Structure, Properties, and Recent Developments in Polysaccharide- and Aliphatic Polyester-Based Packaging—A Review
Abstract
1. Introduction
2. Conventional Food Packaging
2.1. Paper and Paperboard
2.2. Plastic
2.3. Glass
2.4. Metal
3. Biopolymers for Sustainable Food Packaging
3.1. Polysaccharide-Based Biopackaging Materials and Their Applications in the Food Industry
3.1.1. Starch
3.1.2. Cellulose
3.1.3. Chitosan
3.1.4. Pectin
3.1.5. Alginate
3.1.6. Carrageenan
3.2. Aliphatic Polymer-Based Food Packaging
3.2.1. Polylactic Acid (PLA)
3.2.2. Polyhydroxybutyrate (PHB)
4. Challenges and Future Perspectives of Biopackaging in the Food Industry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 22 August 2023).
- Han, J.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Goswami, B. The Role of Food Packaging. In Global Challenges and Innovation in Science and Management, 1st ed.; Rami, A., Jha, P., Shah, P., Eds.; Kaav Publications: Delhi, India, 2019; pp. 157–167. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Nemat, B.; Razzaghi, M.; Bolton, K.; Rousta, K. The Potential of Food Packaging Attributes to Influence Consumers’ Decisions to Sort Waste. Sustainability 2020, 12, 2234. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Nesic, A.; Cabrera-Barjas, G.; Dimitrijevic-Brankovic, S.; Davidovic, S.; Radovanovic, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef]
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S.; Syed, Z.; Sonu, K.; Gupta, N.S.; Kalra, A. Perceiving Biobased Plastics as an Alternative and Innovative Solution to Combat Plastic Pollution for a Circular Economy. Sci. Total Environ. 2023, 874, 162441. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An Overview of Biodegradable Packaging in Food Industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Kumar, K.; Yamunadevi, B.; Poojitha, N.; SuhailMadharHanif, S.; Kannaiyan, S. Biodegradable Packaging Materials. Polym. Bull. 2021, 79, 4467–4496. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-Based Functional Films for Packaging Applications: A Review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Food 2022, 11, 3087. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An Overview of Paper and Paper Based Food Packaging Materials: Health Safety and Environmental Concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, Y.; Fahmy, T.Y.; Mobarak, F.; El-Sakhawy, M.; Fadl, M. Agricultural Residues (Wastes) for Manufacture of Paper, Board, and Miscellaneous Products: Background Overview and Future Prospects. Int. J. ChemTech Res. 2017, 10, 424–448. [Google Scholar]
- Ferdous, T.; Ni, Y.; Quaiyyum, M.A.; Uddin, M.N.; Jahan, M.S. Non-Wood Fibers: Relationships of Fiber Properties with Pulp Properties. ACS Omega 2021, 6, 21613–21622. [Google Scholar] [CrossRef] [PubMed]
- Pydimalla, M.; Reddy, K. Effect of Pulping, Bleaching and Refining Process on Fibers for Paper Making—A Review. Int. J. Eng. Res. Technol. 2020, 9, 310–316. [Google Scholar]
- Hubbe, M.A.; Pruszynski, P. Greaseproof Paper Products: A Review Emphasizing Ecofriendly Approaches. BioResources 2020, 15, 1978–2004. [Google Scholar] [CrossRef]
- Jasmani, L.; Ainun, Z.M.A.; Adnan, S.; Ibrahim, R.; Sapuan, S.M.; Ilyas, R.A. Sustainable Paper-Based Packaging. In Bio-BasedPackaging: Material, Environmental and Economic Aspects, 1st ed.; Sapuan, S.M., Ilyas, R.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 225–244. [Google Scholar] [CrossRef]
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in Packaging Material for Food Products: Historical Background, Current Scenario, and Future Prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef]
- Fadiji, T.; Coetzee, C.; Pathare, P.; Opara, U.L. Susceptibility to Impact Damage of Apples inside Ventilated Corrugated Paperboard Packages: Effects of Package Design. Postharvest Biol. Technol. 2016, 111, 286–296. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Lignou, S. Sustainable Paper-Based Packaging: A Consumer’s Perspective. Foods 2021, 10, 1035. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Sonone, S.S.; Sankhla, M.S.; Kumar, R. Health Risks of Newspaper Ink When Used as Food Packaging Material. Lett. Appl. NanoBioScience 2021, 10, 2614–2623. [Google Scholar] [CrossRef]
- Tajeddin, B.; Arabkhedri, M. Polymers and Food Packaging. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–543. [Google Scholar] [CrossRef]
- Silva, N.; Blumberga, D. Why Biopolymer Packaging Materials Are Better. Environ. Clim. Technol. 2019, 23, 366–384. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Haider, A.; Mohyuddin, A.; Fatima, R.; Salman, M.; Shaheen, A.; Ahmad, H.M.; Al-Hazmi, H.E.; Othman, M.H.D.; Aziz, F.; et al. Tackling Microplastics Pollution in Global Environment through Integration of Applied Technology, Policy Instruments, and Legislation. J. Environ. Manag. 2023, 346, 118971. [Google Scholar] [CrossRef]
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 021. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Nistico, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Mendes, A.C.; Pedersen, G.A. Perspectives on Sustainable Food Packaging—Is Bio-Based Plastics a Solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Rico, B.P.M.; Vieira, M.C. Food Packaging and Migration. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 281–301. [Google Scholar] [CrossRef]
- Lewandowski, K. A Brief Review of Poly (Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V.M. Low-Density Polyethylene Migration from Food Packaging on Cured Meat Products Detected by Micro-Raman Spectroscopy. Microplastics 2022, 1, 428–439. [Google Scholar] [CrossRef]
- Sadighara, P.; Akbari, N.; Mostashari, P.; Yazdanfar, N.; Shokri, S. The Amount and Detection Method of Styrene in Foods: A Systematic Review and Meta-Analysis. Food Chem. X 2022, 13, 100238. [Google Scholar] [CrossRef]
- Tajeddin, B.; Ahmadi, B.; Sohrab, F.; Chenarbon, H.A. Polymers for Modified Atmosphere Packaging Applications. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 457–499. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Kehinde, O.; Ramonu, O.J.; Babaremu, K.O.; Justin, L.D. Plastic Wastes: Environmental Hazard and Instrument for Wealth Creation in Nigeria. Heliyon 2020, 6, e05131. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Islam, M.S.; Haque, M.A.; Rahman, M.M.; Mithu, M.M.R. Toxic Effects of Plastic on Human Health and Environment: A Consequences of Health Risk Assessment in Bangladesh. Int. J. Health 2017, 6, 1. [Google Scholar] [CrossRef]
- Krammer, J. Exploring the Last Phases of Product Development; From Kitchen to Plant Production; Lund University: Lund, Sweden, 2017. [Google Scholar]
- Yaris, A.; Sezgin, A.C. Food Packaging: Glass and Plastic. In Researches on Science and Art in 21st Century Turkey; Gece Publishing: Ankara, Turkey, 2017; Volume 8, pp. 735–740. [Google Scholar]
- Testa, M.; Malandrino, O.; Sessa, M.R.; Supino, S.; Sica, D. Long-Term Sustainability from the Perspective of Cullet Recycling in the Container Glass Industry: Evidence from Italy. Sustainability 2017, 9, 1752. [Google Scholar] [CrossRef]
- Shin, J.; Selke, S.E. Food Packaging. In Food Processing: Principles and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 249–273. [Google Scholar]
- Dunn, T.J. Food Packaging. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R. Review on Metal Packaging: Materials, Forms, Food Applications, Safety and Recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Debeaufort, F. Metal Packaging. In Packaging Materials and Processing for Food, Pharmaceuticals and Cosmetics; ISTE Ltd.: London, UK, 2021; Volume 13, pp. 75–104. [Google Scholar] [CrossRef]
- Sand, C.K. The Role of Metal in Food Packaging. Food Technol. 2021, 7, 78–80. [Google Scholar]
- Pandey, S.; Mishra, K.K.; Ghosh, P.; Singh, A.K.; Jha, S.K. Characterization of Tin-Plated Steel. Front. Mater. 2023, 10, 1113438. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-Based Biodegradable Packaging Materials: A Review of Their Preparation, Characterization and Diverse Applications in the Food Industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Orzan, G.; Cruceru, A.F.; Teodora, C.; Chivu, R. Consumers’ Behavior Concerning Sustainable Packaging: An Exploratory Study on Romanian Consumers. Sustainability 2018, 10, 1787. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, L.G.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, O.A.; Sadiku, E.R.; Babu Reddy, A.; Ndamase, A.S.; Makgatho, G.; Sellamuthu, P.S.; Perumal, A.B.; Nambiar, R.B.; Fasiku, V.O.; Ibrahim, I.D.; et al. The Use of Biopolymers in Food Packaging. In Green Biopolymers and Their Nanocomposites; Springer: Singapore, 2019; pp. 137–158. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.; Andrade, C. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Anis, A.; Ray, S.S.; Kim, D.; Hanh Nguyen, T.T.; Pal, K. Introduction of Biopolymers: Food and Biomedical Applications. In Biopolymer-Based Formulations: Biomedical and Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-Based Materials from Polysaccharides: Properties, Processing, Characterization and Sorption Applications. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, V.; Gupta, R.; Ghosh, T. Advances in Sustainable Polymers; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef]
- Tadini, C.C. Bio-Based Materials from Traditional and Nonconventional Native and Modified Starches; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Cazon, P.; Velazquez, G.; Ramirez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Wang, J.; Hu, P.; Chen, Z.; Liu, Q.; Wei, C. Progress in High-Amylose Cereal Crops through Inactivation of Starch Branching Enzymes. Front. Plant Sci. 2017, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Jayarathna, S.; Andersson, M.; Andersson, R. Recent Advances in Starch-Based Blends and Composites for Bioplastics Applications. Polymers 2022, 14, 4557. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, O.H.P.; Gunathilake, C.; Amaraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.K.; Smith, R.C. Recent Advances in Starch-Based Films toward Food Packaging Applications: Physicochemical, Mechanical, and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Siqueira, L.d.V.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-Based Biodegradable Plastics: Methods of Production, Challenges and Future Perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of Sweet Potato Starch-Based Nanocomposite Films Activated with Thyme Essential Oil on the Shelf-Life of Baby Spinach Leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, S.; Song, K.B. Cowpea Starch Films Containing Maqui Berry Extract and Their Application in Salmon Packaging. Food Packag. Shelf Life 2019, 22, 100394. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging. Polymers 2023, 15, 588. [Google Scholar] [CrossRef]
- Garcia, A.V.; Alvarez-Perez, O.B.; Rojas, R.; Aguilar, C.N.; Garrigós, M.C. Impact of Olive Extract Addition on Corn Starch-Based Active Edible Films Properties for Food Packaging Applications. Foods 2020, 9, 1339. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Environmental Technology & Innovation Intensification of Yam-Starch Based Biodegradable Bioplastic Film with Bentonite for Food Packaging Application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Hou, X.; Cui, Q.; Wu, H.; Shen, G.; Luo, Q.; Li, S.; Liu, X.; Li, M.; et al. Starch-Based Film Functionalized with Zanthoxylum Armatum Essential Oil Improved the Shelf Life of Beef Sauce. LWT 2023, 183, 114930. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ozogul, F.; Dunno, K.D.; Cavender, G.A.; Dawson, P. Development of Starch-Based Films Reinforced with Cellulosic Nanocrystals and Essential Oil to Extend the Shelf Life of Red Grapes. Food Biosci. 2022, 47, 101621. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Manimaran, P.; Sanjay, M.R.; Siengchin, S.; Geetha, M.; Kandakodeeswaran, K.; Boonyasopon, P.; Gorbatyuk, S. Extraction and Development of Starch-Based Bioplastics from Prosopis Juliflora Plant: Eco-Friendly and Sustainability Aspects. Curr. Res. Green Sustain. Chem. 2022, 5, 100296. [Google Scholar] [CrossRef]
- Tafa, K.D.; Satheesh, N.; Abera, W. Mechanical Properties of Tef Starch Based Edible Films: Development and Process Optimization. Heliyon 2023, 9, e13160. [Google Scholar] [CrossRef]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Salmieri, S.; Djidjelli, H.; Lacroix, M. Incorporation of Thymus Vulgaris Essential Oil and Ethanolic Extract of Propolis Improved the Antibacterial, Barrier and Mechanical Properties of Corn Starch-Based Films. Int. J. Biol. Macromol. 2023, 224, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, P.K.; Pattnaik, R.; Kumar, S.; Ojha, S.K.; Srichandan, H.; Parhi, P.K.; Jyothi, R.K.; Sarangi, P.K. Biochemistry, Synthesis, and Applications of Bacterial Cellulose: A Review. Front. Bioeng. Biotechnol. 2022, 10, 780409. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Krochta, J.M. Edible Packaging Materials. Annu. Rev. Offood Sci. Technol. 2016, 1, 415–448. [Google Scholar] [CrossRef]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose Based Polymers in Development of Amorphous Solid Dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef]
- Yemenicioglu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A Review of Current and Future Food Applications of Natural Hydrocolloids. Int. J. Food Sci. Technol. 2019, 1, 1389–1406. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and Characterization of Plant Oil-Incorporated Carboxymethyl Cellulose/Bacterial Cellulose/Glycerol-Based Antimicrobial Edible Films for Food Packaging Applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Luo, W.; Chen, G.; Xiao, N.; Xiao, G.; Liu, C. Development of Functional Hydroxyethyl Cellulose-Based Composite Films for Food Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 989893. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Romao, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of Bacterial Cellulose-Based Active Membranes Containing Herbal Extracts for Shelf-Life Extension of Button Mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2018, 42, e13537. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; Mahmoud, M.; Nada, A.A. Fabrication of Cellulose-Based Adhesive Composite as an Active Packaging Material to Extend the Shelf Life of Cheese. Int. J. Biol. Macromol. 2020, 160, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Yordshahi, A.S.; Moradi, M.; Tajik, H.; Molaei, R. Design and Preparation of Antimicrobial Meat Wrapping Nanopaper with Bacterial Cellulose and Postbiotics of Lactic Acid Bacteria. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of Gallic Acid Loaded Hydroxypropyl Methylcellulose Nanofibers by Electrospinning Technique as Active Packaging Material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Florez, M.; Guerra-Rodríguez, E.; Cazon, P.; Vazquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Luangapai, F.; Peanparkdee, M.; Iwamoto, S. Biopolymer Films for Food Industries: Properties, Applications, and Future Aspects Based on Chitosan. Rev. Agric. Sci. 2019, 7, 59–67. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M.; Martin-gonzalez, F.S.; Jones, O.G.; Garcia-bravo, J.M.; Kaplan, I. Development of Chitosan Films from Edible Crickets and Their Performance as a Bio-Based Food Packaging Material. Polysaccharides 2021, 2, 744–758. [Google Scholar] [CrossRef]
- Zehra, A.; Wani, S.M.; Jan, N.; Bhat, T.A.; Rather, S.A.; Malik, A.R.; Hussain, S.Z. Development of Chitosan—Based Biodegradable Films Enriched with Thyme Essential Oil and Additives for Potential Applications in Packaging of Fresh Collard Greens. Sci. Rep. 2022, 12, 16923. [Google Scholar] [CrossRef]
- Ruiz-cruz, S.; Enrique, M.; Jes, D.; Del-toro-s, C.L.; Gassos-ortega, L.E. Effect of Chitosan–Tomato Plant Extract Edible Coating on the Quality, Shelf Life, and Antioxidant Capacity of Pork during Refrigerated Storage. Coatings 2019, 9, 827. [Google Scholar]
- Wang, K.; Lim, P.N.; Tong, S.Y.; Thian, E.S. Development of Grapefruit Seed Extract-Loaded Poly (ε-Caprolactone)/Chitosan Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Jiang, Q.; Yu, D.; Xu, Y.; Wang, B.; Xia, W. Development and Properties of Bacterial Cellulose, Curcumin, and Chitosan Composite Biodegradable Films for Active Packaging Materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ibresam, O.T.; Al-Hatim, R.R.; Al-Ali, R.M.; Maslekar, N.; Yao, Y.; Agarwal, V. Development of Chitosan/Whey Protein Hydrolysate Composite Films for Food Packaging Application. J. Compos. Sci. 2023, 7, 94. [Google Scholar] [CrossRef]
- Yao, J.; Mao, L.; Wang, C.; Liu, X.; Liu, Y. Development of Chitosan/Poly (Vinyl Alcohol) Active Films Reinforced with Curcumin Functionalized Layered Clay towards Food Packaging. Prog. Org. Coat. 2023, 182, 9–10. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Karkar, B.; Patır, İ.; Eyüboğlu, S.; Şahin, S. Development of an Edible Active Chitosan Film Loaded with Nigella sativa L. Extract to Extend the Shelf Life of Grapes. Biocatal. Agric. Biotechnol. 2023, 50, 102708. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T. Pectic Polysaccharides in Plants: Structure, Biosynthesis, Functions, and Applications. In Extracellular Sugar-Based Biopolymers Matrices; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 487–514. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Vanitha, T.; Khan, M. Role of Pectin in Food Processing and Food Packaging. In Pectins—Extraction, Purification, Characterization and Applications; Masuelli, M., Ed.; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millan, E.; Balandran-Quintana, R.; Lopez-Franco, Y.; Rascon-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review. Int. J. Sci. Res. Mod. Educ. 2016, 1, 188–204. [Google Scholar]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A New Approach to Develop Biodegradable Films Based on Thermoplastic Pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- de Oliveira, A.C.S.; Ferreira, L.F.; Begali, D.d.O.; Ugucioni, J.C.; Neto, A.R.d.S.; Yoshida, M.I.; Borges, S.V. Thermoplasticized Pectin by Extrusion/Thermo-Compression for Film Industrial Application. J. Polym. Environ. 2021, 29, 2546–2556. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Burgos, N.; Jimenez, A.; Garrigos, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Siqueira, R.A.; Veras, J.M.L.; de Sousa, T.L.; de Farias, P.M.; Filho, J.G.d.O.; Bertolo, M.R.V.; Egea, M.B.; Placido, G.R. Pequi Mesocarp: A New Source of Pectin to Produce Biodegradable Film for Application as Food Packaging. Food Sci. Technol. 2022, 42, e71421. [Google Scholar] [CrossRef]
- Pereira, D.G.M.; Vieira, J.M.; Vicente, A.A.; Cruz, R.M.S. Development and Characterization of Pectin Films with Salicornia Ramosissima: Biodegradation in Soil and Seawater. Polymers 2021, 13, 2632. [Google Scholar] [CrossRef] [PubMed]
- Sadadekar, A.S.; Shruthy, R.; Preetha, R.; Kumar, N.; Pande, K.R.; Nagamaniammai, G. Enhanced Antimicrobial and Antioxidant Properties of Nano Chitosan and Pectin Based Biodegradable Active Packaging Films Incorporated with Fennel (Foeniculum vulgare) essential oil and potato (Solanum tuberosum) Peel Extracts. J. Food Sci. Technol. 2023, 60, 938–946. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Utilization of Peel of White Pomelo for the Development of Pectin Based Biodegradable Composite Films Blended with Casein and Egg Albumen. Food Chem. Adv. 2022, 1, 100054. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and Characterisation of a Pectin-Based Edible Film That Contains Mulberry Leaf Extract and Its Bio-Active Components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Pu, Y.; Chen, L.; Cao, J.; Jiang, W. Development and Characterization of a Novel Active and Intelligent Film Based on Pectin and Betacyanins from Peel Waste of Pitaya (Hylocereus undatus). Food Chem. 2023, 404, 134444. [Google Scholar] [CrossRef]
- Kannan, A.; Dheeptha, M.; Sistla, Y.S. Development of Pectin and Sodium Alginate Composite Films with Improved Barrier and Mechanical Properties for Food-Packaging Applications. Eng. Proc. 2023, 37, 80. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Lavinia-florina, C.; Pascuta, M.S.; Varvara, R.; Szabo, K. Development of Pectin and Poly (Vinyl Alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Antioxidant Activities of Mandarin (Citrus unshiu) Peel Pectin Films Containing Sage (Salvia officinalis) Leaf Extract. Int. J. Food Sci. Technol. 2020, 55, 3173–3181. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Castro-Yobal, M.; Contreras-Oliva, A.; Saucedo-Rivalcoba, V.; Rivera-Armenta, J.L.; Hernández-Ramírez, G.; Salinas-Ruiz, J.; Herrera-Corredor, A. Evaluation of Physicochemical Properties of Film-Based Alginate for Food Packing Applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Kruk, K.; Winnicka, K. Alginates Combined with Natural Polymers as Valuable Drug Delivery Platforms. Mar. Drugs 2022, 21, 11. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Reddy, S.G. Alginates –A Seaweed Product: Its Properties and Applications. In Properties and Applications of Alginates; Irem, D., Esra, I., Tugba, K.G., Eds.; IntechOpen: London, UK, 2021; pp. 225–240. [Google Scholar]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Anis, A.; Pal, K. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The Development of a Uniform Alginate-Based Coating for Cantaloupe and Strawberries and the Characterization of Water Barrier Properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of Chitosan and Alginate Based Coatings Enriched with Pomegranate Peel Extract to Extend the Postharvest Quality of Guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Agçeli, G.; Thakur, A.; Singh, S.; Chauhan, P.; Chauhan, P.K. Development of Alginate-Chitosan Based Coating Enriched with ZnO Nanoparticles for Increasing the Shelf Life of Orange Fruits (Citrus sinensis L.). J. Polym. Environ. 2022, 30, 3293–3306. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B. Activating Sodium Alginate-Based Edible Coating Using a Dietary Supplement for Increasing the Shelf Life of Rainbow Trout Fillet during Refrigerated Storage (4 ± 1 °C). J. Food Saf. 2018, 38, e12395. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and Characterization of Sodium Alginate Based Active Edible Films Incorporated with Essential Oils of Some Medicinal Plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Alves, Z.; Ferreira, N.M.; Mendo, S.; Ferreira, P.; Nunes, C. Design of Alginate-based Bionanocomposites with Electrical Conductivity for Active Food Packaging. Int. J. Mol. Sci. 2021, 22, 9943. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.Z.; Tong, W.Y.; Leong, C.R.; Rashid, S.A.; Rozman, N.A.S.; Hamid, N.H.M.; Karim, S.; Tumin, N.D.; Muda, S.A.; Yacob, L.S. Development of Cinnamaldehyde Loaded-Alginate Based Film for Food Packaging. Asia-Pac. J. Mol. Biol. Biotechnol. 2021, 29, 18–25. [Google Scholar] [CrossRef]
- Bata Gouda, M.H.; Zhang, C.; Peng, S.; Kong, X.; Chen, Y.; Li, H.; Li, X.; Luo, H.; Yu, L. Combination of Sodium Alginate-Based Coating with L-Cysteine and Citric Acid Extends the Shelf-Life of Fresh-Cut Lotus Root Slices by Inhibiting Browning and Microbial Growth. Postharvest Biol. Technol. 2021, 175, 111502. [Google Scholar] [CrossRef]
- Montone, A.M.I.; Malvano, F.; Pham, P.L.; Cinquanta, L.C.; Capparelli, R.; Capuano, F.; Albanese, D. Alginate-based Coatings Charged with Hydroxyapatite and Quercetin for Fresh-cut Papaya Shelf Life. Int. J. Food Sci. Technol. 2022, 57, 5307–5318. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Kulandhaivelu, S.V.; Roy, S. Alginate/Carboxymethyl Cellulose/Starch- Based Active Coating with Grapefruit Seed Extract to Extend the Shelf Life of Green Chilli. Ind. Crops Prod. 2023, 199, 99. [Google Scholar] [CrossRef]
- Feng, S.; Tang, Q.; Xu, Z.; Huang, K.; Li, H.; Zou, Z. Development of Novel Co-MOF Loaded Sodium Alginate Based Packaging Films with Antimicrobial and Ammonia-Sensitive Functions for Shrimp Freshness Monitoring. Food Hydrocoll. 2023, 135, 108193. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Durairaj, R.B.; Mageshwaran, G.; Joseph, G.B. A Brief Review on Edible Food Packaging Materials. J. Glob. Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Karbowiak, T.; Debeaufort, F.; Champion, D.; Voilley, A. Wetting Properties at the Surface of Iota-Carrageenan-Based Edible Films. J. Colloid Interface Sci. 2006, 294, 400–410. [Google Scholar] [CrossRef]
- Lindstrom, T.; Osterberg, F. Evolution of Biobased and Nanotechnology Packaging—A Review. Nord. Pulp Pap. Res. J. 2020, 35, 491–515. [Google Scholar] [CrossRef]
- Bhat, K.M.; Jyothsana, R.; Sharma, A.; Rao, N.N. Carrageenan-Based Edible Biodegradable Food Packaging: A Review. Int. J. Food Sci. Nutr. 2020, 5, 69–75. [Google Scholar]
- Li, F.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhe, T.; Guo, Z.; Sun, X.; Wang, Q.; Wang, L. Copper Sulfide Nanoparticle-Carrageenan Films for Packaging Application. Food Hydrocoll. 2020, 109, 106094. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-Based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent Advances in Carrageenan-Based Films for Food Packaging Applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; de Souza, P.K.; Silva, B.D.Z.; Martiny, T.R.; Moraes, C.C.; Morais, M.M.; Raghavan, V.; da Rosa, G.S. Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging. Molecules 2020, 25, 5563. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and Characterization of K-Carrageenan/Konjac Glucomannan/TiO2 Nanocomposite Film with Efficient Anti-Fungal Activity and Its Application in Strawberry Preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Ramos, A.; Luís, Â.; Amaral, M.E. Production and Characterization of K-Carrageenan Films Incorporating Cymbopogon Winterianus Essential Oil as New Food Packaging Materials. Foods 2023, 12, 2169. [Google Scholar] [CrossRef] [PubMed]
- Panatarani, C.; Praseptiangga, D.; Widjanarko, P.I.; Azhary, S.Y.; Nurlilasari, P.; Rochima, E.; Joni, I.M. Synthesis, Characterization, and Performance of Semi-Refined Kappa Carrageenan-Based Film Incorporating Cassava Starch. Membranes 2023, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bang, Y.J.; Yoon, K.S.; Priyadarshi, R.; Rhim, J.W. Pine Needle (Pinus densiflora) Extract-Mediated Synthesis of Silver Nanoparticles and the Preparation of Carrageenan-Based Antimicrobial Packaging Films. J. Nanomater. 2022, 2022, 8395302. [Google Scholar] [CrossRef]
- Mahajan, K.; Kumar, S.; Bhat, Z.F.; Naqvi, Z.; Mungure, T.E.; Bekhit, A.E.D.A. Functionalization of Carrageenan Based Edible Film Using Aloe Vera for Improved Lipid Oxidative and Microbial Stability of Frozen Dairy Products. Food Biosci. 2021, 43, 101336. [Google Scholar] [CrossRef]
- You, S.; Zhang, X.; Wang, Y.; Jin, Y.; Wei, M.; Wang, X. Development of Highly Stable Color Indicator Films Based on κ-Carrageenan, Silver Nanoparticle and Red Grape Skin Anthocyanin for Marine Fish Freshness Assessment. Int. J. Biol. Macromol. 2022, 216, 655–669. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer Hybrid Materials: Development, Characterization, and Food Packaging Applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Khodaei, D.; Álvarez, C.; Mullen, A.M. Biodegradable Packaging Materials from Animal Processing Co-Products and Wastes: An Overview. Polymers 2021, 13, 2561. [Google Scholar] [CrossRef]
- Galgano, F.; Condelli, N.; Favati, F.; Di Bianco, V.; Perretti, G.; Caruso, M.C. Biodegradable Packaging and Edible Coating for Fresh-Cut Fruits and Vegetables. Ital. J. Food Sci. 2015, 27, 1–20. [Google Scholar]
- Maurizzi, E.; Bigi, F.; Quartieri, A.; De Leo, R.; Volpelli, L.A.; Pulvirenti, A. The Green Era of Food Packaging: General Considerations and New Trends. Polymers 2022, 14, 4257. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly (Lactic Acid) (PLA) and Polyhydroxyalkanoates (PHAs), Green Alternatives to Petroleum-Based Plastics: A Review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Sharma, S. Polylactic Acid (PLA) and Its Composites: An Eco-friendly Solution for Packaging. In Sustainable Food Packaging Technology; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 107–132. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Torres-giner, S.; Figueroa-lopez, K.J.; Melendez-rodriguez, B.; Prieto, C.; Pardo-figuerez, M.; Lagaron, J.M. Emerging Trends in Biopolymers for Food Packaging. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 1–32. [Google Scholar]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of Active Agents Filled Polylactic Acid Films for Food Packaging Application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Khanjari, A.; Esmaeili, H.; Hamedi, M. Shelf-Life Extension of Minced Squab Using Poly-Lactic Acid Films Containing Cinnamomum Verum Essential Oil. Int. J. Food Microbiol. 2023, 385, 109982. [Google Scholar] [CrossRef] [PubMed]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Fadhel, Y.B.; Djidjelli, H.; Lacroix, M. Development of Antimicrobial Films Based on Poly(Lactic Acid) Incorporated with Thymus Vulgaris Essential Oil and Ethanolic Extract of Mediterranean Propolis. Int. J. Biol. Macromol. 2021, 185, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hao, Y.; Liu, B.; Chen, Y.; Li, L. Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation. Foods 2023, 12, 3397. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Torres-Giner, S. Quality and Shelf-Life Stability of Pork Meat Fillets Packaged in Multilayer Polylactide Films. Foods 2022, 11, 426. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Xu, Y.; Huang, H.; Zhao, H.; Wang, J.; Wang, S. Synthesis and Characterization of Antibacterial Polylactic Acid Film Incorporated with Cinnamaldehyde Inclusions for Fruit Packaging. Int. J. Biol. Macromol. 2020, 164, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly (Lactic Acid)/Poly (Butylene-Succinate-Co-Adipate) (PLA/PBSA) Blend Films Containing Thymol as Alternative to Synthetic Preservatives for Active Packaging of Bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, R.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Kang, L.; Cheng, Z.; Gao, W.; Chen, K. Biodegradable Sandwich-Architectured Films Derived from Pea Starch and Polylactic Acid with Enhanced Shelf-Life for Fruit Preservation. Carbohydr. Polym. 2021, 251, 117117. [Google Scholar] [CrossRef] [PubMed]
- Wongthanaroj, D.; Jessmore, L.A.; Lin, Y.; Bergholz, T.M.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Sustainable and Eco-Friendly Poly (Lactic Acid)/Cellulose Nanocrystal Nanocomposite Films for the Preservation of Oxygen-Sensitive Food. Appl. Food Res. 2022, 2, 100222. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Smart Packaging Based on Polylactic Acid: The Effects of Antibacterial and Antioxidant Agents from Natural Extracts on Physical—Mechanical Properties, Colony Reduction, Perishable Food Shelf Life, and Future Prospective. Polymers 2023, 15, 4103. [Google Scholar] [CrossRef]
- Gabor, D.; Tita, O. Biopolymers Used in Food Packaging: A Review. Acta Univ. Cibiniensis Ser. E Food Technol. 2012, XVI, 3–19. [Google Scholar]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and Characterization of Environmentally Safe and Highly Biodegradable Microbial Polyhydroxybutyrate (PHB) Based Graphene Nanocomposites for Potential Food Packaging Applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- Pawar, P.A.; Purwar, A.H. Bioderadable Polymers in Food Packaging. Am. J. Eng. Res. 2013, 2, 151–164. [Google Scholar]
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Balart, R.; Torres-Giner, S.; Arrieta, M.P. Innovative Solutions and Challenges to Increase the Use of Poly(3-Hydroxybutyrate) in Food Packaging and Disposables. Eur. Polym. J. 2022, 178, 111505. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Facile Fabrication and Characterization of Novel Antimicrobial and Antioxidant Poly (3-Hydroxybutyrate)/Essential Oil Composites for Potential Use in Active Food Packaging Applications. Int. J. Biol. Macromol. 2023, 252, 98. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Bassani, A.; Zaccone, M.; Luana, M.; Díaz, E.; Apodaca, D.; Spigno, G. Active Coated PLA-PHB Film with Formulations Containing a Commercial Olive Leaf Extract to Improve Quality Preservation of Fresh Pork Burgers. Chem. Eng. Trans. 2023, 102, 85–90. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced Mechanical and Antioxidant Properties of Biodegradable Poly (Lactic) Acid-Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Film Utilizing α-Tocopherol for Peach Storage. Packag. Technol. Sci. 2021, 34, 187–199. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin Nanocomposite Based on Plasticized Poly (Lactic Acid)/Poly(3-Hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers 2022, 14, 3177. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Walton, W.C.; Wang, L.; Li, L.; Wang, Y. Characterization of Polylactic Acids-Polyhydroxybutyrate Based Packaging Film with Fennel Oil, and Its Application on Oysters. Food Packag. Shelf Life 2019, 22, 100388. [Google Scholar] [CrossRef]
- Singh, P.; Chatli, A.S.; Mehndiratta, H.K. Development of Starch Based Edible Films. Int. J. Dev. Res. 2018, 8, 23501–23506. [Google Scholar]
- Yang, S.-Y.; Cao, L.; Kim, H.; Beak, S.E.; Song, K.B. Utilization of Foxtail Millet Starch Film Incorporated with Clove Leaf Oil for the Packaging of Queso Blanco Cheese as a Model Food. Starch/Staerke 2018, 70, 1700171. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karim, R.; Sultan, M.T.; Paykary, M.; Johnson, S.K.; Shekarforoush, E. Comparison of Starch Films and Effect of Different Rice Starch-Based Coating Formulations on Physical Properties of Walnut during Storage Time at Accelerated Temperature. J. Food Process Eng. 2018, 41, e12607. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An Active Packaging Film Based on Yam Starch with Eugenol and Its Application for Pork Preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.W.; Kim, J. Cellulose Nanofiber-Based Nanocomposite Films Reinforced with Zinc Oxide Nanorods and Grapefruit Seed Extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mattos, A.V.d.A.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with Chitosan-Based Edible Films for Mechanical/Biological Protection of Strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan Films Incorporated with Apricot (Prunus Armeniaca) Kernel Essential Oil as Active Food Packaging Material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Bajić, M.; Ročnik, T.; Oberlintner, A.; Scognamiglio, F.; Novak, U.; Likozar, B. Natural Plant Extracts as Active Components in Chitosan-Based Films: A Comparative Study. Food Packag. Shelf Life 2019, 21, 100365. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A. Chitosan Edible Films Enhanced with Pomegranate Peel Extract: Study on Physical, Biological, Thermal, and Barrier Properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Development of Active Edible Coating of Alginate and Aloe Vera Enriched with Frankincense Oil for Retarding the Senescence of Green Capsicums. LWT 2021, 145, 111341. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of Sodium Alginate-Based Films Incorporated with Thymol for Fresh-Cut Apple Packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of Thyme Oil–Alginate-Based Coating on Quality and Microbial Safety of Fresh-Cut Apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Wang, J.; Dong, M.; Jia, P.; Bu, T.; Wang, Q.; Wang, L. Sodium Alginate-Based Nanocomposite Films with Strong Antioxidant and Antibacterial Properties Enhanced by Polyphenol-Rich Kiwi Peel Extracts Bio-Reduced Silver Nanoparticles. Food Packag. Shelf Life 2021, 29, 100741. [Google Scholar] [CrossRef]
- Ravishankar, S.; Jaroni, D.; Zhu, L.; Olsen, C.; McHugh, T.; Friedman, M. Inactivation of Listeria Monocytogenes on Ham and Bologna Using Pectin-Based Apple, Carrot, and Hibiscus Edible Films Containing Carvacrol and Cinnamaldehyde. J. Food Sci. 2012, 77, M377–M382. [Google Scholar] [CrossRef]
- Romero, J.; Cruz, R.M.S.; Díez-Méndez, A.; Albertos, I. Valorization of Berries’ Agro-Industrial Waste in the Development of Biodegradable Pectin-Based Films for Fresh Salmon (Salmo salar) Shelf-Life Monitoring. Int. J. Mol. Sci. 2022, 23, 8970. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active Edible Films Based on Semi-Refined κ-Carrageenan: Antioxidant and Color Properties and Application in Chicken Breast Packaging. Food Packag. Shelf Life 2020, 24, 100476. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite Coating Based on Carrageenan and ZnO Nanoparticles to Maintain the Storage Quality of Mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-Based Biodegradable Active Packaging and Its Application to Salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA Nanocomposite Films Containing Bergamot Essential Oil, TiO2 Nanoparticles, and Ag Nanoparticles on Shelf Life of Mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active Packaging Based on PLA and Chitosan-Caseinate Enriched Rosemary Essential Oil Coating for Fresh Minced Chicken Breast Application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Ajesh Kumar, V.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of Biodegradable Materials in Food Packaging: A Review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Krithika, P.L.; Ratnamala, K.V. Modification of Starch: A Review of Various Techniques. Int. J. Res. Anal. Rev. 2019, 6, 32–45. [Google Scholar]
- Suri, S.; Singh, A. Modification of Starch by Novel and Traditional Ways: Influence on the Structure and Functional Properties. Sustain. Food Technol. 2023, 1, 348–362. [Google Scholar] [CrossRef]
- Peramune, D.L.; Ranaweera, S.; Marasinghe, W.N.; Manatunga, D.C.; Jayathunge, K.G.L.R.; Dassanayake, R.S. Polysaccharides-Based Food Packaging Films: An Overview. In Food Packaging-Safety, Management and Quality; Lai, W.-F., Ed.; Nova Science Publishers: New York, NY, USA, 2022; pp. 3–40. [Google Scholar] [CrossRef]
- Popyrina, T.N.; Demina, T.S.; Akopova, T.A. Polysaccharide-Based Films: From Packaging Materials to Functional Food. J. Food Sci. Technol. 2023, 60, 2736–2747. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bangar, S.P.; Thaku, N.; Trif, M. Recent Advancements in Smart Biogenic Packaging: Reshaping the Future of the Food Packaging Industry. Polymers 2022, 14, 829. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent Trends in Nanotechnology Applications of Bio-Based Packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]




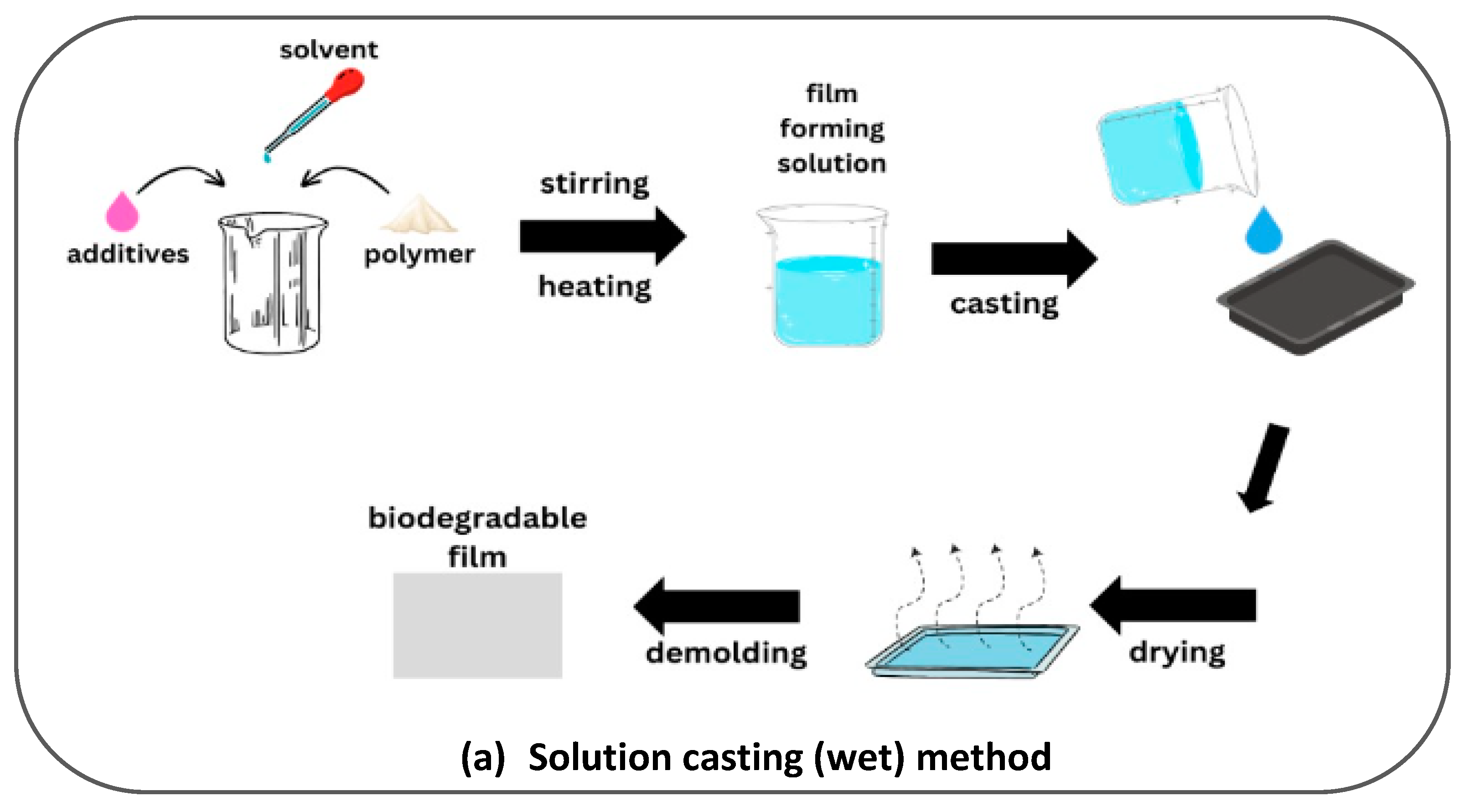
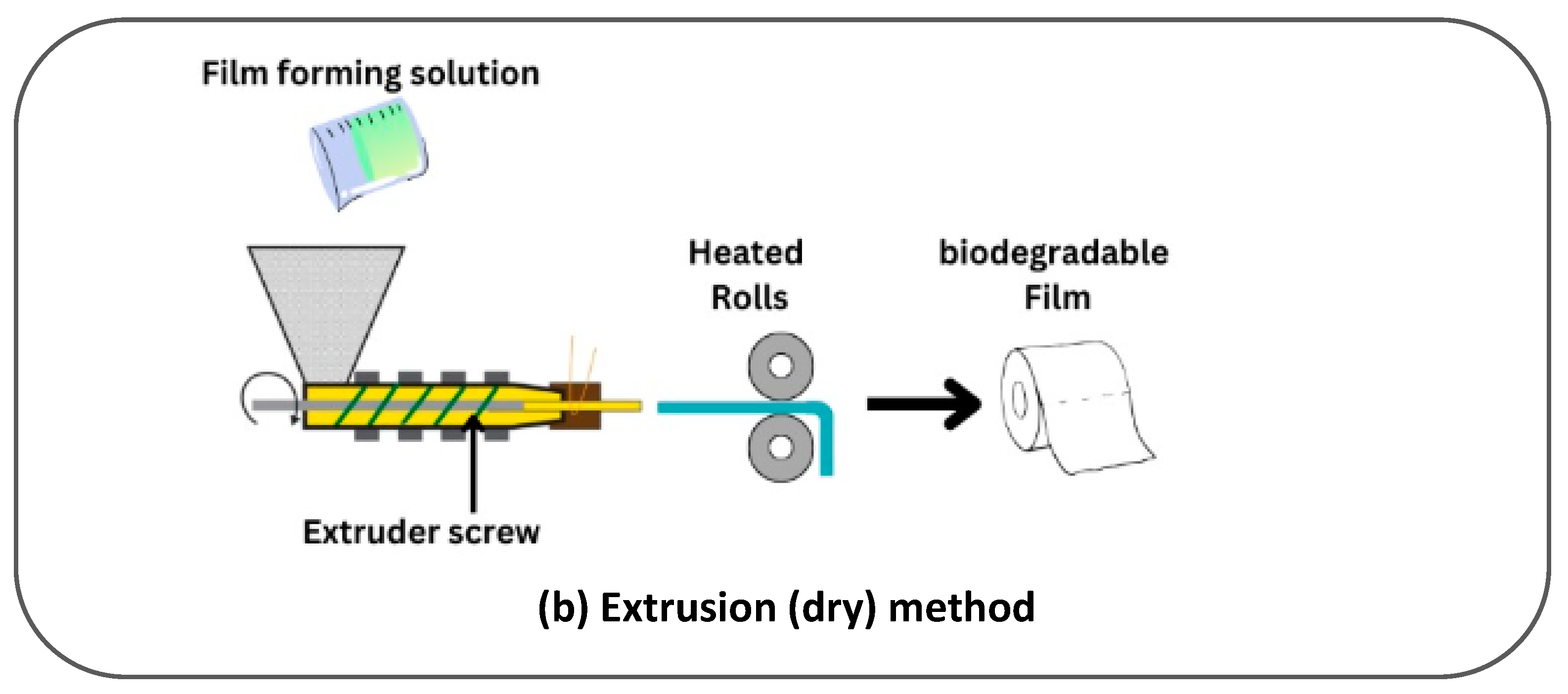
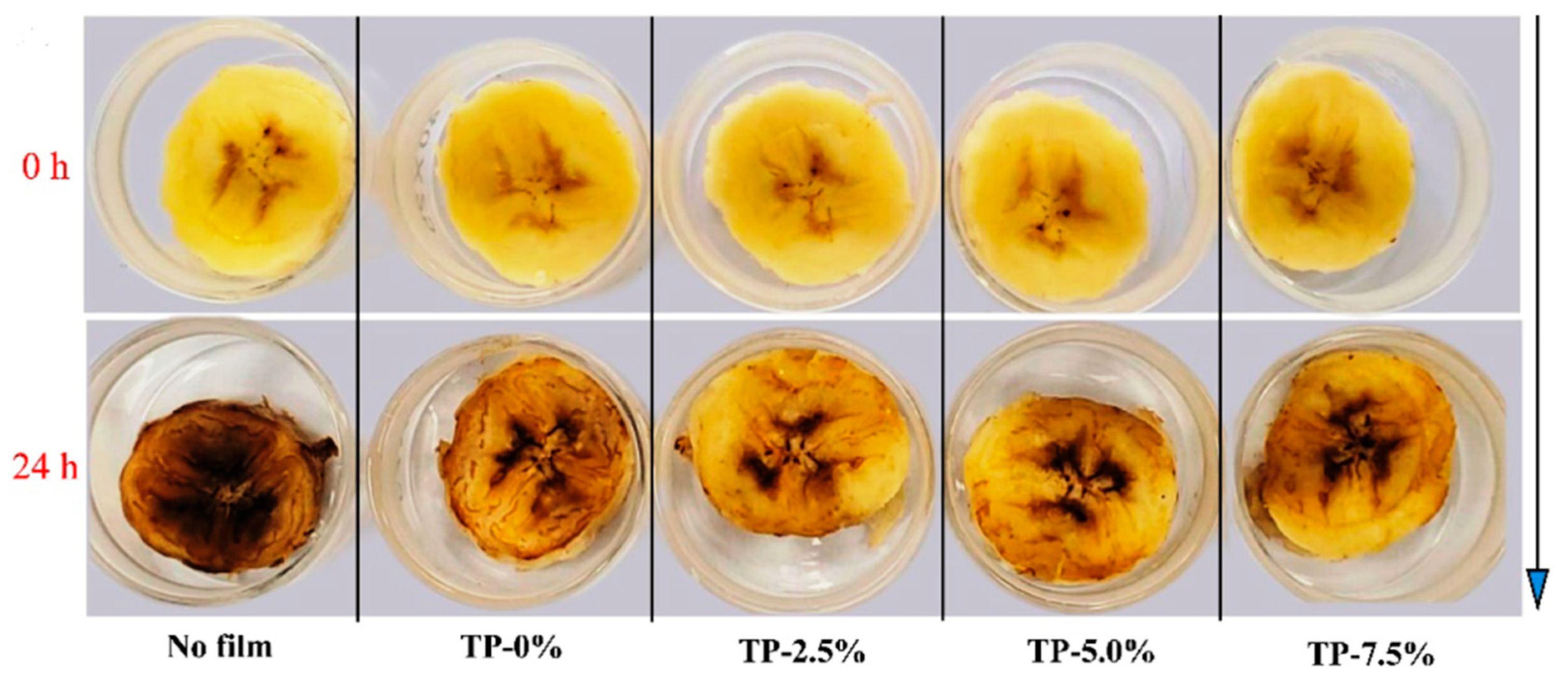



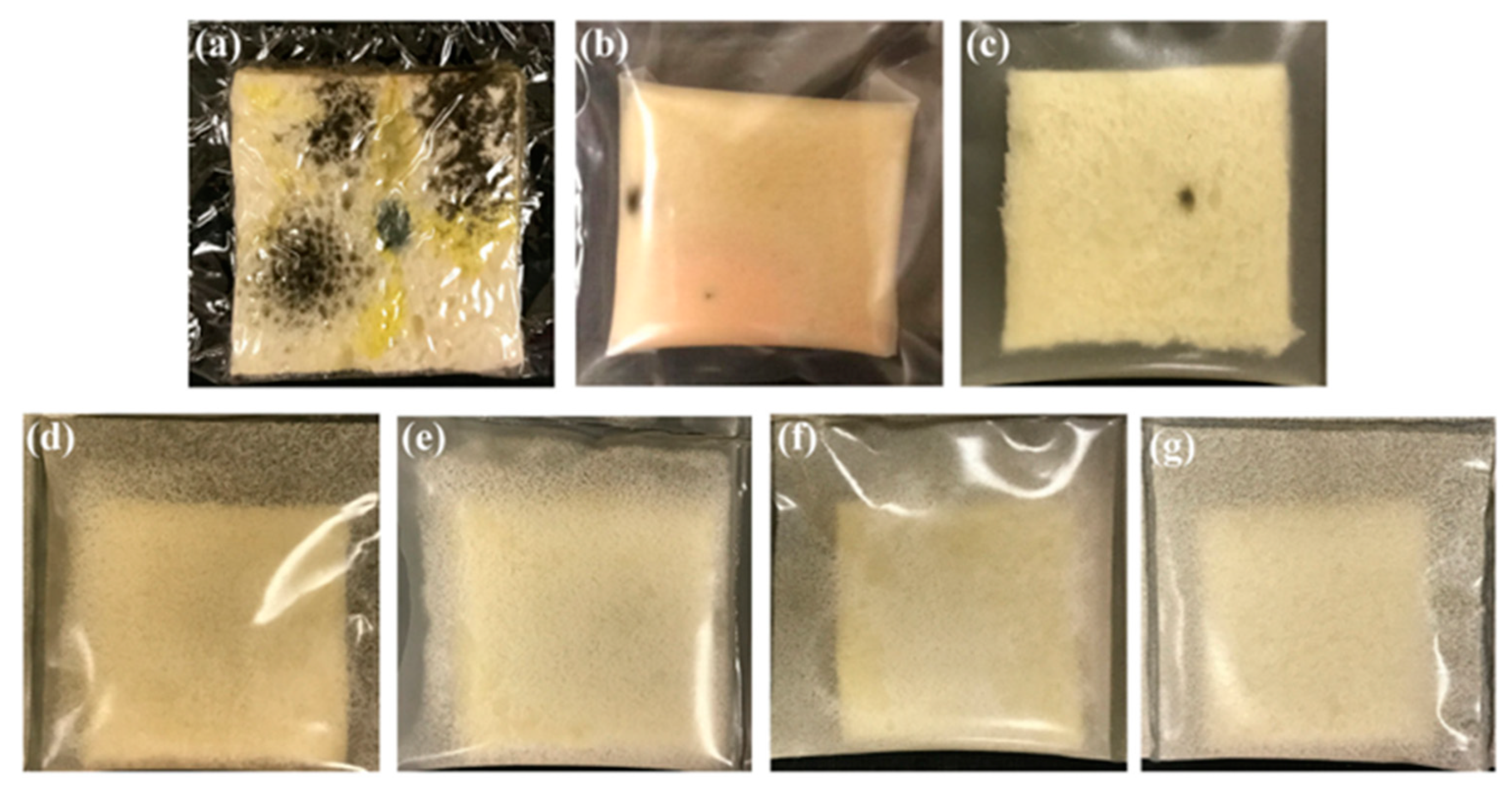







| Paper Type | Properties | Application | Ref. |
|---|---|---|---|
| Kraft paper | Type of coarse, high-strength, economical, porous, tear-resistant paper with a rough surface that can be coated or laminated | Beverage carriers, boxes, sacks, cartons, packages for flour, dried fruits, sugar | [15] |
| Greaseproof paper | Translucent, machine-finished, resistant to oils | Wraps cookies, confectionary, snack foods, highly oily foods | [20] |
| Parchment paper | Made from acid-treated pulp, not heat sealable, poor air and moisture barrier properties, high wet strength, greaseproof | Layer between pastry or meat slices, labels for fatty foods, cheese wrapping | [15] |
| Glassine paper | Glassy, smooth surface; transparent sheet; good grease and oil resistance; high density | Liner for baked goods, biscuits, cookies, cooking fats | [21] |
| Bleached paper | Soft and white, weaker compared to unbleached paper, expensive | Food labels, flour, sugar, fruits and vegetables | [22] |
| Paperboards | Thicker than paper, rigid, foldable; different types are available: whiteboard, liner board, food board, carton board, chipboard, corrugated board | Rigid boxes, beverage cartons, boxes for fruits and vegetables | [22,23] |
| Plastic | Properties | Application | Ref. |
|---|---|---|---|
| Polyethylene terephthalate (PET) | Good barrier to gases and moisture; resistant to heat, mineral oils, solvents, and acids; transparent; tough | Beverage and mineral water bottles, jars, tubes, trays | [32,33] |
| High-density polyethylene (HDPE) | Good barrier to solvents and moisture, high tensile strength, opaque, high-temperature capability | Beverage and milk bottles, shopping bags, ice cream containers | [30,34] |
| Polyvinyl chloride (PVC) | High resistance to chemicals, high strength, good oil barrier properties, good heat sealability | Bottles, food wraps | [35] |
| Low-density polyethylene (LDPE) | Good heat sealing; resistant to acid, oils, and bases; rigid; flexible; transparent | Bakery, frozen, fresh produce, and meat packing; soft squeeze bottles | [36] |
| Polypropylene (PP) | Good water vapor barrier, resistant to gases and odors, high strength, puncture resistance | Containers for ice cream, margarine, yogurt, snack packs, and biscuit packs | [34] |
| Polystyrene (PS) | Brittle, rigid, poor barrier to moisture and gases, good insulation properties | Cutlery, food insulation boxes, meat trays, egg containers | [37,38] |
| Packaging Material | Advantages | Disadvantages | References |
|---|---|---|---|
| Paper and paperboard | Low production cost, biodegradable, lightweight, flexible, printable, renewable, and recyclable | Combined with other packing materials, limited barrier properties, less durable, susceptible to damage, and unsustainable | [15,24] |
| Plastic | Versatile, lightweight, flexible, chemically resistant, low-cost, better physical properties, inert characteristics, easily processed, and recyclable | Non-biodegradable, causes environmental pollution, leaches many hazardous chemicals into foods, and dependent on fossil fuels | [22,41,44] |
| Glass | Durable, chemically inert, recyclable, transparent, good barrier properties, heat-resistant, and high-strength | Fragile, heavier than other materials, high production cost, and more energy consumption in production | [44,47] |
| Metal | Durable, excellent barrier properties, good physical protection, recyclable, good formability, good heat resistance, and versatility | High production cost, corrosive, and non-biodegradable | [48,50] |
| Packaging Material | Additives | Preparation Method | Food Sample | Properties of Packaging | Role as Food Packaging | Ref. |
|---|---|---|---|---|---|---|
| Potato starch-based film | Sodium Alginate Glycerol Essential oil | Casting method | Perishable food products | Water vapor transmission rate was 0.00254 g/m2/h for the films. | Shelf-life extension and inhibition of the spoilage organisms E. coli and B. cereus. | [199] |
| Foxtail millet starch-based film | Clove leaf oil Sorbitol | Casting method | Cheese | Possessed ultraviolet light barrier properties, tensile strength (6.78–4.00 MPa), and elongation at break (66.26–99.48%) when the essential oil content was increased. | Reduced lipid oxidation and microbial growth compared to LDPE. | [200] |
| Corn, wheat, and rice starch-based coating | Chitosan | Coating method | Walnut | Thickness of starch films ranged between 0.19 ± 0.01 and 0.21 ± 0.02 mm. Water vapor permeability of films ranged from 20.63 ± 0.27 to 23.96 ± 0.25 g mm/m2 d kPa. Tensile strength ranged between 0.27 ± 0.04 and 0.89 ± 0.2 MPa. | Shelf-life extension due to reduced effects of oxygen, moisture, and temperature. | [201] |
| Chinese yam starch-based film | Sorbitol Glycerol Eugenol | Casting method | Pork | Plasticizer enhanced the mechanical strength and barrier to moisture and oxygen. | Due to its superior barrier and antibacterial qualities. increased the shelf-life of pork beyond 50%. | [202] |
| Cellulose nanofiber-based film | Zinc oxide nanorods Grapefruit seed extract | Casting method | - | Highly transparent, nanocomposite films with an enhanced vapor barrier (ranged from 0.46 ± 0.01 to 0.56 ± 0.02 × 10−9 g·m/m2·Pa·s) and UV blocking qualities. | Exhibited antimicrobial activity against food-borne pathogens and good antioxidant activity. | [203] |
| Chitosan-based coating | Glycerol | Coating method | Strawberry | - | Excellent antibacterial and antifungal activity for one week and maintained the appearance of strawberries. | [204] |
| Chitosan-based film | Apricot kernel essential oil | Casting method | Sliced bread | With addition of essential oil, water vapor transmission rate was decreased from 1394 ± 47 to 821 ± 31 g m−2 d−1 and tensile strength increased from 9.45 ± 0.53 to 19.36 ± 1.06 MPa. | Enhanced the shelf-life of bread, with antioxidant and antimicrobial activity against E. coli, B. subtilis, and fungal growth. | [205] |
| Chitosan-based films | Plant extracts obtained from oak, hop, and brown algae | Casting method | - | Blended films showed increasing moisture content (21.5–28.3%), total soluble matter (23.8–28.9%), and elongation at break (14.0–31.0%) for oak and algal extract-containing films but decreasing tensile strength (12.7 MPa–5.5 MPa) and Young’s modulus (230.8 MPa–19.4 MPa) | - | [206] |
| Chitosan-based films | Pomegranate peel extract Glycerol | Casting method | Fruits and vegetables | Thickness (0.142–0.159 mm), tensile strength (32.45–35.23 MPa), opacity (0.039–0.061%), water barrier effect (1.32–1.60 g·mm/m2), and gas barrier properties (93.81–103.45 meq/kg) of the films increased with increasing volume of pomegranate peel extract. | Extended storage life and improved quality. | [207] |
| Alginate-based films | Glycerol Aloe vera Frankincense oil | Casting method | Green capsicum | Mechanical properties and thermal stability were increased in the presence of aloe vera and frankincense oil. Water vapor permeability was decreased in the film containing aloe vera and oil from 21.53 ± 1.43 g mm/m2 day kPa for alginate to 8.18 ± 0.24 g mm/m2 day kPa. | Senescence retardation and resistance to the mass loss of green capsicums. | [208] |
| Alginate-based film | Glycerol Thymol | Two-stage cross- linking method | Fresh-cut apple | In comparison to sodium alginate films without thymol, thymol/sodium alginate composite films were shown to have poor water vapor permeability, water solubility, and swelling ratios but good tensile strength, elongation at break, and UV–vis light blocking capabilities. | Inhibited the growth of Staphylococcus aureus and E. coli and maintained apple weight, color, and appearance. | [209] |
| Alginate-based coating | Glycerol Thyme oil | Dipped method | Fresh-cut apple | - | Prevented bacteria growth, respiration, weight loss, and browning reaction while preserving firmness. | [210] |
| Alginate-based film | Kiwi peel extract Silver nanoparticles | Casting method | Cherry | Films exhibited high UV barrier qualities, water vapor resistance, and tensile strength. | Increased cherries’ shelf-life by preventing moisture loss and protecting against microbial deterioration with strong antimicrobial and antioxidant properties. | [211] |
| Pectin-based film | Carvacrol Cinnamaldehyde | Casting method | Ham and bologna | Thickness of the films varied: apple films, from 0.128 to 0.135 mm; carrot films, from 0.041 to 0.049 mm; and hibiscus films, from 0.049 to 0.056 mm. | Improved microbial food safety by reducing the L. monocytogenes population with essential oil. | [212] |
| Pectin-based film | Glycerol Berry extract | Casting method | Salmon fillets | With the addition of berry extract, the thickness of the films was increased from 0.128 mm to 0.248 mm. | Improved shelf-life due to antioxidant and barrier properties. | [213] |
| Carrageenan-based film | Water extract of germinated fenugreek seeds Sorbitol | Casting method | Chicken breast | - | Improved the shelf-life of meat by controlling the growth of microorganisms on the surface of chicken breast. | [214] |
| Carrageenan-based film | ZnO nanoparticles Glycerol | Dipping method | Mango | Water vapor transmission rate of the film ranged from 65.88 ± 1.55 to 59.94 ± 0.87 g m−2 24 h−1, tensile strength ranged from 84.83 ± 4.67 to 121.53 ± 6.57 MPa, and elongation ranged from 60.94 ± 6.03 to 65.91 ± 2.49% with the addition of ZnO. | Maintained firmness and delayed the discoloration and decay of mango. | [215] |
| PLA-PHB based films | Glycerol Cinnamaldehyde | Casting method | Salmon | PLA-PHB based film showed better tensile strength and excellent oxygen permeability rate compared to ethylene vinyl alcohol copolymer-based based film. Ethylene vinyl alcohol copolymer-based films had reduced water vapor transmission rates compared to PLA-PHB-based films. | Reduced the total bacterial count of the sample. | [216] |
| PLA-based film | Bergamot essential oils Nano-TiO2 Nano-Ag | Casting method | Mango | - | PLA nanocomposite films effectively extended the postharvest life and delayed the loss of mango firmness during the entire storage period. | [217] |
| PLA- and chitosan–caseinate-based film | Rosemary essential oil | Casting method | Fresh minced chicken breast | Elastic modulus of films ranged from 1133 ± 136 MPa for control sample to 2073 ± 89 MPa for chitosan- and oil-incorporated film. The tensile strength of the control film was 93 ± 9 MPa, whereas the value of the chitosan, caseinate, and essential oil-incorporated film was 160 ± 28 MPa. | Provided antioxidant effects and improved the shelf-life of fresh meat products. | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasinghe, W.N.; Jayathunge, K.G.L.R.; Dassanayake, R.S.; Liyanage, R.; Bandara, P.C.; Rajapaksha, S.M.; Gunathilake, C. Structure, Properties, and Recent Developments in Polysaccharide- and Aliphatic Polyester-Based Packaging—A Review. J. Compos. Sci. 2024, 8, 114. https://doi.org/10.3390/jcs8030114
Marasinghe WN, Jayathunge KGLR, Dassanayake RS, Liyanage R, Bandara PC, Rajapaksha SM, Gunathilake C. Structure, Properties, and Recent Developments in Polysaccharide- and Aliphatic Polyester-Based Packaging—A Review. Journal of Composites Science. 2024; 8(3):114. https://doi.org/10.3390/jcs8030114
Chicago/Turabian StyleMarasinghe, Wasana N., K. G. L. R. Jayathunge, Rohan S. Dassanayake, Rumesh Liyanage, Pasan C. Bandara, Suranga M. Rajapaksha, and Chamila Gunathilake. 2024. "Structure, Properties, and Recent Developments in Polysaccharide- and Aliphatic Polyester-Based Packaging—A Review" Journal of Composites Science 8, no. 3: 114. https://doi.org/10.3390/jcs8030114
APA StyleMarasinghe, W. N., Jayathunge, K. G. L. R., Dassanayake, R. S., Liyanage, R., Bandara, P. C., Rajapaksha, S. M., & Gunathilake, C. (2024). Structure, Properties, and Recent Developments in Polysaccharide- and Aliphatic Polyester-Based Packaging—A Review. Journal of Composites Science, 8(3), 114. https://doi.org/10.3390/jcs8030114








