for meat preservation. Int. J. Biol. Macromol. 2024, 261, 129706. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Khalil, H.A. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef]
- Hu, H.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Effect of chitosan/starch aldehyde-catechin conjugate composite coating on the quality and shelf life of fresh pork loins. J. Sci. Food Agric. 2022, 102, 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Santos, P.; dos Ouros, L.F. Starch-Based Packaging and Coating Polymers for Food; Academic Press: Cambridge, MA, USA, 2024; pp. 295–310. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhang, J.; Yang, F.; Wang, W.; Li, W.; Qin, C. Properties and biological activity of chitosan-coix seed starch films incorporated with nano zinc oxide and Artemisia annua essential oil for pork preservation. LWT 2022, 164, 113665. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Tang, J.; He, B.; Yu, H.; Xu, X.; Li, C.; Wang, C.; Liu, Y.; Su, Y. Pork preservation by antimicrobial films based on potato starch (PS) and polyvinyl alcohol (PVA) and incorporated with clove essential oil (CLO) Pickering emulsion. Food Control 2023, 154, 109988. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, W.; Qin, X.; Wang, H.; Tan, L.; Liu, X. Chitosan/oxidized Konjac Glucomannan films incorporated with Zanthoxylum Bungeanum essential oil: A novel approach for extending the shelf life of meat. Int. J. Biol. Macromol. 2024, 262, 129683. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Phoopanasaeng, P.; Sripahco, T.; Soykeabkaew, N.; Pripdeevech, P. Application of chitosan films incorporated with Zanthoxylum limonella essential oil for extending shelf life of pork. Int. J. Biol. Macromol. 2024, 262, 129703. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; He, S.; Chen, L.; Chen, H.; Ouyang, K.; Wang, W. Effect of gelatin-chitosan-Cyclocarya paliurus flavonoids edible coating film on the preservation of chilled beef. LWT 2024, 199, 116138. [Google Scholar] [CrossRef]
- Elsherif, W.M.; Zayed, G.M.; Tolba, A.O. Antimicrobial activity of chitosan- edible films containing a combination of carvacrol and rosemary nano-emulsion against Salmonella enterica serovar Typhimurium and Listeria monocytogenes for ground meat. Int. J. Food Microbiol. 2024, 418, 110713. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Lin, L.; Peng, S.; Shi, C.; Li, C.; Hua, Z.; Cui, H. Preparation and characterization of cassava starch/sodium carboxymethyl cellulose edible film incorporating apple polyphenols. Int. J. Biol. Macromol. 2022, 212, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, X.; Zhang, Y.; Liu, G. Development and characterization of a new potato starch/watermelon peel pectin composite film loaded with TiO2 nanoparticles and microencapsulated Lycium barbarum leaf flavonoids and its use in the Tan mutton packaging. Int. J. Biol. Macromol. 2023, 252, 126532. [Google Scholar] [CrossRef]
- Nandi, S.; Guha, P. Development, characterization and application of starch-based film containing polyphenols of piper betle L. waste in chicken meat storage. Food Chem. 2024, 431, 137103. [Google Scholar] [CrossRef]
- Pramitasari, R.; Gunawicahya, L.N.; Anugrah, D.S.B. Development of an Indicator Film Based on Cassava Starch–Chitosan Incorporated with Red Dragon Fruit Peel Anthocyanin Extract. Polymers 2022, 14, 4142. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.W.; Lin, M.; Mustapha, A. Chitosan/acetylated starch composite films incorporated with essential oils: Physiochemical and antimicrobial properties. Food Biosci. 2021, 43, 101287. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Das, S.; Dwivedi, A.; Dubey, N.K. Application of chitosan and other biopolymers based edible coatings containing essential oils as green and innovative strategy for preservation of perishable food products: A review. Int. J. Biol. Macromol. 2023, 253, 127688. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Karua, C.S.; Sahoo, A. Synthesis and characterization of starch/chitosan composites. Mater. Today Proc. 2020, 33, 5179–5183. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Ezati, P.; Ezati, P.; Rhim, J.-W.; Rhim, J.-W. Chitosan/Starch-Based Active Packaging Film with N, P-Doped Carbon Dots for Meat Packaging. ACS Appl. Bio Mater. 2023, 6, 1294–1305. [Google Scholar] [CrossRef]
- Silva, O.A.; Pellá, M.C.G.; Friedrich, J.C.C.; Pellá, M.G.; Beneton, A.G.; Faria, M.G.I.; Colauto, G.A.L.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effects of a Native Cassava Starch, Chitosan, and Gelatin-Based Edible Coating over Guavas (Psidium guajava L.). ACS Food Sci. Technol. 2021, 1, 1247–1253. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Karaś, M.; Kordowska-Wiater, M.; Skrzypek, T.; Kazimierczak, W. Inherently acidic films based on chitosan lactate-doped starches and pullulan as carries of nisin: A comparative study of controlled-release and antimicrobial properties. Food Chem. 2023, 404, 134760. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, S.; Bayram, G.M.U.; Khati, P.; Simsek, H. Developments and application of chitosan-based adsorbents for wastewater treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef] [PubMed]
- Oyekunle, D.T.; Omoleye, J.A. New process for synthesizing chitosan from snail shells. J. Physics Conf. Ser. 2019, 1299, 012089. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Omoleye, J.A. Extraction, characterization and kinetics of demineralization of chitin produced from snail shells of different particle sizes using 1.2 M HCl. Int. J. Mech. Eng. Technol. 2019, 10, 2011–2020. [Google Scholar]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Yu, D.; Yu, Z.; Zhao, W.; Regenstein, J.M.; Xia, W. Advances in the application of chitosan as a sustainable bioactive material in food preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3782–3797. [Google Scholar] [CrossRef] [PubMed]
- Petroni, S.; Tagliaro, I.; Antonini, C.; D’arienzo, M.; Orsini, S.F.; Mano, J.F.; Brancato, V.; Borges, J.; Cipolla, L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Mar. Drugs 2023, 21, 147. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.D.A.; Cadaval, T.R.S.A., Jr.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems, In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2020; pp. 457–489. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Sallam, K.I.; Malak, N.M. Improvement of the microbial quality, antioxidant activity, phenolic and flavonoid contents, and shelf life of smoked herring (Clupea harengus) during frozen storage by using chitosan edible coating. Food Control 2021, 130, 108317. [Google Scholar] [CrossRef]
- ES-288; Egyptian Standard for Smoked Fish. Egyptian Organization of Standardization and Quality: Cairo Governorate, Egypt, 2005.
- Arshad, M.S.; Batool, S.A. Natural antimicrobials, their sources and food safety. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Fernando, S.S.; Jo, C.; Mudannayake, D.C.; Jayasena, D.D. An overview of the potential application of chitosan in meat and meat products. Carbohydr. Polym. 2024, 324, 121477. [Google Scholar] [CrossRef] [PubMed]
- Alboghbeish, H.; Khodanazary, A. The Comparison of Quality Characteristics of Refrigerated Carangoides coeruleopinnatus Fillets with Chitosan and Nanochitosan Coating. Turk. J. Fish. Aquat. Sci. 2018, 19, 957–967. [Google Scholar]
- Kulawik, P.; Jamróz, E.; Tkaczewska, J.; Vlčko, T.; Zając, M.; Guzik, P.; Janik, M.; Tadele, W.; Golian, J.; Milosavljević, V. Application of antimicrobial chitosan-Furcellaran-hydrolysate gelatin edible coatings enriched with bioactive peptides in shelf-life extension of pork loin stored at 4 and −20 °C. Int. J. Biol. Macromol. 2024, 254, 127865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Wang, D.; Tang, J.; Xu, M. Synergistic stabilization of garlic essential oil nanoemulsions by carboxymethyl chitosan/Tween 80 and application for coating preservation of chilled fresh pork. Int. J. Biol. Macromol. 2024, 266, 131370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, B.; Zhang, Y.; Hu, J.; Sun, T.; Dong, T.; Yun, X. Development of high barrier poly(l-actic acid)/chitosan/graphene oxide flexible films for meat packaging by layer-by-layer. Food Biosci. 2024, 60, 104304. [Google Scholar] [CrossRef]
- Elsherief, M.F.; Devecioglu, D.; Saleh, M.N.; Karbancioglu-Guler, F.; Capanoglu, E. Chitosan/alginate/pectin biopolymer-based Nanoemulsions for improving the shelf life of refrigerated chicken breast. Int. J. Biol. Macromol. 2024, 264, 130213. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Liang, S.; Li, Y. Enhanced antioxidant and antibacterial activities of chitosan/zein nanoparticle Pickering emulsion-incorporated chitosan coatings in the presence of cinnamaldehyde and tea polyphenol. Int. J. Biol. Macromol. 2024, 266, 131181. [Google Scholar] [CrossRef]
- Yan, R.; Liu, M.; Zeng, X.; Du, Q.; Wu, Z.; Guo, Y.; Tu, M.; Pan, D. Preparation of modified chitosan-based nano-TiO2–nisin composite packaging film and preservation mechanism applied to chilled pork. Int. J. Biol. Macromol. 2024, 269, 131873. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Chitosan/gelatin-based multifunctional films integrated with sulfur-functionalized chitin for active packaging applications. Food Hydrocoll. 2024, 149, 109537. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, H.; Wang, Y. Nanocomplexes film composed of gallic acid loaded ovalbumin/chitosan nanoparticles and pectin with excellent antibacterial activity: Preparation, characterization and application in coating preservation of salmon fillets. Int. J. Biol. Macromol. 2024, 259, 128934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xu, Z.; Li, H.; Xie, J. Effects of active film based on chitosan/polyvinyl alcohol on the quality of refrigerated sea bass (Lateolabrax Japonicus) fillets. Food Biosci. 2024, 59, 103854. [Google Scholar] [CrossRef]
- Riahi, Z.; Khan, A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Sustainable chitosan/polyvinyl alcohol composite film integrated with sulfur-modified montmorillonite for active food packaging applications. Prog. Org. Coat. 2024, 192, 108474. [Google Scholar] [CrossRef]
- Sheerzad, S.; Khorrami, R.; Khanjari, A.; Gandomi, H.; Basti, A.A.; Khansavar, F. Improving chicken meat shelf-life: Coating with whey protein isolate, nanochitosan, bacterial nanocellulose, and cinnamon essential oil. LWT 2024, 197, 115912. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, X.; Zhong, H.; Li, C.; Shi, C.; Cui, H.; Lin, L. Ethyl cellulose/gelatin-carboxymethyl chitosan bilayer films doped with Euryale ferox seed shell polyphenol for cooked meat preservation. Int. J. Biol. Macromol. 2024, 256, 128286. [Google Scholar] [CrossRef]
- Thakur, R.; Wickramarachchi, S.; Pal, K.; Sarkar, P. Gelatin/chitosan-lactate/curcuma hydroethanolic extract-based antimicrobial films: Preparation, characterization, and application on chicken meat. Food Hydrocoll. 2024, 154, 110075. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Janik, M.; Tkaczewska, J.; Krzyściak, P.; Skóra, M.; Guzik, P.; Milosavljević, V.; Tadele, W. Antimicrobial and antioxidant properties of chitosan-furcellaran-gelatin hydrolysate coatings enhanced with bioactive peptides. Food Control 2023, 153, 109931. [Google Scholar] [CrossRef]
- Li, H.; Qu, S.; Ma, P.; Zhang, J.; Zhao, K.; Chen, L.; Huang, Q.; Zou, G.; Tang, H. Effects of chitosan coating combined with thermal treatment on physicochemical properties, bacterial diversity and volatile flavor of braised duck meat during refrigerated storage. Food Res. Int. 2023, 167, 112627. [Google Scholar] [CrossRef]
- Liu, W.; Kang, S.; Xue, J.; Chen, S.; Yang, W.; Yan, B.; Liu, D. Self-assembled carboxymethyl chitosan/zinc alginate composite film with excellent water resistant and antimicrobial properties for chilled meat preservation. Int. J. Biol. Macromol. 2023, 247, 125752. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Sheikh, M.; Hasnain, A. Effects of cross linking and/or acetylation on sorghum starch and film characteristics. Int. J. Biol. Macromol. 2020, 155, 786–794. [Google Scholar] [CrossRef]
- Subroto, E. Review on the Analysis Methods of Starch, Amylose, Amylopectinin Food and Agricultural Products. Int. J. Emerg. Trends Eng. Res. 2020, 8, 3519–3524. [Google Scholar] [CrossRef]
- Putri, T.R.; Adhitasari, A.; Paramita, V.; Yulianto, M.E.; Ariyanto, H.D. Effect of different starch on the characteristics of edible film as functional packaging in fresh meat or meat products: A review. Mater. Today Proc. 2023, 87, 192–199. [Google Scholar] [CrossRef]
- Govindaraju, I.; Zhuo, G.-Y.; Chakraborty, I.; Melanthota, S.K.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mahato, K.K.; Mazumder, N. Investigation of structural and physico-chemical properties of rice starch with varied amylose content: A combined microscopy, spectroscopy, and thermal study. Food Hydrocoll. 2022, 122, 107093. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef]
- Stawski, D. New determination method of amylose content in potato starch. Food Chem. 2008, 110, 777–781. [Google Scholar] [CrossRef]
- Huang, X. Modification of Potato Starch Granule Structure and Morphology in Planta by Expression of Starch Binding Domain Fusion Proteins Xingfeng Huang. PhD. Thesis, Wageningen University, Wageningen, The Netherlands, 2010. Available online: https://core.ac.uk/download/pdf/29241345.pdf (accessed on 19 May 2024).
- Abioye, V.; Adeyemi, I.; Akinwande, B.; Kulakow, P.; Maziya-Dixon, B. Effect of steam cooking and storage time on the formation of resistant starch and functional properties of cassava starch. Cogent Food Agric. 2017, 3, 1296401. [Google Scholar] [CrossRef]
- Yu, J.K.; Moon, Y.S. Corn Starch: Quality and Quantity Improvement for Industrial Uses. Plants 2021, 11, 92. [Google Scholar] [CrossRef]
- Colussi, R.; do Nascimento, L.Á.; Singh, J. Potential Use of Starch From Different Sources in the Preparation of Mucoadhesive Films Uso Potencial De Amido De Diferentes Fontes Na Preparação De Filmes Mucoadesivos. Rev. CIATEC 2021, 13, 1–9. [Google Scholar]
- Sondari, D.; Falah, F.; Suryaningrum, R.; Sari, F.P.; Septefani, A.A.; Restu, W.K.; Sampora, Y. Biofilm Based on Modified Sago Starch: Preparation and Characterization. Reaktor 2019, 19, 125–130. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Solikhah, A.F.; Rahmawati, A.Y. Tensile Strength and Elongation Testing for Starch-Based Bioplastics using Melt Intercalation Method: A Review. J. Phys. Conf. Ser. 2021, 1858, 012028. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Chandla, N.K.; Khatkar, S.K.; Singh, S.; Saxena, D.C.; Jindal, N.; Bansal, V.; Wakchaure, N. Tensile Strength and Solubility Studies of Edible Biodegradable Films Developed from Pseudo-cereal Starches: An Inclusive Comparison with Commercial Corn Starch. Asian J. Dairy Food Res. 2020, 39, 139–146. [Google Scholar] [CrossRef]
- Suh, J.H.; Ock, S.Y.; Park, G.D.; Lee, M.H.; Park, H.J. Effect of moisture content on the heat-sealing property of starch films from different botanical sources. Polym. Test. 2020, 89, 106612. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Hu, X.; Huang, X.; Zhang, X.; Zou, X.; Shi, J. Preparation of edible antibacterial films based on corn starch /carbon nanodots for bioactive food packaging. Food Chem. 2024, 444, 138467. [Google Scholar] [CrossRef] [PubMed]
- Quilez-Molina, A.I.; Merino, D.; Dumon, M. Porous starch embedded with anthocyanins-CMC coating as bifunctional packaging with seafood freshness monitoring properties. Food Hydrocoll. 2024, 154, 110114. [Google Scholar] [CrossRef]
- Vidal, N.P.; Charlampita, M.C.; Spotti, M.J.; Martinez, M.M. Multifunctional phloroglucinol-loaded pea starch coating for refrigerated salmon. Food Packag. Shelf Life 2024, 43, 101277. [Google Scholar] [CrossRef]
- Hernández-Nolasco, Z.; Ríos-Corripio, M.A.; Hidalgo-Contreras, J.V.; Castellano, P.H.; Rubio-Rosas, E.; Hernández-Cázares, A.S. Optimization of sodium alginate, taro starch and lactic acid based biodegradable films: Antimicrobial effect on a meat product. LWT 2024, 192, 115718. [Google Scholar] [CrossRef]
- Adame, M.Y.; Shi, C.; Li, C.; Aziz, T.; Alharbi, M.; Cui, H.; Lin, L. Fabrication and characterization of pullulan/tapioca starch-based antibacterial films incorporated with Litsea cubeba essential oil for meat preservation. Int. J. Biol. Macromol. 2024, 268, 131775. [Google Scholar] [CrossRef]
- Chandrasekar, C.M.; Nespoli, L.; Bellesia, T.; Ghaani, M.; Farris, S.; Romano, D. Fabrication of double layer nanoparticle infused starch-based thermoplastic food packaging system for meat preservation. Int. J. Biol. Macromol. 2024, 254, 127689. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Yu, R.; Zheng, H.; Wang, P. Acylated pectin/gelatin-based films incorporated with alkylated starch crystals: Characterization, antioxidant and antibacterial activities, and coating preservation effects on golden pomfret. Int. J. Biol. Macromol. 2023, 241, 124532. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Aminzare, M.; Azar, H.H.; Rostamizadeh, K. Effect of corn starch coating incorporated with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on microbial quality of fresh chicken meat and fate of inoculated Listeria monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.A.R.; Camelo-Silva, C.; da Silva Carvalho, C.; de Mello, J.R.; Barroso, N.G.; da Silva Barros, E.L.; Silva, P.P.; Pertuzatti, P.B. Active packaging with starch, red cabbage extract and sweet whey: Characterization and application in meat. LWT 2021, 135, 110275. [Google Scholar] [CrossRef]
- Wu, L.; Lv, S.; Wei, D.; Zhang, S.; Zhang, S.; Li, Z.; Liu, L.; He, T. Structure and properties of starch/chitosan food packaging film containing ultra-low dosage GO with barrier and antibacterial. Food Hydrocoll. 2023, 137, 108329. [Google Scholar] [CrossRef]
- Bi, S.; Qin, D.; Yuan, S.; Cheng, X.; Chen, X. Homogeneous modification of chitin and chitosan based on an alkali/urea soluble system and their applications in biomedical engineering. Green Chem. 2021, 23, 9318–9333. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Omoleye, J.A. Effect of particle sizes on the kinetics of demineralization of snail shell for chitin synthesis using acetic acid. Heliyon 2019, 5, e02828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, R.; Xu, C.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr. Polym. 2021, 264, 118050. [Google Scholar] [CrossRef]
- Zou, W.; Gu, J.; Li, J.; Wang, Y.; Chen, S. Tailorable antibacterial and cytotoxic chitosan derivatives by introducing quaternary ammonium salt and sulfobetaine. Int. J. Biol. Macromol. 2022, 218, 992–1001. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H.; Xie, T.; Chen, L.; Hu, S.; Tian, H.; Wang, Y.; Wang, J. Characterization and antibacterial effect of quaternized chitosan anchored cellulose beads. Int. J. Biol. Macromol. 2020, 155, 1325–1332. [Google Scholar] [CrossRef]
- Ji, N.; Qin, Y.; Xi, T.; Xiong, L.; Sun, Q. Effect of chitosan on the antibacterial and physical properties of corn starch nanocomposite films. Starch-Starke 2016, 69, 1600114. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Bof, M.J.; Bordagaray, V.C.; Locaso, D.E.; García, M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar] [CrossRef]
- Navarro, Y.M.; Soukup, K.; Jandová, V.; Gómez, M.M.; Solis, J.L.; Cruz, J.F.; Siche, R.; Šolcová, O.; Cruz, G.J.F. Starch/chitosan/glycerol films produced from low-value biomass: Effect of starch source and weight ratio on film properties. J. Phys. Conf. Ser. 2019, 1173, 012008. [Google Scholar] [CrossRef]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Abraham, Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch–chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Babaee, M.; Garavand, F.; Rehman, A.; Jafarazadeh, S.; Amini, E.; Cacciotti, I. Biodegradability, physical, mechanical and antimicrobial attributes of starch nanocomposites containing chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 195, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Garavand, Y.; Taheri-Garavand, A.; Garavand, F.; Shahbazi, F.; Khodaei, D.; Cacciotti, I. Starch-Polyvinyl Alcohol-Based Films Reinforced with Chitosan Nanoparticles: Physical, Mechanical, Structural, Thermal and Antimicrobial Properties. Appl. Sci. 2022, 12, 1111. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydr. Polym. 2019, 210, 204–209. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Basha, R.K.; Naim, M.N. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bao, Y.; Li, J.; Bi, J.; Chen, Q.; Cui, H.; Wang, Y.; Tian, J.; Shu, C.; Wang, Y. A sub-freshness monitoring chitosan/starch-based colorimetric film for improving color recognition accuracy via controlling the pH value of the film-forming solution. Food Chem. 2022, 388, 132975. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, Z.; Mirmoghtadaie, L.; Abdolmaleki, K.; Khani, M.R.; Farhoodi, M.; Moradi, E.; Shokri, B.; Shojaee-Aliabadi, S. Characterization of physicochemical and antimicrobial properties of plasma-treated starch/chitosan composite film. Packag. Technol. Sci. 2021, 34, 385–392. [Google Scholar] [CrossRef]
- Anisimov, I.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Renewable Hybrid Biopolymer/Polyaniline Composites for Humidity Sensing. ACS Appl. Polym. Mater. 2022, 4, 7204–7216. [Google Scholar] [CrossRef]
- Kusrini, E.; Wilson, L.D.; Padmosoedarso, K.M.; Mawarni, D.P.; Sufyan, M.; Usman, A. Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications. J. Compos. Sci. 2023, 7, 52. [Google Scholar] [CrossRef]
- Yusof, W.R.W.; Awang, N.Y.F.; Laile, M.A.A.; Azizi, J.; Husaini, A.A.S.A.; Seeni, A.; Wilson, L.D.; Sabar, S. Chemically modified water-soluble chitosan derivatives: Modification strategies, biological activities, and applications. Polym. Technol. Mater. 2023, 62, 2182–2220. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Varma, R.S.; Thakur, V.K. Nano/Micro-Structural Supramolecular Biopolymers: Innovative Networks with the Boundless Potential in Sustainable Agriculture. Nano-Micro Lett. 2024, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kubota, R.; Aoyama, T.; Urayama, K.; Hamachi, I. Four distinct network patterns of supramolecular/polymer composite hydrogels controlled by formation kinetics and interfiber interactions. Nat. Commun. 2023, 14, 1696. [Google Scholar] [CrossRef]
- Xing, J.; Jin, S.; Yu, Y.; Zeng, G.; Zhang, F.; Xiao, H.; Yang, R.; Li, K.; Li, J. Supramolecular and double network strategy toward strong and antibacterial protein films by introducing waterborne polyurethane and quaternized chitosan. Ind. Crops Prod. 2023, 205, 117445. [Google Scholar] [CrossRef]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Llamazares, S.R. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Istiqomah, A.; Prasetyo, W.E.; Firdaus, M.; Kusumaningsih, T. Valorisation of lemongrass essential oils onto chitosan-starch film for sustainable active packaging: Greatly enhanced antibacterial and antioxidant activity. Int. J. Biol. Macromol. 2022, 210, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Udoetok, I.A.; Szpunar, J.A.; Wilson, L.D. Hydrolyzed Forms of Cellulose and Its Metal Composites for Hydrogen Generation: An Experimental and Theoretical Investigation. J. Compos. Sci. 2024, 8, 262. [Google Scholar] [CrossRef]
- Kusrini, E.; Safira, A.I.; Usman, A.; Prasetyanto, E.A.; Nugrahaningtyas, K.D.; Santosa, S.J.; Wilson, L.D. Nanocomposites of Terbium Sulfide Nanoparticles with a Chitosan Capping Agent for Antibacterial Applications. J. Compos. Sci. 2023, 7, 39. [Google Scholar] [CrossRef]



| Chitosan-Based Coating/Film | Coated Meat Sample | Key Findings | Ref. |
|---|---|---|---|
| Chitosan (Cht) films incorporated with Zanthoxylum limonella (Zl) oil | Fresh pork | The incorporation of Zanthoxylum limonella (Zl) oil into chitosan film efficiently preserves pork, prolonging its shelf life and sustaining its physical attributes. Moreover, the inclusion of the essential oil resulted in a significant antibacterial effect against E. coli and S. aureus, in contrast to the chitosan film without essential oil, owing to the synergistic effects on antibacterial activity. The chitosan films without Zl oil had a tensile strength (TS) of 10.21 MPa, while the chitosan films that contained 2 and 4% of Zl essential oil had tensile strength values of 12.54 and 13.64 MPa, respectively. Furthermore, % elongation at break (EB) values for chitosan films integrated with 0%, 2%, and 4% Zl essential oil were determined to be 72.14%, 81.24%, and 84.23%, respectively. The water vapor permeability (WVP) of chitosan-free films was 1.50 × 10−9 ± 1.80 × 10−10 g/s⋅m⋅Pa, whereas films containing 2% and 4% Zl essential oil showed similar values of WVP 1.51 × 10−9 ± 1.30 × 10−10 g/s⋅m⋅Pa and 1.53 × 10−9 ± 1.78 × 10−10 g/s⋅m⋅Pa. The incorporation of essential oil improved flexibility and mechanical strength, and no significant effects occurred in relation to thermal stability, WVP, or water solubility. | [39] |
| Chitosan–furcellaran–hydrolysate gelatin edible coatings enriched with bioactive peptides | Pork loin | Microbiological tests demonstrated the resistance of the coatings against yeasts, microscopic fungi, and aerobic bacteria, especially in the early stages of storage when coated samples showed microbial reductions of 0.5–2 log CFU/g versus the controls. When stored in a freezer or refrigerator, the coatings did not seem to affect water activity. Applying edible coatings refined with bioactive peptides extended the pork loin’s acceptability by up to seven days. | [70] |
| Edible chitosan (Cht) films containing a combination of carvacrol and rosemary nanoemulsion (NE) | Ground meat | The chitosan-free films’ water vapor permeability (WVP) was estimated at 3102 g/m2/day. However, when the carvacrol nanoemulsion (NE) (1.56%) was added to the chitosan films, the WVP significantly reduced (2648 g/m2/day). Moreover, compared to the control films, the chitosan films with carvacrol NE (1.56%) showed higher tensile strength (61.42 ± 0.06 MPa) and elongation (30.56 ± 0.07%). Furthermore, chitosan-based films containing essential oil NEs, such as carvacrol (0.78%) and rosemary (1.56%), effectively reduced pathogenic contamination and improved the quality of the meat. The constant 1.56% carvacrol NE release into the chitosan layer guaranteed continued antibacterial activity. | [41] |
| Garlic essential oil nanoemulsions (GEO-NEs) fabricated by mixing carboxymethyl chitosan (CMCht), Tween 80 (TW 80), and the oil phase of garlic essential oil (GEO). | Fresh pork (hind-leg muscle) | Garlic essential oil nanoemulsions (GEO-NEs) displayed enhanced antioxidant capacity in scavenging free radicals of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), diammonium salt, and 1,1-diphenyl-2-picrylhydrazyl (DPPH), and had greater antibacterial activity against S. aureus and E. coli than free GEO. Moreover, the loading of GEO-NEs with 3% GEO significantly increased the chilled pork’s shelf life by ca. 1 week. | [71] |
| Poly(lactic acid)/chitosan/graphene oxide (PLA/Cht/GO) flexible films. GO was deposited layer-by-layer (LbL) on PLA-poly (butylene itaconate) (PBI) copolymers with chitosan (Cht) as the bonding layer | Pork loin meat | The addition of PBI (poly (butylene itaconate)) prevents PLA (poly(l-lactic acid)) from physically aging and crystallizing while increasing its hydrophilicity and flexibility. The oxygen transmission rate (OTR) of the films showed a decreasing trend with the LBL (layer-by-layer) deposition of GO (graphene oxide). The OTR of the PLBI/Cht/(GO-Cht)10 film decreased to 1.6 cm3/m2⋅d and the oxygen transmission coefficient (OTC) decreased to 0.4 × 10−8 cm3 m/m2⋅h⋅Pa. The water vapor permeability (WVP) of the PLBI/Cht/(GO-Cht)10 films decreased to 4.48 × 10−8 cm3 m/m2⋅h⋅Pa, a 55.0% reduction compared to the PLBI film. The utility of chitosan as the outermost component resulted in anti-microbial properties of the generated LBL films. Based on the results, PLBI/Cht/(GO-Cht)x with a higher number of GO layers considerably reduced the oxidation and microbiological development of chilled meat, while preserving its superior texture and color. Most significantly, owing to the incredibly low OTR, the PLBI/Cht/(GO-Cht)10 film outperformed a commercially available polyacrylate/polyethylene film for the preservation of chilled meat. | [72] |
| Chitosan–alginate–pectin (Cht/Alg/Pct) biopolymer-based NEs | Chicken breast | The most effective coating to reduce cooking loss was an essential oil (EO)–nanoemulsion (EO-NE)–chitosan (Cht) coating; the synthesized coatings, such as EO–NE–chitosan, EO–NE–alginate, and EO–NE–pectin, showed promising results in maintaining color stability. Microbiological analysis showed that the EO–NE–chitosan coating significantly inhibited the growth of mesophilic and psychrophilic bacteria, as well as yeasts, extending the shelf life of chicken breasts. | [73] |
| Chitosan/oxidized konjac glucomannan (KGM) films incorporated with Zanthoxylum Bungeanum (ZB) essential oil | Pork | Treatment with Zanthoxylum Bungeanum essential oil (ZB-EO) increases water vapor permeability (WVP) and reduces mechanical properties. The addition of 1% ZB-EO increased tensile strength (TS) by 18.92% and decreased water solubility by 10.05%, WVP by 6.60%, and moisture content by 1.03%. The TS levels of film were increased as the ZB-EO concentration increased, and the highest value (36.52 MPa) was observed in sliced meat wrapped in chitosan/oxidized KGM (OKGM with ozone) film with 1% ZB-EO. As the ZB-EO concentration exceeded 1.5%, a decrease in TS occurred. In addition, the elongation at break (EB) was at maximum with the addition of 1% ZB-EO (55.8%), whereas the EB decreased above 1.5%. Increasing the ZB-EO content improved the properties of the composite films (antibacterial, antioxidant, and UV barrier). The inclusion of ZB-EO dramatically reduced the thiobarbituric acid, total volatile basic nitrogen, redness, total viable count, and pH value. This formulation increased pork meat hardness after 10 days of preservation. | [38] |
| Chitosan/zein nanoparticle (NP) Pickering emulsion incorporating chitosan (Cht) coatings in the presence of cinnamaldehyde and tea polyphenol | Pork loin meat | Tea polyphenols greatly increased the antioxidant potential of chitosan coatings from 2.09% to 57.61% in terms of DPPH (1,1-diphenyl-2-picrylhydrazyl) value and from 2.63% to 38.85% in terms of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) value. Cinnamaldehyde greatly enhanced the antibacterial efficacy of chitosan coatings against E. coli and S. aureus. The inhibition zones rose from 3.03 ± 0.23 mm to 18.39 ± 1.22 mm and from 7.66 ± 1.61 mm to 15.70 ± 1.75 mm, respectively, under 20% oil content. Pickering emulsions with micro-scale particle size and the addition of cinnamaldehyde and tea polyphenol may alter droplet dispersion. The shelf life of fresh pork may increase by more than 4 days, further confirming the preservation effect of chitosan coatings. | [74] |
| Chitosan (Cht)-based nano-TiO2–nisin composite packaging film | Chilled pork | Nisin encouraged nano-TiO2 dispersion within chitosan, and the components of the film (nisin, nano-TiO2, and chitosan) were linked together by hydrogen bonding. The film tensile strength (TS) was evaluated for chitosan (31.63 ± 0.90 MPa) and nisin–chitosan (34.35 ± 1.01 MPa) versus films for nano-TiO2–chitosan (48.19 ± 1.13 MPa) and nano-TiO2–nisin–chitosan (49.38 ± 0.63 MPa). As a result, the nano-TiO2–chitosan film had a greater TS at break than the chitosan film. The mechanical strength of the composite film grew dramatically as the nano-TiO2 content in the film matrix increased, but the rate of water vapor permeability and light transmittance reduced, while the antibacterial activity steadily increased. According to the bacterial phase results, the active composite film considerably slowed down the growth of Acinetobacter in chilled pork. Furthermore, transcriptome analysis revealed that photo-catalytic nano-TiO2 can work in concert to enhance preservation by reducing spoilage-related gene expression, upregulating secondary metabolite synthesis in A. johnnii XBB1, and dramatically blocking cell autoregulation and membrane wall system repair. | [75] |
| Chitosan–gelatin (Cht/Gel)-based active packaging films containing S-chitin (Cht/Gel@S-chitin) | Chicken meat | The introduction of S-chitin improved the chitosan–gelatin film’s tensile strength (by 18.4%) and elongation at break (by 42.2%), while somewhat reducing the film’s transparency and boosting UV blocking with 98.7% UV-A and 100% UV-B light screening. The composite films demonstrated significant antioxidant activity. The films demonstrated efficacious antibacterial action, effectively impeding the development of L. monocytogenes and E. coli after 3 and 12 h of incubation, respectively. The chicken packaged with Cht/Gel@S-chitin films maintained its appearance, total viable colony count, thiobarbituric acid (TBA) reactive substance, and pH after 20 days of storage at 4 °C. | [76] |
| Pectin film incorporated with gallic acid (GA)-loaded ovalbumin (OVA)/chitosan NPs | Salmon fillets | The pectin film integrated with gallic acid (GA)-loaded OVA/chitosan NPs demonstrated good mechanical and light-barrier properties. The film’s tensile strength (TS) and elongation at break (EB) were 15.97 ± 1.55 MPa and 7.29 ± 0.42%, respectively, and its opacity value was 1.65 ± 0.06 UA/mm. The TVB-N (total volatile base nitrogen), pH, and microbial growth analysis results showed that the nanocomposite (NC) films effectively delayed the spoiling of salmon fillets over their 12-day refrigerated storage period. Salmon fillets treated with pectin film infused with GA-loaded OVA/chitosan NPs had a 3-day longer shelf life than the control group, and their sensory quality also improved. Additionally, the growth of amine-producing bacteria (E. coli and Morganella morganii) and the formation of biogenic amines (particularly histamine) in salmon fillets were postponed by the NC films. | [77] |
| Active films (CPB) were developed based on chitosan/polyvinyl alcohol (PVA) integrated with ginger essential oil (GEO) loaded with bacterial cellulose | Sea bass fillets | Following 12 days of storage, the sample enveloped in CPB0.8 film exhibited a reduced microbial load in contrast to the active film sample where ginger essential oil (GEO) was absent. In particular, CPB0.8 film demonstrated potent antioxidant and antibacterial properties that could hinder the spread of microorganisms and absorb exudates, preventing the oxidation of lipids and protein while being stored in the refrigerator. Thus, the depreciation of sea bass fillets during storage could be effectively delayed by CPB0.8 film. | [78] |
| Chitosan/polyvinyl alcohol (Cht/PVA) composite film integrated with sulfur-modified montmorillonite (S-MMT) | Chicken fillets | In comparison to chitosan/polyvinyl alcohol (Cht/PVA) films, Cht/PVA/S-MMT composite films revealed enhanced hydrophobicity, strength, and flexibility with better moisture barrier qualities. The Cht/PVA/S-MMT4% composite film exhibited a notable improvement over the neat Cht/PVA film in terms of tensile strength (~25.1%), elongation at break (~94.1%), and UV-blocking performance (98.2% UV-A and 100% UV-B). In addition, the Cht/PVA/S-MMT composite films revealed strong bactericidal and antioxidant properties (100% ABTS and 65.4% DPPH scavenging activity) against E. Coli and L. monocytogenes. For a 20-day storage period, the chicken in the composite film exhibited a notable decrease in total viable colonies, TBA reactive substances, pH, and physical appearance. | [79] |
| Coating with whey protein isolate (WPI), nano-chitosan, bacterial nanocellulose, and cinnamon essential oil | Chicken meat fillets | The antibacterial activity of the WPI-NCht (whey protein isolate, nano-chitosan, and bacterial nanocellulose) coating was greatly enhanced by the addition of CEO (cinnamon essential oil), which effectively inhibited the growth of yeast, mold, S. aureus, lactic acid bacteria, Enterobacteriaceae, Pseudomonas spp., psychrotrophic bacteria, and mesophilic bacteria. Additionally, the coating decreased the rate of decomposition by inhibiting the rise in thiobarbituric acid (TBA) level, peroxide value, and total volatile base nitrogen level. Sensory tests showed that coated fillets retained their outstanding flavor, color, and odor versus the control group. Relative to the uncoated (control) group, the chicken breast fillets’ shelf life was prolonged beyond 5 days via the application of WPI-NC+ 1.5% cinnamon EO coating. | [80] |
| Gelatin–chitosan–Cyclocarya paliurus flavonoid edible coating film (Gel–Cht–CPF) | Fresh beef | During storage, gelatin–chitosan–Cyclocarya paliurus (Gel–Cht–CPF) films effectively maintained the freshness of chilled beef, and the preservation impact grew as the CPF (C. paliurus flavonoid) concentration increased. Compared to the gelatin–chitosan (Gel–Cht) film treatment group, the addition of CPF slowed the rate at which the pH and weight of the beef increased, and to some extent also conserved the meat’s color. In addition, CPF-added films, versus the Gel–Cht film, suppressed the growth of microorganisms and subsequent increases in TBB (2-thiobarbitone) and TVB-N (total volatile base nitrogen) in beef samples, and also impeded the oxidation of proteins and lipids in beef. On day 14, the TVB-N was 15.517 mg/100 g, which was 36.75 mg/100 g less than the control group. In summary, the Gel-Cht-CPF film successfully increased the life span of beef, with an excellent preservation effect on chilled meat. | [40] |
| Ethyl cellulose–Gel–CM–Cht bilayer films doped with Euryale ferox (EF) seed shell polyphenol | Cooked beef and chicken | L. monocytogenes was effectively inhibited by Euryale ferox (EF) polyphenol, resulting in superior antibacterial and antioxidant properties. The presence of EP improved the barrier and mechanical properties of the bilayer film. Furthermore, the active bilayer film preparation demonstrated good protection against L. monocytogenes and delayed lipid oxidation in ready-for-consumption meat products with considerably delayed changes in color, moisture loss, pH, and texture in cooked beef/chicken, according to preservation tests. | [81] |
| Chitosan (Cht)/anthocyanin intelligent packaging film fortified by cellulose nanocrystal | Shrimp | Cellulose nanocrystals (CNCs) and natural blueberry anthocyanin (AN) were added to the film as property enhancers and color indicators, respectively. The film’s constituent parts were intricately connected by a profusion of ionic and hydrogen bonds. By varying the quantity of added CNCs, Cht-AN-CNCs 9% film was obtained, demonstrating exceptional antibacterial, antioxidant, barrier, and mechanical capabilities. The tensile strength of the Cht-AN-CNCs 9% film was significantly increased from 15 MPa to 35 MPa; whereas the water vapor permeability, oxygen permeability, and swelling properties decreased from 31.6 × 10−12 g/(m⋅s⋅Pa) to 1.6 × 10−12 g/(m⋅s⋅Pa), from 51.7 g/(m2d) to 12.2 g/(m2d), and from 159.2% to 92.0%, respectively. In addition, the intelligent film demonstrated good biodegradability in a natural setting. The composite film caused a discernible color change when it was used to preserve fresh shrimp, which closely matched changes in the TVB-N (total volatile base nitrogen) levels and pH levels of the shrimp meat. The findings demonstrate that the ecologically friendly intelligent packaging film can be used to visually monitor food freshness via color changes to enable the detection of deterioration by consumers. | [17] |
| Gelatin–chitosan-lactate–curcuma hydroethanolic extract-based antimicrobial films | Chicken meat | In comparison to gelatin film alone, its combination with chitosan–lactate produces an intricate network structure and improves hydrogen bonding in the film structure. This enhanced the optical, physicochemical, rheological, and active properties of the gelatin–chitosan–lactate (Gel/ChtL) films, but the chitosan–lactate-added films did not exhibit any antimicrobial activity. Moreover, the incorporation of curcuma hydroethanolic extract (CEE) into the Gel/ChtL-based film signified connections that occurred between the phenolic chemicals found in CEE and the biopolymer matrix. These interactions had a major impact on the antimicrobial, antioxidant, physical, optical, barrier, mechanical, and morphological properties of the gelatin–chitosan–lactate–CEE-based film. The water vapor transfer rate (WVTR) of the Gel1.5–chitosan–lactate (ChtL)1-based film reduced significantly (p < 0.05) from 7.95 ± 0.06 (Gel1.5/ChtL1) to 6.58 ± 0.13 g/h·m2 (Gel1.5/ChtL1/CEE200) with the incorporation of CEE. The tensile strength of the Gel/ChtL/CEE films increased from 20.60 ± 0.58 MPa (for Gel1.5/ChtL1) to 33.89 ± 3.29 MPa (for Gel1.5/ChtL1/CEE200). Also, the elastic modulus (EM) increased from 13.15 ± 1.75 MPa (for Gel1.5/ChtL1) to 17.87 ± 1.14 MPa (for Gel1.5/ChtL1/CEE200), while the flexibility simultaneously reduced from 42.64 ± 2.28% to 21.85 ± 2.04%. Simultaneously, minor modifications were noted in thermal and rheological characteristics. Furthermore, when compared to an unwrapped chicken kept at 4 °C for 10 days, the films successfully maintained the freshness of the chicken meat. | [82] |
| Polysaccharide film containing cinnamaldehyde–chitosan nano- particles (NPs) | Burgers prepared with minced meat | A variety of film characteristics were assessed, including transmittance in the 200–800 nm range and water in the chitosan film. Moreover, the findings showed that the development of Listeria had a significant impact on hamburgers without film. However, the CFU/g level was lower in burgers with the film. The entirety of cinnamaldehyde was released in vitro during the first 5 days, which was crucial for safeguarding the meat against potential bacterial growth. When the films’ antibacterial qualities were examined, the results showed that, over 20 days, L. monocitogenes’ total aerobic value (4.85 log CFU/g) decreased, the total coliform value of 1.26 log CFU/g decreased, and the potential growth value was less than 0.5 log10. | [83] |
| Chitosan–furcellaran–gelatin hydrolysate (GelH) coatings enhanced with bioactive peptides | Smoked pork ham and fresh pork loin | The incorporation of GelH into the biopolymer structure resulted in coatings containing peptides that had strong antibacterial and antioxidant capabilities, as demonstrated by tests conducted on two distinct preserved meat products: fresh pork loin and smoked pork ham. All studied food pathogens were suppressed in growth by the peptide coatings, except for A. flavus. A reduction in total viable counts by more than 3.5 log CFU/g occurred, effectively inhibiting their proliferation. The most promising coatings, containing RW4 and LL37 (1.25–2.5 μg/mL), were efficient in suppressing the total viable counts for fresh pork loin. | [84] |
| Chitosan coating combined with thermal treatment | Duck-leg meat | The findings demonstrated that the application of chitosan coating in conjunction with thermal treatment significantly enhanced the quality and extended the shelf life of braised duck meat by lowering carbonyl concentrations and restricting Enterobacteriaceae counts, total viable counts, and the occurrence of 4 primary spoilage organisms (Pseudomonas, Acinetobacter, Weissella, and Brochothrix). Furthermore, an examination of the volatile taste compound composition showed that the mixed treatment greatly increased the primary contributors to the main aroma. | [85] |
| Carboxymethyl (CM) chitosan/zinc alginate (CMCht/Zn-Alg) composite film | Pork | The incorporation of Zn ions into the composite structure conferred exceptional antibacterial and water resistance properties to the film, as evidenced by the water vapor permeability (WVP) and antibacterial tests conducted on E. coli and S. aureus. In addition, the freshly prepared composite film displayed an improved mechanical property owing to chelation bond formation between the carboxyl groups and Zn ions. The solubility of the composite film CMCht/SA (Na-Alg) was almost 100%, and its thickness was roughly 58 μm. Following Zn-ion post-treatment, the thickness of the CMCht/SA-Zn1, CMCht/SA-Zn2, and CMCht/SA-Zn3 composite films increased to 112 μm, 118 μm, and 121 μm, respectively. Furthermore, the CMCht/SA WVTR was very high, ca. 1.91 × 10−11 g⋅s−1⋅m−1⋅Pa−1. After Zn-ion post-treatment, the WVP of the composite films CMCht/SA-Zn1, CMCht/SA-Zn2, and CMCht/SA-Zn3 was reduced to 1.27 × 10−11 g⋅s−1⋅m−1⋅Pa−1, 1.19 × 10−11 g⋅s−1⋅m−1⋅Pa−1, and 1.17 × 10−11 g⋅s−1⋅m−1⋅Pa−1, respectively. Additionally, the chilled meat preservation test showed that the composite film may considerably increase the lifespan of pork by 5 days, demonstrating its exceptional ability for effective preservation. | [86] |
| Starch-Based Coating/Film | Coated Meat Sample | Key Findings | Ref. |
|---|---|---|---|
| Carbon dots (CDs), prepared with carrot as a precursor, were introduced into corn starch (CS) to construct a bio-based CS-CD composite film | Deep-fried meatballs | The thermal stability and elasticity of the composite film were enhanced by the high carbon content and dense surface produced by the uniform doping of carbon dots (CDs). The films with a low concentration of CDs exhibited superior solubility, water vapor permeability (WVP), tensile strength (TS), elongation at break (EB), and heat resistance in comparison to the corn starch (CS) films. Furthermore, the CS-CD composite films exhibited strong techno-functional characteristics like antibacterial and antioxidant activities. These films successfully prevented the growth of microbes when used to store and preserve fried meatballs, preserving the meatballs’ flavor, texture, and appearance. | [102] |
| Porous starch embedded with anthocyanins–carboxymethyl cellulose (PS-ACMC) coating | Shrimp | The porous citrate–starch displayed improved mechanical and water properties with an esterification degree of 32.0% ± 0.8% and substitution degree of 0.44% ± 0.0%. The composite was stabilized and given structural support by the porous starch matrix, which also gave it exceptional mechanical resistance—up to 5-fold that of typical expanded polystyrene foam. Carboxymethyl cellulose (CMC) functioned as a host complex, providing efficient colorimetric pH-sensing characteristics and guaranteeing a sustained reaction by successfully anchoring the active natural anthocyanins. These biopolymers exhibit perfect synergy, with no gaps or cavitation between the two phases, which would have compromised some of the biocomposite’s functional characteristics. When shrimp were utilized as test items, PS-ACMC composites showed a rapid and efficient color reaction, changing from violet to greenish blue, enabling a visual and instantaneous freshness evaluation. | [103] |
| Phloroglucinol (Phg)-loaded pea starch coating | Atlantic salmon (Salmo salar) | The properties of the control (without phloroglucinol—Phg) and Phg-loaded films varied with different Phg levels (2%, 4%, and 8%), denoted as Phg2%, Phg4%, and Phg8%, respectively. For instance, the water vapor permeability (WVP) of the control films was found to be 6.58 ± 1.49 × 10−10 g·m/m2·s·Pa, compared to 7.04 × 10−10 g·m/m2·s·Pa, 6.23 × 10−10 g·m/m2·s·Pa, and 6.30 × 10−10 g·m/m2·s·Pa reported for Phg2%, Phg4%, and Phg8%, respectively. The Young’s modulus was found to decrease from 474.53 MPa (control) to 326.47 MPa (Phg2%), 189.343 MPa (Phg4%), and 103.92 MPa (Ph8%). The tensile strength also decreased from 12.63 MPa (control) to 9.67 MPa (Phg2%), 8.64 MPa (Phg4%), and 7.62 MPa (Phg8%). Phg caused a notable, dose-dependent delay in the proteolysis of meat. The slower rise in pH, trimethylamine, and flesh softening during storage (4 °C for up to 17 days) served as supporting evidence. Furthermore, the development of oxidation indicators, such as sulfur-derived volatiles and methyl and ethyl ester volatiles, was also suppressed or delayed (in a dose-dependent manner) by Phg (solid-phase microextraction—SPME; gas chromatography/mass spectrometry—GC/MS). On the other hand, these molecules were marginally more abundant at the maximum Phg concentration (Phg 8%). | [104] |
| Sodium alginate, taro starch and lactic acid-based biodegradable films | Spanish chorizo-type meat product | As the concentration of lactic acid (LA) increased, water vapor permeability (WVP), solubility, humidity, and thickness increased, whereas mechanical properties reduced. The optimal composition was determined as 1.25% v/v LA, 0.75% w/v glycerol, and 1.04% w/v SA, with a WVP of 1.05 g⋅mm/kPa⋅h⋅m2 and a thickness of 0.14 mm. The optimized biodegradable film (OBF) evidenced in vitro antibacterial performance against L. monocytogenes, Salmonella, and E. coli. Spanish chorizo-type meat samples packaged with OBF (T3), control biodegradable film, CBF (T2), and samples without biodegradable film (T1) showed significant physicochemical changes (humidity, weight loss, pH, acidity, and hardness) during the first 9 days. | [105] |
| Starch-based functional film embedded with polyphenolic extract of waste petioles of betel leaf (BLP). | Chicken meat | Loading the extract increased the intermolecular interactions between potato St, guar gum, and the extract, which in turn improved flexibility, thickness, water solubility, DPPH radical-scavenging activity, and UV light protection ability. The integration of betel leaf petiole (BLPE) extract increased the water vapor permeability of the films from 1.89 ± 0.16 to 2.84 ± 0.18 (×10−7 g·mm/m2·Pa·s). With the incorporation of 8% betel leaf petiole extract, the tensile strength and elastic modulus of the potato starch–guar gum–BLPE (PSGG-BLPE) composite films decreased from 8.29 ± 0.17 MPa to 1.02 ± 0.04 MPa and 2.11 ± 0.06 GPa to 0.48 ± 0.09 GPa, respectively. The produced film exhibited optimum water and mechanical barrier qualities. The extract-embedded film preserved the quality of chicken flesh ca. 4 °C for up to 12 days throughout the shelf-life investigation. The extract-blended films’ biodegradation time was significantly shortened from 28 days for the original film to 14 days for the blended film. This suggests that these films are a good substitute for non-biodegradable film when it comes to preserving raw meat. | [46] |
| Pullulan (P)/tapioca starch (TSt)-based antibacterial films incorporated with Litsea cubeba essential oil | Beef meat | The film showed important barrier qualities by decreasing water vapor and oxygen permeability (OP; by 38.19% and 32.14%, respectively) and increasing antioxidant activity (by 21.19%). The main characteristic of this packaging material is the controlled and gradual release of L. cubeba essential oil (LC-EO), which prolongs shelf life and helps maintain the quality of food products. On the other hand, the tensile strength and elongation at break decreased (from 28.94 MPa to 11.29 MPa, and from 15.36% to 12.19%, respectively) when LC-EO was used. Most remarkably, the film showed a strong antibacterial effect (with substantial inhibition diameters of 17.32 mm and 18.59 mm, respectively) against foodborne pathogens, namely E. coli and S. aureus. Bacterial growth, pH, texture, color, and TBARS (thiobarbituric acid-reactive substances) values all showed that the film successfully maintained the quality of beef meat at 4 °C, inhibiting deterioration and prolonging the term of storage. | [106] |
| Double-layer (FeO and ZnO) nano-particle-infused starch-based thermoplastic food packaging system | Mutton and chicken meat | FeO and ZnO NP-infused bio-thermoplastic films demonstrated strong oxygen-scavenging and antibacterial activity, respectively. Consequently, a double-layer nano-biothermoplastic (NBP) packaging technique for food preservation was created by combining the two films. Thus, the tensile strength, Young’s modulus, swelling index, and water vapor permeability of the tamarind seed St films were found to be 10.22 MPa, 16.43 MPa, 62.41%, and 0.61 g s−1m−1Pa−1, respectively. The amorphous nature of starch (St) and the film’s swelling index were found to have an impact on the distribution and diffusion of NPs in the St-based films, respectively. In the NBP films, the crystalline features of the NPs were obscured by the amorphous nature of St. Hence, it was discovered that the color, chemical, and microbial properties of mutton and chicken meat kept at 4 °C were influenced by the dissemination of NPs from the NBP packaging system. | [107] |
| Potato starch (PS)/watermelon peel pectin (Wpp) composite film with Lycium barbar-um microencapsulated leaf flavonoids (MLFs) and nano-TiO2 (Pst/Wpp/MLF/TiO2) | Tan mutton | The concentration of nano-TiO2 influenced the composite film’s water vapor permeability. When 0.03% TiO2 was employed, the water vapor permeability was the lowest, at roughly 2.06 × 10−9 (g·m/m2⋅Pa⋅s). In addition, the combined effects of MLFs and nano-TiO2 enhanced the composite film’s thermal stability, UV-blocking capabilities, and mechanical strength whilst enhancing its antioxidant and antibacterial properties. Additionally, there was a regulated and continuous release of flavonoids from the composite film onto the meat surface when tan mutton was coated with the composite film containing MLFs and nano-TiO2. The results showed that all wrapped treatments, particularly Pst/Wpp/MLF/TiO2, significantly reduced the increments of TVC (total viable count), TBARS (thiobarbituric acid-reactive substances), and pH values in tan mutton. The color and texture were preserved throughout the entire storage period at 4 °C. | [45] |
| Antimicrobial film based on potato starch (PS) and polyvinyl alcohol (PVA) incorporated with clove essential oil (CLO) Pickering emulsion | Pork meat | The films’ crystallinity was established based on hydrogen bonding and electrostatic interactions reducing the elongation (375.3–91.6%) and tensile strength (22.4–6.80 MPa). The color difference, opacity, water vapor permeability, water absorption, and moisture content of the antimicrobial films were 5.06–7.15, 3.32–8.95 A/mm, 1.70–2.20 × 10−12 g·cm/cm2⋅s⋅Pa, 38.6–65.9%, and 8.80–10.5%, respectively. Moreover, the antimicrobial film exhibited strong antibacterial qualities, preventing the development and reproduction of S. aureus and E. coli, which have consistent and stable structures. The film was very transparent with minimal permeability, and barely any color difference. Raising the PECEO (Pickering emulsion based on clove essential oil) concentration further enhanced the antibacterial and antioxidant qualities of the composite film. Fresh pork was kept for an additional 6–10 days by the application of the antimicrobial film, indicating the film’s potential for pork preservation. | [37] |
| Acylated pectin–gelatin-based films incorporated with alkylated starch crystals (AP/G-ASC) | Golden pomfret (Trachinotus blochii) fillets | It was shown that the AP/G-ASC-3% composite film had significantly better mechanical qualities, with a surface that was compact, dense, and uncrackable. The evaluated composite films showed a noticeable improvement in barrier efficiency, and the AP/G/ASC-10% composite film showed a considerable increase in contact angle to 94.02°. In addition, the composite film solutions demonstrated potent antibacterial and antioxidation properties against S. aureus and E. coli. Furthermore, the results from the preservation experiments showed that the composite coatings—particularly AP/G-ASC-3%—could successfully extend the lifespan of golden pomfret (T. blochii) fillets during storage at 4 °C, giving the fish better texture and better antioxidant qualities. | [108] |
| Ginger starch-based edible films incorporated with coconut shell liquid smoke (CSLS) by ultrasound treatment | Ground beef | The samples coated with ginger starch films with the incorporation of 0, 5, 10, or 15% CSLS (CF, LSF1, LSF2, or LSF3, respectively) demonstrated varying tensile strength (14.28 MPa, 15.35 MPa, 15.81 MPa, and 15.74 MPa, respectively), elongation at break (27.55%, 33.34%, 35.92%, and 38.95%, respectively), and water vapor permeability (1.54, 1.38, 1.35, 1.33 (g·mm/m2h·kPa), respectively). Antibacterial, thermal, mechanical, and barrier properties were all improved in the CSLS–ginger starch films treated with ultrasound. Upon ultrasonic treatment, the antibacterial efficacy of CSLS against B. cereus, S. Enteritidis, L. monocytogenes, E. coli O157:H7, S. aureus, and E. coli rose dramatically. Over the course of a 12-day storage period, the E. Coli O157:H7 populations in ground beef were lowered by 1.33 log CFU/g in the films comprising 15% CSLS. During the period of refrigeration, the ground beef samples’ lipid oxidation was successfully suppressed by the CSLS–starch films. These findings suggest that CSLS–ginger St film treated with ultrasound has potential use as a novel antimicrobial active food packaging material. | [23] |
| Cassava starch/sodium carboxymethyl cellulose (CMC) edible film with apple polyphenols | Chicken breast | Adding apple polyphenol enhances the film’s flexibility initially, then causes it to decline, while the barrier ability increases dramatically, and the tensile strength marginally drops. When the concentration of AP was 70 mg/mL, the film’s tensile strength (TS) reduced from 5.61 ± 0.45 to 3.36 ± 0.19, its water vapor transmittance dropped from 7.17 ± 0.17 to 4.97 ± 0.07, and its peroxide value dropped from 1.896 ± 0.04 to 0.53 ± 0.04. It was discovered that hydrogen bonds developed between apple polyphenol, cassava starch, and carboxymethyl cellulose (CMC), and these interactions displayed high compatibility, improving the crystallinity of the cassava starch/CMC/AP-4 film microstructure, with the film becoming more compact with less roughness. The rise in blocking ability is exactly proportional to the increase in compactness. Simultaneously, the rise in crystallinity is credited with improving thermal stability. | [44] |
| Starch–polyester (PLA:PHBV blend) bilayer films incorporating phenolic acids (ferulic, p-coumaric, and protocatechuic acid) into the polyester layer | Pork meat | The tensile strength (TS), elongation at break (EB), oxygen transmission rate (OTR), oxygen permeability (OP), water transmission rate, and water vapor permeability (WVP) values of the bilayer films coated with ferulic acid were 9.0 MPa, 2.0%, 0.347 cm3/dm2, 0.76 cm3·m−1·s−1·Pa−1, 70 g/d·m2, and 0.4 g·mm·kPa−1·h−1·m−2; with p-coumaric acid were 8.0 MPa, 3.0%, 0.325 cm3/dm2, 0.73 cm3·m−1·s−1·Pa−1, 58 g/d·m2, and 0.41 g·mm·kPa−1·h−1·m−2; and with protocatechuic acid were 8.0 MPa, 2.0%, 0.280 cm3/dm2, 0.62 cm3·m−1·s−1·Pa−1, 86 g/d·m2, and 0.54 g·mm·kPa−1·h−1·m−2, respectively. Incorporating phenolic acids decreased the stiffness and break-resistant properties of the bilayers while increasing their capacity to withstand water vapor and oxygen, primarily in the case of proto-catechuic acid. The bilayer films’ antioxidant capacity was greatly increased by phenolic acids, which also decreased the amount of packaged meat that oxidized during storage. The meat microbial counts were similarly decreased by phenolic acid-loaded bilayers, primarily in terms of lactic acid bacteria. Throughout storage, these impacts were favorable for the sample pH and for color parameter development. Active starch–polyester bilayer films show tremendous promise for enhancing the quality preservation and shelf life of pork meat. | [109] |
| Pregelatinized high-dissolution (HD) and low-dissolution (LD) cassava starch with different water solubilities were incorporated with papain | Chilled Australian grass-fed lean beef (Bos taurus) sirloin steaks | The incorporation of papain altered the shape, permeability, mechanical properties, and physical characteristics of starch sheets. Papain-containing edible films, especially LD films, showed decreased water dissolution and disintegration in the water. The mechanical properties of the HD and LD films varied depending on the increase in papain concentration. In addition to producing rougher surface microstructures from enhanced starch crystallization in HD films, papain significantly decreased oxygen and water vapor permeability through polymer matrices by limiting diffusivity. Consequently, the addition of 5–15% papain to edible starch-based films resulted in functional packaging that altered the texture of the meat. | [43] |
| Starch-based film was developed by incorporating carbon dots (CDs) from soy protein isolate (SPI) and anthocyanin extracted from clitoria ternatea flower extract (CTE) | Pork | For starch/carbon dot/clitoria ternatea (CT) flower extract (SED), SED1 and SED2 were synthesized with carbon dot concentrations of 9% (w/v) and 17% (w/v), and it was revealed that the moisture content, swelling degree, and solubility were 17.30%, 73.20, and 28.21% for SED1 and 12.40%, 62.60, and 27.15%, for SED2, respectively. This contrasted films without carbon dots (20.20%, 76.69, and 29.12%) and films without carbon dots and CT flower extract (18.45%, 51.75, and 30.78%). The starch/carbon dot/CT flower extract films (SED) demonstrated the best mechanical, barrier, thermal, and antioxidant qualities, owing to the complementary effects of carbon dots and CTE. In addition, the SED films showed color changes at varying pH levels because CTE contains anthocyanin. Therefore, the SED film can be employed as a low-cost visual indicator to check the freshness of packed pork samples. The study demonstrated how the color changed from purple to green as the amount of storage time grew. The films might be used to keep an eye on the freshness of food products, such as pork. | [42] |
| Corn starch coatings incorporated with Zataria multiflora essential oil (ZEO) and cinnamaldehyde (CIN) in conventional nanoemulsion (NZEO) and fortified nanoemulsion (NZEOC) forms | Chicken meat | Over 20 days of storage at 4 ± 1 °C, starch coatings containing Zataria multiflora essential oil (ZEO) nanoemulsions showed stronger antibacterial effects on certain spoilage microorganisms and pathogenic bacteria of chicken flesh compared to coatings containing traditional forms of ZEO. In addition, the best antibacterial properties were found in chicken meat coated with a starch solution containing a nanoemulsion of ZEO fortified with cinnamon (NZEOC). At the end of storage, NZEOC treatment exhibited elevated antibacterial qualities, according to the following outcomes: 7.96 log10 CFU/g for total viable count, 7.29 log10 CFU/g for psychrotrophic count, 6.51 log10 CFU/g for lactic acid bacteria, 6.98 log10 CFU/g for Enterobacteriaceae count, 5.16 log10 CFU/g for mold and yeast count, and 6.51 log10 CFU/g for inoculated L. monocytogenes. However, when cinnamaldehyde (CIN) was introduced to ZEO during the nanoemulsion production process (NZEOC), the antimicrobial effects of coating solutions were enhanced in comparison to when NZEO and CIN were added separately to the St solution (NZEO + CIN). | [110] |
| Starch, red cabbage extract (RCE), glycerol, sweet whey (SW), and water. | Ground beef | Two independent variables, red cabbage extract (RCE) and sweet whey (SW), were optimized, and it was observed that T2, T7, and T10 films composed of (64.18% RCE and 4.36% SW), (50% RCE and 0.0% SW), and (50% RCE and 15% SW), respectively, presented good mechanical properties, high antioxidant capacity (due to the presence of phenolic compounds and anthocyanins), low solubility, and low water vapor permeability. The moisture content, water vapor permeability, solubility, tensile strength, elongation at break, and Young’s modulus of the T2, T7, and T10 films were (22.95%, 0.34 g·mm·h−1·m−2·kPa−1, 28.23%, 3.14 MPa, 28.73%, 10.87 MPa), (14.20%, 0.41 g·mm·h−1·m−2·kPa−1, 26.14%, 6.28 MPa, 54.44%, 12.15 MPa), and (17.35%, 0.35 g·mm·h−1·m−2·kPa−1, 25.43%, 4.88 MPa, 22.06%, 22.42 MPa), respectively. Ground beef packaged with the T2 film showed the least change in quality parameters. | [111] |
| Chitosan–Starch Coating/Films | Coated Meat Sample | Key Findings | Ref. |
|---|---|---|---|
| Antibacterial packing film based on amylose starch and 2-hydroxypropyl-trimethylammonium chloride chitosan (HTCCht) | Meat | The mechanical characteristics of the amylose films were enhanced by a composite of HTCCht (2-hydroxypropyl-trimethylammonium chloride chitosan) and amylose starch. The antibacterial efficacy of the HTCCht/amylose films was HTCCht dose-dependent and showed good antibacterial activity against both S. aureus and E. coli. The ideal mass ratio of HTCCht to amylose was found to be 1:4, based on the antibacterial and mechanical properties and quantity of HTCCht required. In contrast to amylose films, the ideal HTCCht/amylose films exhibited an elongation at break and tensile strength of 53.86% (a 109.59% increase) and 16.13 MPa (a 266.65% increase), respectively. It was found that the HTCCht/amylose films displayed bacteriostatic activity, comparatively low cytotoxicity, reduced UV transmittance, and the capacity to improve the durability of fresh meat. | [31] |
| Nitrogen, phosphorus-doped green-tea-derived carbon nanodots (CNDs) incorporated with chitosan–starch (Cht/St) | Pork meat | With varying concentrations of carbon nanodots (CNDs), various chitosan–starch–CND films were produced at 0%, 1%, 2%, and 3% to yield water vapor permeability values of 1.15, 1.28, 1.31, and 1.35 (×10−9 g·m/m2·Pa·s), respectively. The addition of CNDs (nitrogen, phosphorus-doped, green-tea-derived carbon dots) increased the blockage of UV light (93.1% of UV-A and approximately 99.7% of UV-B) without considerably altering the water vapor permeability (WVP) or transparency of the films. Moreover, the addition of CNDs to the chitosan–starch films increased the antioxidant activity (71.4% for DPPH and 98.0% for ABTS) and demonstrated high antibacterial activity against S. aureus, E. coli, and L. monocytogenes. It was established that wrapping the meat in the resulting film at 20 °C reduces bacterial growth (below 2.5 Log CFU/g after 48 h) with no appreciable distortion in the color of the wrapped meat. | [52] |
| Chitosan–starch-based colorimetric film synthesized based on potato starch (PS), chitosan (Cht), and Lonicera caerulea L. anthocyanins (LCA) | Fresh shrimp | The colorimetric film exhibiting the lowest water solubility (33.11%) and the highest tensile strength (6.43 MPa) showed a sub-freshness indication effect when the pH of the film-forming solution was set to 2.5. Hydrogen bonds favored the binding of chitosan to the surface of the PS in the film matrix, where LCA was firmly embedded. The shrimp freshness evaluations showed that when the storage time was extended to 4 °C, the PS-CH-LCA pH 2.5 film showed noticeable color changes (red → grey-pink/grey → grey-green). This film was highly correlated with shrimp degeneration indices (pH, TVC, TBARS, and TVB-N), enabling the authors to determine if the shrimp were fresh, sub-fresh, or spoiled. | [129] |
| Chitosan–coix seed starch films incorporated with nano zinc oxide and Artemisia annua essential oil (AAEO) | Pork | The addition of ZnO significantly improved the films’ antibacterial, barrier, hydrophobic, and mechanical activity, but did not affect their antioxidant capacity. Increasing ZnO from 0% to 5% resulted in a significant drop in water vapor permeability (WVP) and oxygen permeability (OP), which rapidly reduced from 385 g/m2⋅day and 1950 cm3/m2⋅day to 218 g/m2⋅day and 1624 cm3/m2⋅day, respectively. A further increase in the ZnO content from 5% to 7% also reduced the WVP and OP of the film by 4.58% and 2.77%, respectively. The incorporation of AAEO (A. annua essential oil) enhanced the films’ flexibility, hydrophobicity, barrier qualities, antibacterial activity, and antioxidant activity. When Artemisia annua essential oil was increased from 0 to 8%, the OP and WVP of the films significantly reduced from 1624 cm3/m2⋅day and 218 g/m2⋅day to 1388 cm3/m2⋅day and 125 g/m2⋅day, respectively. The structural analysis revealed that ZnO and AAEO were evenly distributed and well incorporated into the film matrix with good inter-compatibility among all the constituents. When the ZnO and AAEO contents were 5% and 8%, respectively, the film’s physio-chemical and biological qualities attained an optimum state. The results of the pork preservation tests indicated that the pork samples coated with the 5% ZnO-8% AAEO film were able to effectively suppress (p < 0.05) the growth of microorganisms and lipid oxidation, hence extending the pork’s shelf life. | [36] |
| Chitosan–starch aldehyde–catechin conjugate (SACC) composite | Pork loins | The chitosan and starch aldehyde–catechin conjugate (SACC) exhibited synergistic antimicrobial and antioxidant properties. On day 14, the chitosan–SACC-coated pork loins displayed lower shear force (27.40 N), protein oxidation level (0.047 mmol free thiol group g−1), lipid oxidation level (0.47 mg malondialdehyde kg−1), total volatile base nitrogen content (130.2 mg kg−1), total viable count (7.11 log CFU g−1), pH value (5.99), and weight loss (7.16%)) compared to the uncoated and chitosan-coated pork loins. In the meantime, during the chilled storage period, the pork loins’ sensory, microstructure, and color properties were successfully preserved by the chitosan–SACC composite coating. The application of a chitosan–SACC composite coating increased the shelf life of pork loins from 8 days (uncoated) to 14 days. | [33] |
| Chitosan–acetylated starch composite films incorporated with essential oils | Beef | The addition of essential oils to the films remarkably enhanced protection against oxygen, water vapor, and light; this enhancement may have been brought about by greater bonding connections with the chitosan–acetylated starch (Cht/ACS) matrix. The addition of 2.50% essential oil reduced the films’ moisture content and water solubility by 22.7% and 21.6%, respectively. The addition of 2.00% essential oil reduced the films’ elongation at break and tensile strength by 40.4% and 25.6%, respectively. The film opacity increased by 795.8% when the essential oil concentration reached 2.50%, while the peroxide value and water vapor permeability fell by 52.6%, and 35.5%, respectively. In a beef model, the films exhibited stronger antimicrobial properties against a Gram-negative bacterial pathogen (E. coli O157:H7) and spoilage bacteria when higher concentrations of essential oils were incorporated. This was because the films had higher concentrations of antimicrobial components and an enhanced oxygen barrier. | [48] |
| Plasma-treated starch–chitosan (St/Cht) composite film | Chicken breast fillet | The mechanical properties of the starch–chitosan composite film were significantly enhanced by low-pressure argon and air plasma treatment. The composite film treated with plasma exhibited an increase in tensile strength from 10.59 to 22.09 MPa after 12 min, resulting in the creation of a steady cross-linked network on the biopolymer’s surface, which made argon plasma more effective. Moreover, after being exposed to air and argon plasma for 12 min each, the starch–polyester oxygen transmission rate (OTR) values for the film were found to be 0.024 ± 0.015 and 0.015 ± 0.013 cm3 mm m−2 day−1, respectively, in contrast to the untreated film, which had an OTR of 0.062 ± 0.02 cm3 mm m−2 day−1. The ductility of the film increased due to the functionalization and degradation of the biopolymers caused by air plasma. After plasma treatment, there was an increase in solubility and hydrophilicity but no discernible change in water vapor transmission rate (VTR). The film’s oxygen permeability increased following both plasma treatments. However, there was no discernible variation between the total viable count in the fillets packaged for treated and untreated films. Consequently, the chicken breast fillets’ shelf life was not extended by cold plasma therapy-treated films. | [130] |
| Chitosan/curcumin nanoparticle-based zein and potato starch composite film (CCN/zein/PS films) | Schizothorax prenati fillet | Relative to the curcumin-loaded chitosan nanoparticle (CCN)-free film, the water vapor permeability (WVP) and oxygen permeability (OP) reduced with higher CCN volume. The zein/CCN film (with 5/5 mass ratio) had the lowest WVP (1.39 ± 0.03 10−4 g·mm)/(h·m2·kPa), while the zein/CCN film (with 3/7 mass ratio) had the lowest OP (2.10 ± 0.15 10−14 cm3/m s Pa). Meanwhile, the composite films (CCN/zein/PS films) revealed high oxidation resistance, barrier performance, mechanical properties, and broad relative release efficiency, especially for the effective relative release of curcumin (CUR). As an indication of CCN’s antioxidant and antimicrobial properties, the CCN/zein/potato starch composite films were able to preserve the treated fish muscle’s sensorial, chemical, and microbial quality. The shelf life of Schizothorax prenati fillets was extended by up to 15 days by the CCN/zein/PS composite film, which also held off physicochemical changes in the meat. | [128] |
| Chitosan–starch film containing pomegranate peel extract and Thymus kotschyanus essential oil | Fresh beef (Quadriceps femoris muscle) | In comparison to chitosan–starch (Cht-St) films, films containing Thymus kotschyanus essential oil (TKEO) and pomegranate peel extract (PPE) had lower elongation values and tensile strength. The chitosan–starch films had a water vapor permeability (WVP) of 15.01 g/m2/h. The water solubility for chitosan–starch film was 12.54% compared to 23.77% and 23.29 for the Cht-St-PPE (1%) and TKEO (2%) films, respectively. The average elongation value of 16.06% for the chitosan–starch group decreased to 10.33% and 11.18% in the Cht-St-PPE (1%) and Cht-St-PPE (1%) groups with TKEO (2%), respectively. The results showed that composite films comprising TKEO and PPE have anti-listeria effects. The utilization of TKEO and PPE effectively minimizes lipid oxidation and bacterial counts. The amounts of TKEO or PPE additives directly influenced the impact of chitosan–starch films on chilled meat. All additives showed promising outcomes in terms of antibacterial and antioxidant activity, including sensory characteristics (e.g., color, odor, and broad acceptability). Nevertheless, the Cht-St-PPE groups (1%) with TKEO (2%) produced the most favorable outcomes overall, indicating that a mixture of with TKEO and PPE can be used to extend the shelf life of meat. | [35] |
| Cassava starch–chitosan incorporated with red dragon fruit peel anthocyanin extract | White leg shrimp (Litopen-aeus vannamei) | Antioxidant activity was boosted to 94.44% when red dragon fruit peel anthocyanin was added, according to the results. Its flexibility was also increased, as evidenced by the lowest Young’s modulus (0.14 ± 0.01 MPa), smallest tensile strength (3.89 ± 0.15 MPa), and maximum elongation at break (27.62 ± 0.57%). The indicator film’s sensitivity to pH was demonstrated by a color change from red to yellow as pH rose. The film’s color was also altered when it was employed to evaluate packaged shrimp’s freshness at room temperature and in a chiller. | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyekunle, D.T.; Nia, M.H.; Wilson, L.D. Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review. J. Compos. Sci. 2024, 8, 302. https://doi.org/10.3390/jcs8080302
Oyekunle DT, Nia MH, Wilson LD. Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review. Journal of Composites Science. 2024; 8(8):302. https://doi.org/10.3390/jcs8080302
Chicago/Turabian StyleOyekunle, Daniel T., Marzieh Heidari Nia, and Lee D. Wilson. 2024. "Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review" Journal of Composites Science 8, no. 8: 302. https://doi.org/10.3390/jcs8080302
APA StyleOyekunle, D. T., Nia, M. H., & Wilson, L. D. (2024). Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review. Journal of Composites Science, 8(8), 302. https://doi.org/10.3390/jcs8080302








