Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polypropylene/TiO2 Nonwoven Fiber
2.3. Characterization
2.4. Measurements of UV-Protection Ability
- (a)
- The wavelength is corrected using a holmium oxide filter, which is a filter for ultraviolet wavelengths, as an absorption band filter.
- (b)
- The transmission linearity is corrected with a calibrated mesh with a transmission close to 6.7%, 3.3%, and 2.0%.
3. Results and Discussion
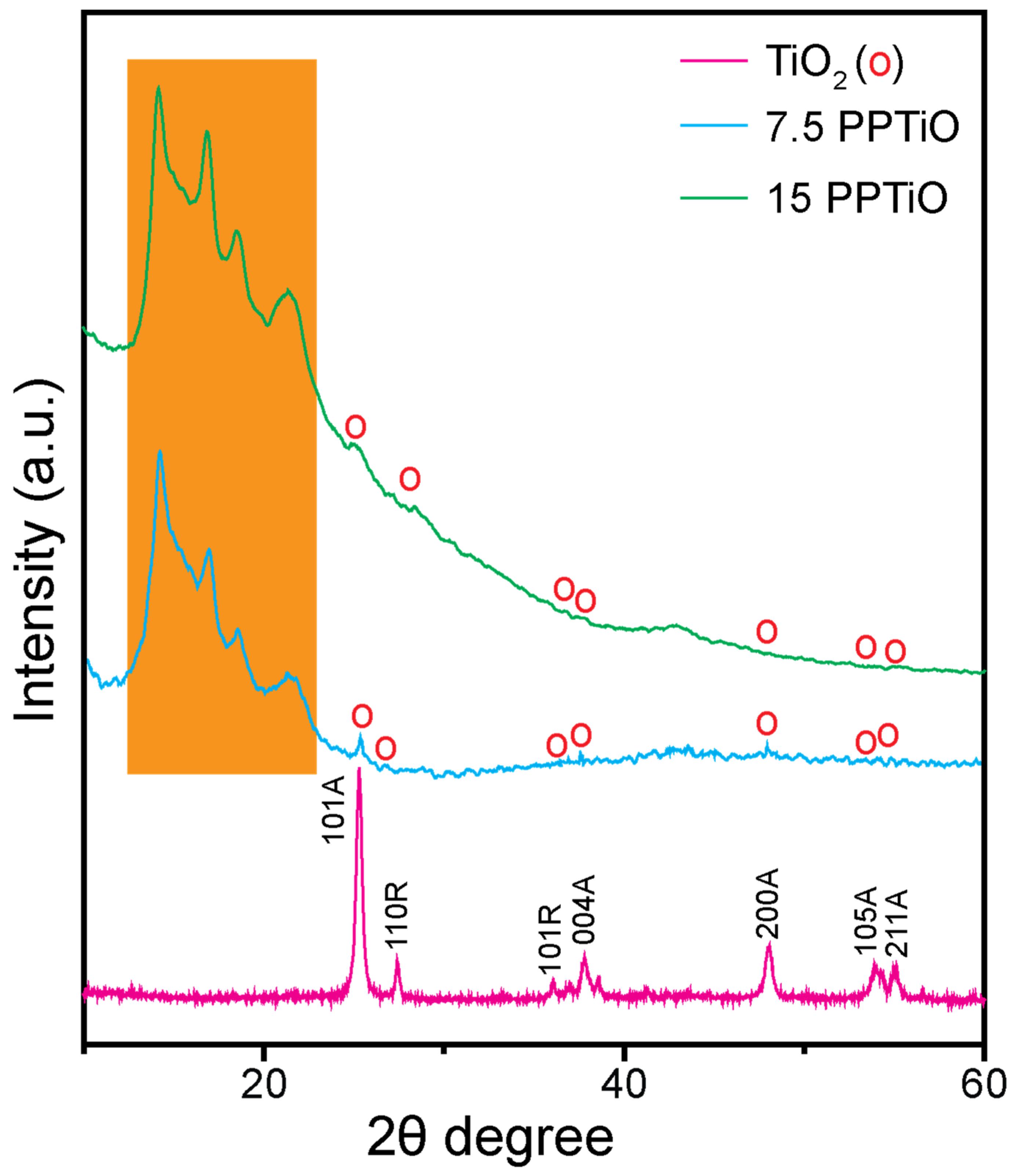
3.1. Structural Properties
3.2. Thermal Properties
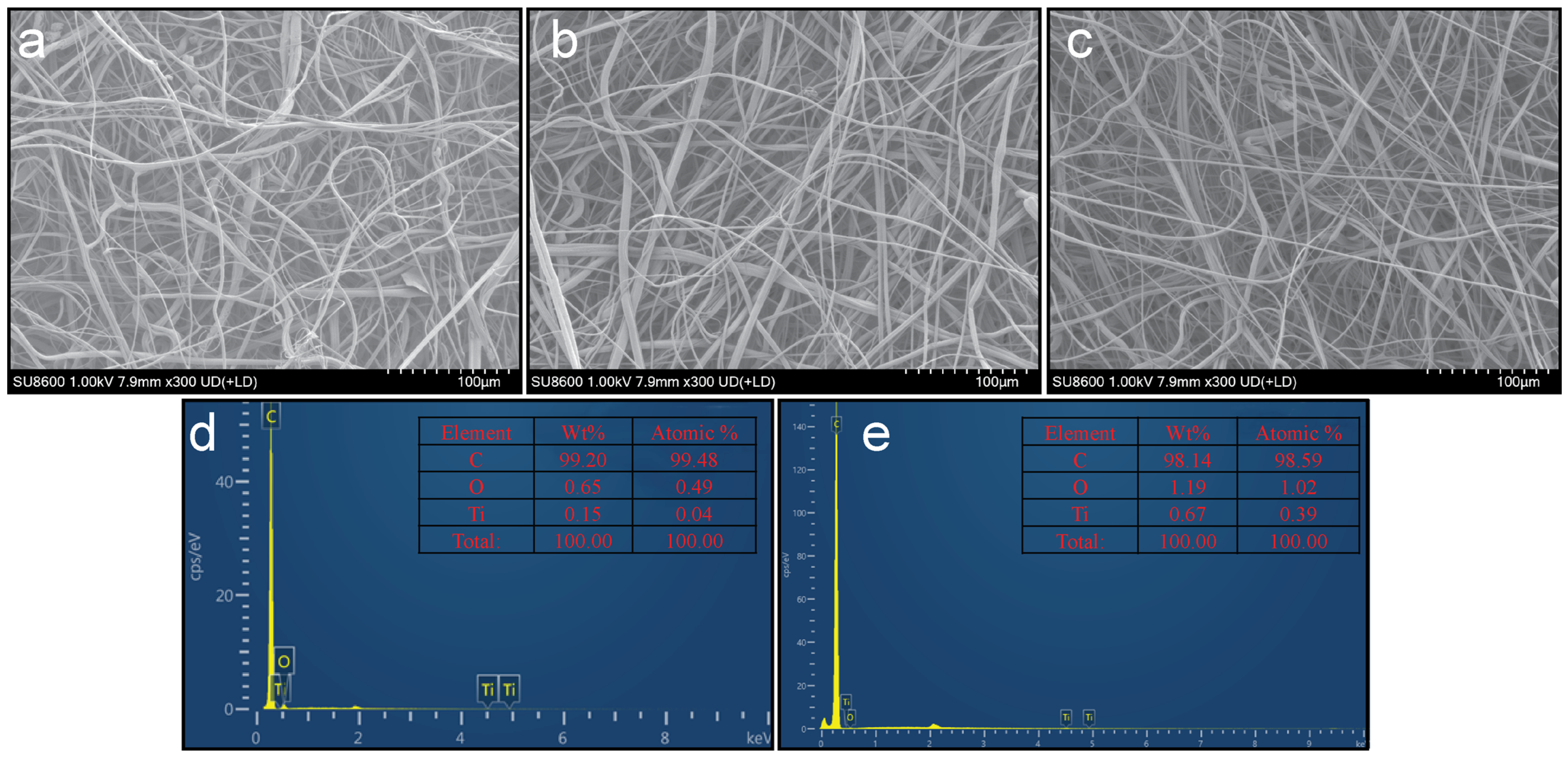
3.3. Morphological Properties
3.4. Wettability Measurement
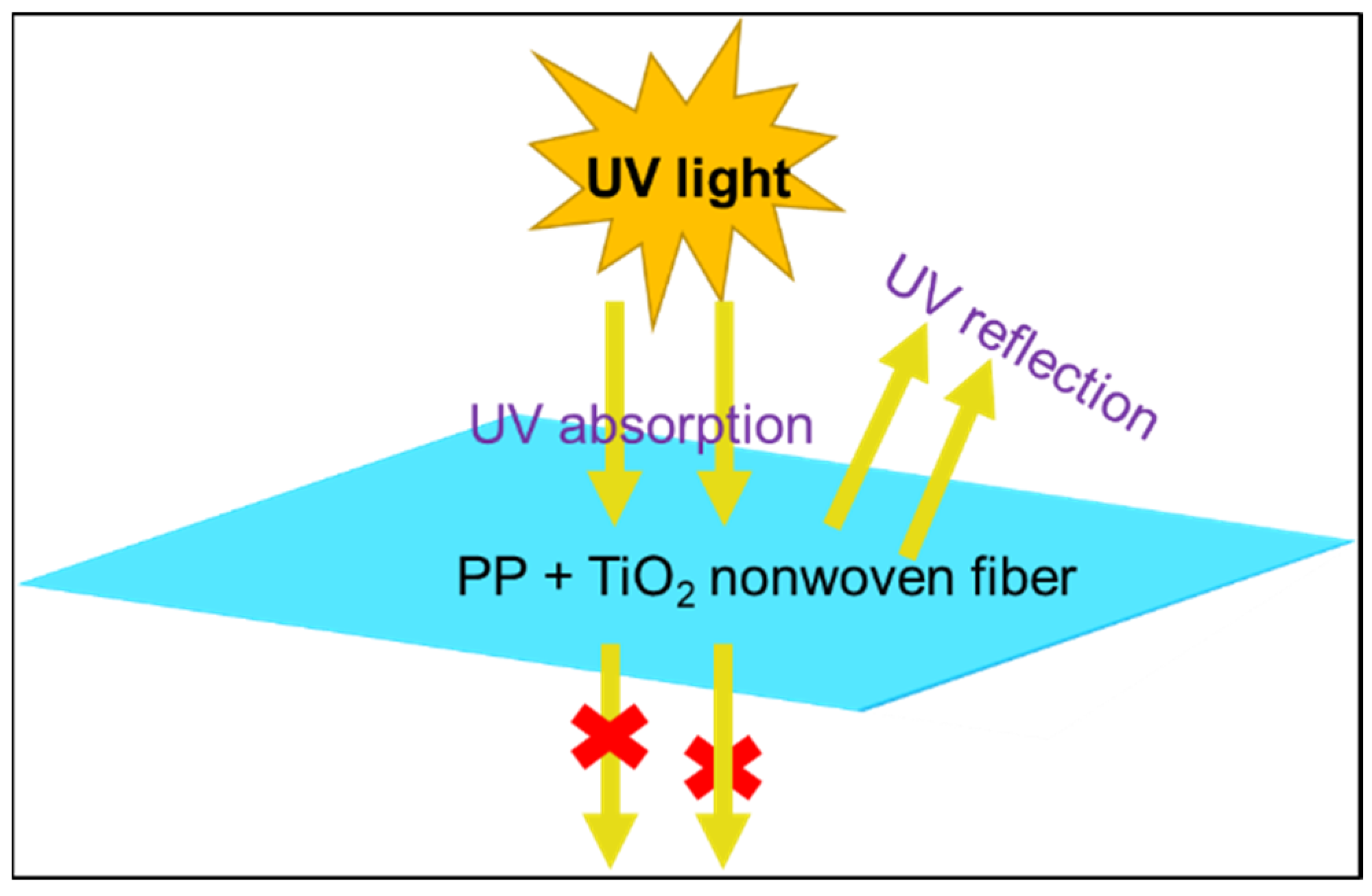
3.5. UV-Protection Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, M.; Hu, X.; Qu, L.; Du, M.; Zhu, S.; Sun, Y.; Han, G. Ultraviolet Protection Cotton Fabric Achieved via Layer-by-layer Self-assembly of Graphene Oxide and Chitosan. Appl. Surf. Sci. 2016, 377, 141–148. [Google Scholar] [CrossRef]
- Mavrić, Z.; Tomšič, B.; Simončič, B. Recent advances in the ultraviolet protection finishing of textiles. Tekstilec 2018, 61, 201–220. [Google Scholar] [CrossRef]
- González, S.; Fernández-Lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin. Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.H.; Daoud, W.A.; Kong, Y.Y. A new approach to UV-blocking treatment for cotton fabrics. Text. Res. J. 2004, 74, 97–100. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Alhashmi Alamer, F.; Beyari, R.F. The Influence of Titanium Oxide Nanoparticles and UV Radiation on the Electrical Properties of PEDOT:PSS-Coated Cotton Fabrics. Materials 2023, 16, 1738. [Google Scholar] [CrossRef] [PubMed]
- Karian, H. Handbook of Polypropylene and Polypropylene Composites, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Lewin, M. Handbook of Fiber Chemistry, 3rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 812–958. [Google Scholar]
- Khan, M.Z.; Taghavian, H.; Fijalkowski, M.; Militky, J.; Tomkova, B.; Venkataraman, M.; Adach, K. Effect of microwave power on bactericidal and UV protection properties of the ZnO nanorods grown cotton fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131135. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ashraf, M.; Hussain, T.; Rehman, A.; Malik, M.M.; Raza, Z.A.; Nawab, Y.; Zia, Q. In situ deposition of TiO2 nanoparticles on polyester fabric and study of its functional properties. Fibers Polym. 2015, 16, 1092–1097. [Google Scholar] [CrossRef]
- Bogdan, J.; Jackowska-Tracz, A.; Zarzyńska, J.; Pławińska-Czarnak, J. Chances and limitations of nanosized titanium dioxide practical application in view of its physicochemical properties. Nanoscale Res. Lett. 2015, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Topcu, S.; Jodhani, G.; Gouma, P. Optimized Nanostructured TiO2 Photocatalysts. Front. Mater. 2016, 3, 35. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Sánchez-González, S.; García-Antón, J. Photoelectrochemical characterization of anatase-rutile mixed TiO2 nanosponges. Int. J. Hydrogen Energy 2016, 41, 18380–18388. [Google Scholar] [CrossRef]
- Catone, D.L. Raw Materials-Nano-particle additives for PET. Chem. Fibers Int. 2004, 54, 25. [Google Scholar]
- Rottstegge, J.; Zhang, X.; Zhou, Y.; Xu, D.; Han, C.C.; Wang, D. Polymer nanocomposite powders and melt spun fibers filled with silica nanoparticles. J. Appl. Polym. Sci. 2007, 103, 218–227. [Google Scholar] [CrossRef]
- Erdem, N.; Cireli, A.A.; Erdogan, U.H. Flame retardancy behaviors and structural properties of polypropylene/nano-SiO2 composite textile filaments. J. Appl. Polym. Sci. 2009, 111, 2085–2091. [Google Scholar] [CrossRef]
- Han, K.; Yu, M. Study of the preparation and properties of UV-blocking fabrics of a PET/TiO2 nanocomposite prepared by in situ polycondensation. J. Appl. Polym. Sci. 2006, 100, 1588–1593. [Google Scholar] [CrossRef]
- Akter, J.; Hanif, M.A.; Islam, M.A.; Sapkota, K.P.; Hahn, J.R. Selective growth of Ti3+/TiO2/CNT and Ti3+/TiO2/C nanocomposite for enhanced visible-light utilization to degrade organic pollutants by lowering TiO2-bandgap. Sci. Rep. 2021, 11, 9490. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Rhee, S.W.; Choi, H.W. Preparation of TiO2 nanotube/nanoparticle composite particles and their applications in dye-sensitized solar cells. Nanoscale Res. Lett. 2012, 7, 48. [Google Scholar] [CrossRef]
- Hanif, M.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Kim, Y.S. Photocatalytic VOCs Degradation Efficiency of Polypropylene Membranes by Incorporation of TiO2 Nanoparticles. Membranes 2023, 13, 50. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Bhadrachari, G.; Al-Mesri, A.; Thomas, J. A Facile Approach of Thin Film Coating Consisted of Hydrophobic Titanium Dioxide over Polypropylene Membrane for Membrane Distillation. J. Membr. Sci. 2019, 6, 196–202. [Google Scholar]
- Sun, F.; Li, T.-T.; Ren, H.; Jiang, Q.; Peng, H.-K.; Lin, Q.; Lou, C.-W.; Lin, J.-H. PP/TiO2 Melt-Blown Membranes for Oil/Water Separation and Photocatalysis: Manufacturing Techniques and Property Evaluations. Polymers 2019, 11, 775. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Hu, J.-J.; Wang, G.-F.; Cui, J.-Q.; Zhang, Y.-F.; Zhen, Q. Facile Preparation of Hydrophobic PLA/PBE Micro-Nanofiber Fabrics via the Melt-Blown Process for High-Efficacy Oil/Water Separation. Polymers 2022, 14, 1667. [Google Scholar] [CrossRef] [PubMed]
- Topala, I.; Dumitrascu, N.; Pohoata, V. Influence of plasma treatments on the hemocompatibility of PET and PET+ TiO2 films. Plasma Chem. Plasma Process. 2008, 28, 535–551. [Google Scholar] [CrossRef]
- Placinta, G.; Arefi-Khonsari, F.; Gheorghiu, M.; Amouroux, J.; Popa, G. Surface properties and the stability of poly(ethylene terephtalate) (PET) films treated in plasmas of He–O2 mixtures. J. Appl. Polym. Sci. 1997, 66, 1367–1376. [Google Scholar] [CrossRef]
- Wu, J.; Xia, J.; Jing, C.; Lei, W.; Wang, B.P. Formation of hierarchical ZnO nanostructure on tinfoil substrate and the application on wetting repellency. Appl. Phys. A 2011, 105, 221–224. [Google Scholar] [CrossRef]
- Shaik, U.P.; Kshirsagar, S.; Krishna, M.G.; Tewari, S.P.; Dhar Purkayastha, D.; Madhurima, V. Growth of superhydrophobic zinc oxide nanowire thin films. Mater. Lett. 2012, 75, 51–53. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Zha, F.; Lei, Z. Facile fabrication of superhydrophobic ZnO surfaces from high to low water adhesion. Mater. Lett. 2012, 75, 71–73. [Google Scholar] [CrossRef]
- Tsuzuki, T.; He, R.; Wang, J.; Sun, L.; Wang, X.; Hocking, R. Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int. J. Nanotechnol. 2012, 9, 1017–1029. [Google Scholar] [CrossRef]
- Bouzidi, A.; Jilani, W.; Yahia, I.S.; Zahran, H.Y.; Assiri, M.A. Optical Analysis and UV-Blocking Filter of Cadmium Iodide-Doped Polyvinyl Alcohol Polymeric Composite Films: Synthesis and Dielectric Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3940–3952. [Google Scholar] [CrossRef]
- Algaba, I.; Riva, A. In vitro measurement of the ultraviolet protection factor of apparel textiles. Color Technol. 2002, 118, 52. [Google Scholar] [CrossRef]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, J.; Sun, Q.; Chen, L.; Feng, X.; Zhao, Y. Mussel-inspired multifunctional coating for enhancing the UV-resistant property of polypropylene fibers. Macromol. Res. 2017, 25, 431–438. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Sivaraj, D. Synergistic Antibacterial Activity of Barium Doped TiO2 Nanoclusters Synthesized by Microwave Processing. RSC Adv. 2016, 6, 9663–9671. [Google Scholar] [CrossRef]
- Luo, Y.S.; Yang, J.P.; Dai, X.J.; Yang, Y.; Fu, S.Y. Preparation and optical properties of novel transparent Al-doped-ZnO/epoxy nanocomposites. J. Phys. Chem. C 2009, 113, 9406–9411. [Google Scholar] [CrossRef]
- Kavya, H.V.; Nithin, K.S.; Kendagannaswamy, B.K.; Sachhidananda, S.; Chamaraja, N.A. Optical performance appraisal of mechanically flexible and visibly clear PVP-PVA/calcium doped zirconium oxide nanocomposites for UV shielding applications. Optik 2021, 227, 166008–166029. [Google Scholar] [CrossRef]
- Arunadevi, R.; Kavitha, B.; Rajarajan, M.; Suganthi, A.; Jeyamurugan, A. Investigation of the drastic improvement of photocatalytic degradation of Congo red by monoclinic Cd, Ba-CuO nanoparticles and its antimicrobial activities. Surf. Interfaces 2018, 10, 32–44. [Google Scholar] [CrossRef]
- Soumya, S.; Sheemol, V.N.; Amba, P.; Mohamed, A.P.; Ananthakumar, S. Sn and Ag doped ZnO quantum dots with PMMA by in situ polymerization for UV/IR protective, photochromic multifunctional hybrid coatings. Sol. Energy Mater. Sol. Cells 2018, 174, 554–565. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Devendiran, M.; Yun, J.H.; Kim, G.C.; Kalaiarasan, S.; Lee, I.H. Role of carbon quantum dots and film thickness on enhanced UV shielding capability of flexible polymer film containing carbon quantum dots/N-doped ZnO nanoparticles. Opt. Mater. 2018, 84, 771–777. [Google Scholar] [CrossRef]





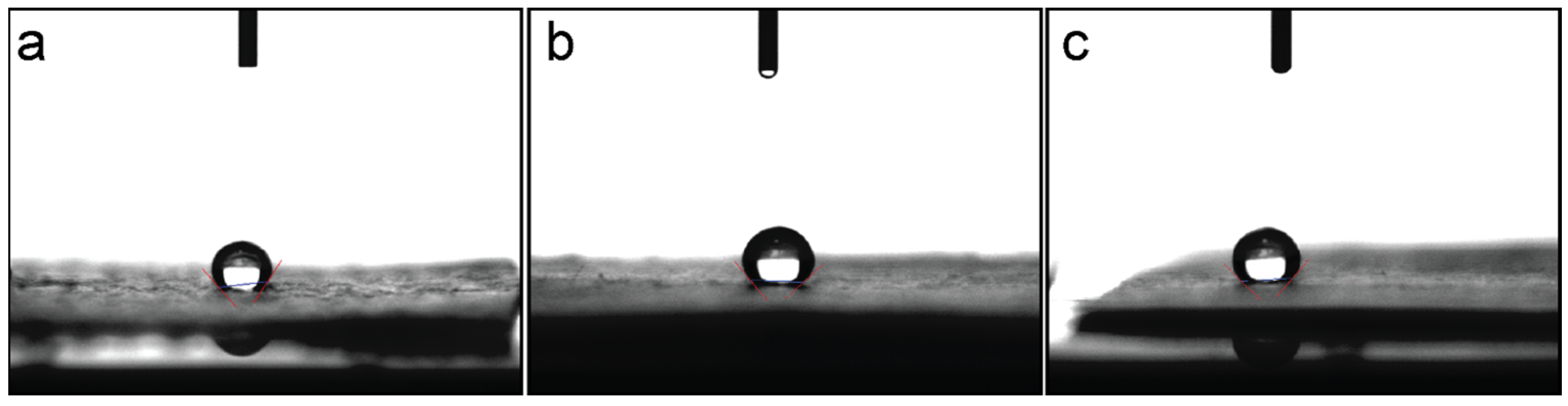


| Serial No. | Sample Name, CA | ||
|---|---|---|---|
| PPNF, θ (°) | 7.5 PPTO, θ (°) | 15 PPTO, θ (°) | |
| 1st run | 131.65 | 136.40 | 145.30 |
| 2nd run | 127.03 | 136.89 | 146.02 |
| 3rd run | 130.81 | 141.60 | 141.32 |
| 4th run | 129.17 | 135.04 | 134.24 |
| 5th run | 128.79 | 133.01 | 141.77 |
| Average + STDEV | 129.49 ± 1.62 | 136.59 ± 2.84 | 141.73 ± 4.18 |
| Wavelength | UV-Protection Ability (%) | ||
|---|---|---|---|
| PPNF | 7.5 PPTO | 15 PPTO | |
| (280~400) UV-R | 48.6 ± 0.44 | 62.0 ± 1.92 | 79.7 ± 1.18 |
| (315~400) UV-A | 48.3 ± 0.44 | 60.9 ± 1.86 | 77.5 ± 1.16 |
| (280~315) UV-B | 49.5 ± 0.45 | 65.8 ± 1.91 | 87.4 ± 1.24 |
| Sample Name | UV-Protection Ability (%) | |||
|---|---|---|---|---|
| 280 nm | 320 nm | 360 nm | Ref. | |
| Ba doped TiO2 nanoparticles | - | 53 | 18 | [34] |
| Al doped ZnO/Epoxy nanocomposite | 50 | 45 | 40 | [35] |
| PVA/CAN doped ZnO composite film | 58 | 56 | 40 | [36] |
| Cd doped CuO nanoparticles | 64 | 70 | 76 | [37] |
| Sn doped ZnO quantum dots | 75 | 38 | 25 | [38] |
| polymer and carbon/N doped ZnO nanomaterials | 70 | 85 | 75 | [39] |
| 15 PPTO | 88.99 | 86.18 | 75.34 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, M.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Han, S.-W.; Kang, S.-S.; Kim, Y.S. Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber. J. Compos. Sci. 2024, 8, 86. https://doi.org/10.3390/jcs8030086
Hanif MA, Shin H, Chun D, Kim HG, Kwac LK, Han S-W, Kang S-S, Kim YS. Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber. Journal of Composites Science. 2024; 8(3):86. https://doi.org/10.3390/jcs8030086
Chicago/Turabian StyleHanif, Md. Abu, Hyokyeong Shin, Danbi Chun, Hong Gun Kim, Lee Ku Kwac, Sang-Won Han, Sung-Soo Kang, and Young Soon Kim. 2024. "Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber" Journal of Composites Science 8, no. 3: 86. https://doi.org/10.3390/jcs8030086
APA StyleHanif, M. A., Shin, H., Chun, D., Kim, H. G., Kwac, L. K., Han, S.-W., Kang, S.-S., & Kim, Y. S. (2024). Development of Highly Ultraviolet-Protective Polypropylene/TiO2 Nonwoven Fiber. Journal of Composites Science, 8(3), 86. https://doi.org/10.3390/jcs8030086








