Polymer Composites of Low-Density Polyethylene (LDPE) with Elongated Hematite (α-Fe2O3) Particles of Different Shapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hematite Particles
2.2. Preparation of LDPE/Hematite Composites
2.3. Characterization
3. Results and Discussion
3.1. Properties of Hematite Particles
3.2. FT-IR Spectroscopy
3.3. Mechanical Properties
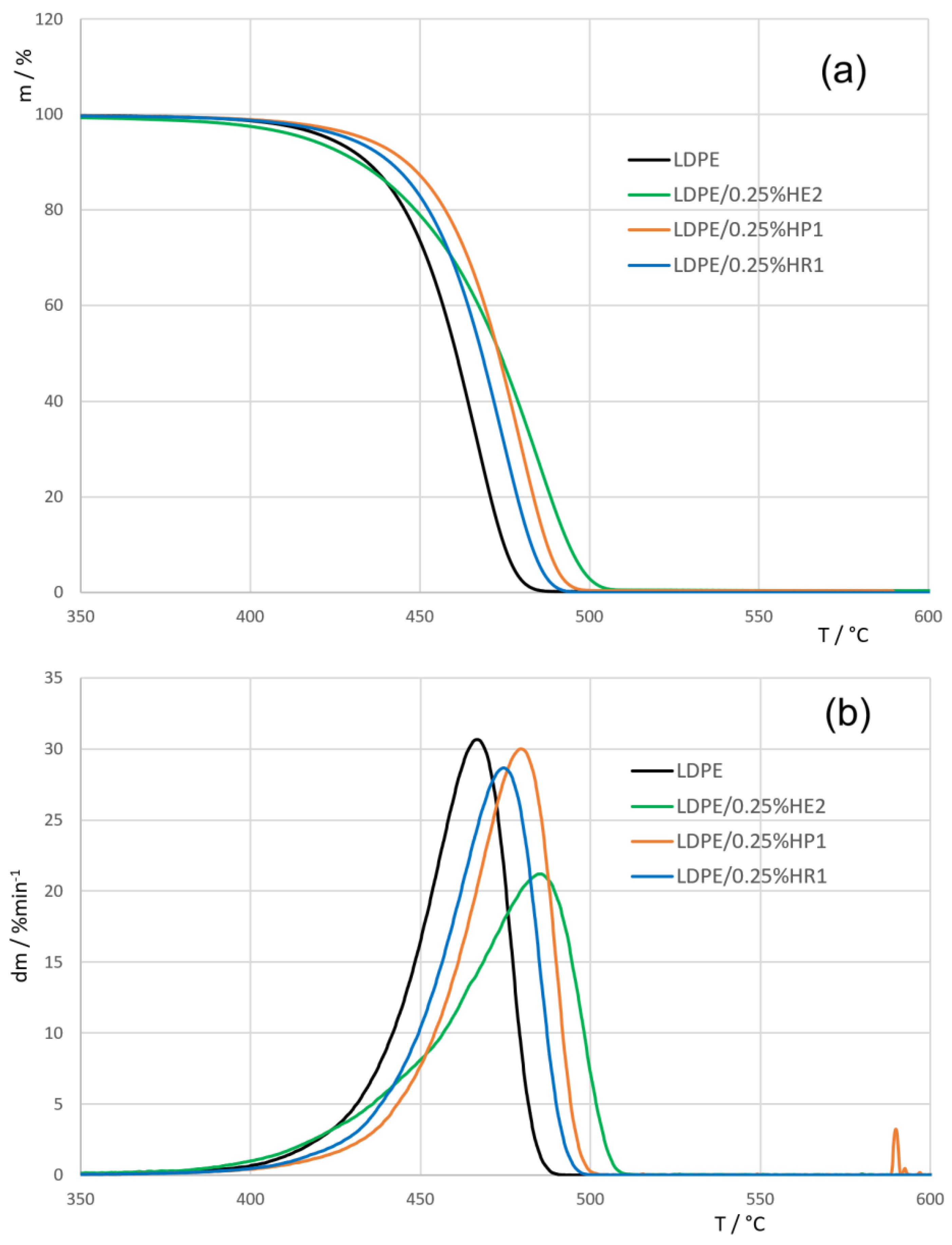
3.4. Thermogravimetric Analysis (TGA)
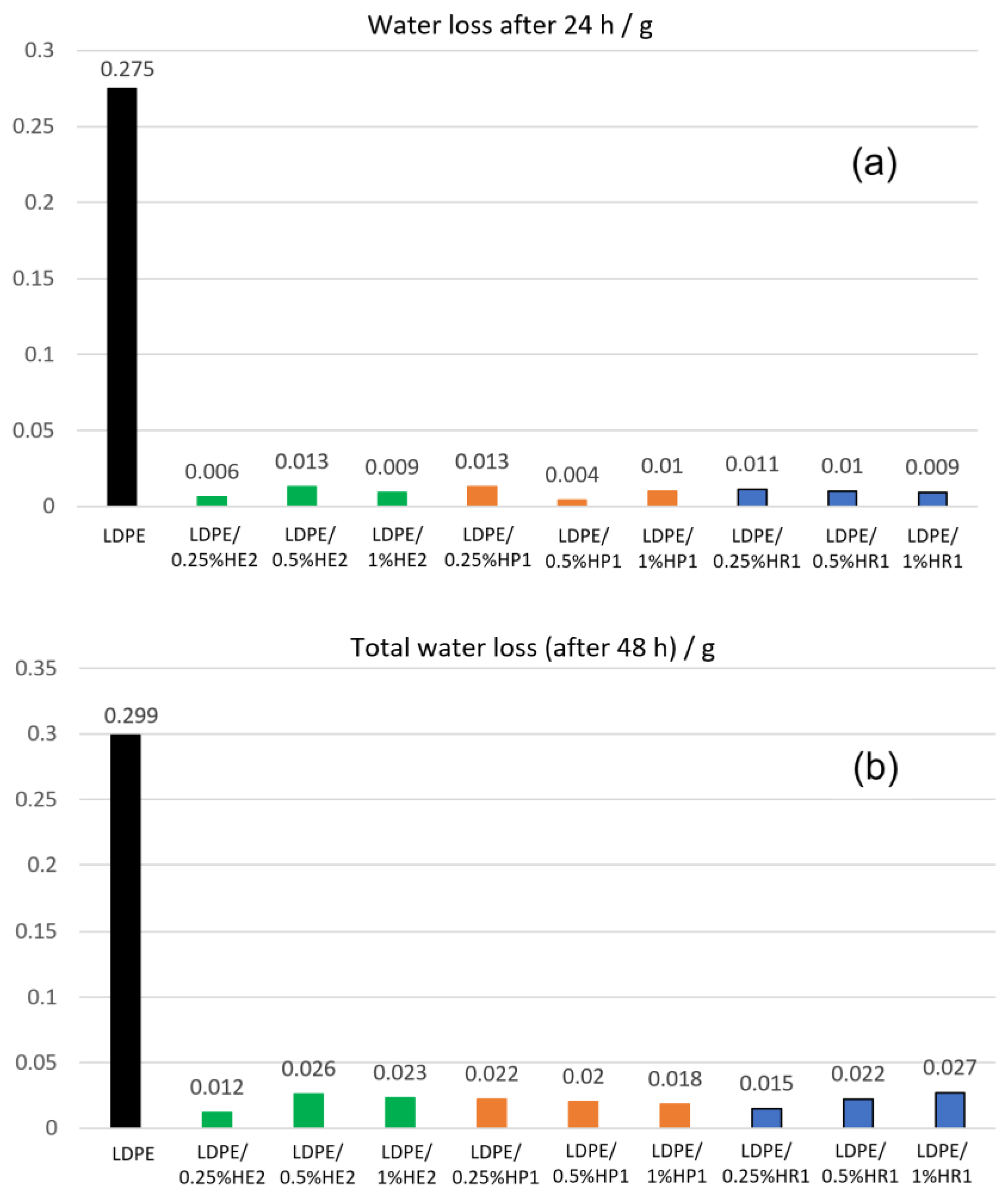
3.5. Barrier Properties
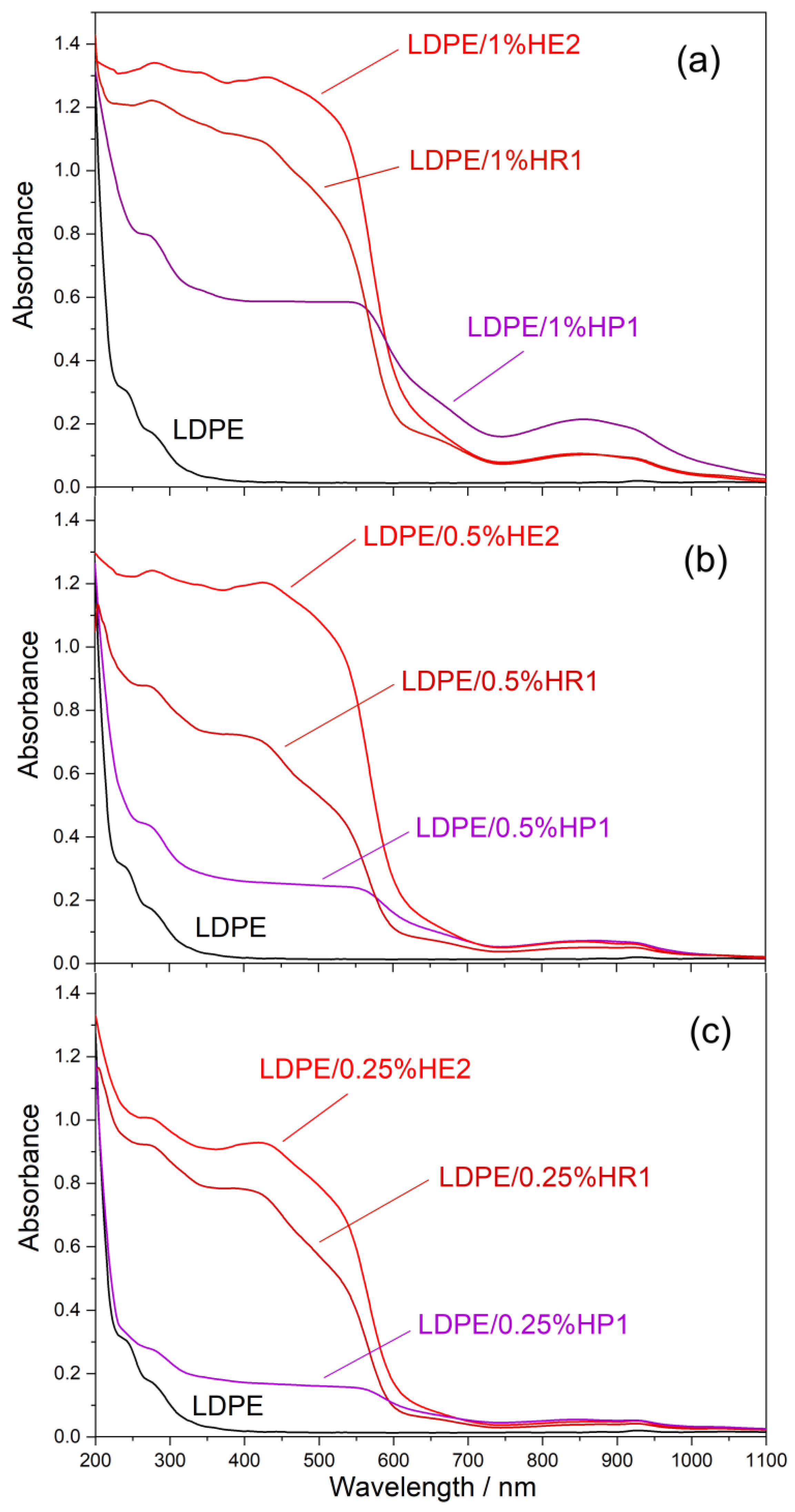
3.6. UV-Vis-NIR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerstle, F.P. Composites. In Encyclopedia of Polymer Science and Technology, 2nd ed.; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; J. Wiley & Sons: New York, NY, USA, 1985; Volume 6, pp. 776–820. [Google Scholar]
- Rothon, R.N. (Ed.) Particulate-Filled Polymer Composites; Rapra Technology Limited: Shawbury, UK, 2003. [Google Scholar]
- Rothon, R. (Ed.) Fillers for Polymer Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Siva Prasanna, S.R.V.; Balaji, K.; Pandey, S.; Rana, S. Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites, 1st ed.; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. [Google Scholar]
- Yadav, R.; Singh, M.; Shekhawat, D.; Lee, S.-Y.; Park, S.-J. The role of fillers to enhance the mechanical, thermal, and wear characteristics of polymer composite materials: A review. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107775. [Google Scholar] [CrossRef]
- Sahu, S.K.; Boggarapu, V.; Sreekanth, P.S.R. Improvements in the mechanical and thermal characteristics of polymer matrix composites reinforced with various nanofillers: A brief review. Mater. Today Proc. 2023, in press. [CrossRef]
- Kausar, A. Polymeric materials filled with hematite nanoparticle: Current state and prospective application. Polym. Plast. Technol. Mater. 2020, 59, 323–338. [Google Scholar] [CrossRef]
- Djoković, V.; Nedeljković, J.M. Stress relaxation in hematite nanoparticles-polystyrene composites. Macromol. Rapid Commun. 2000, 21, 994–997. [Google Scholar] [CrossRef]
- Marinović-Cincović, M.; Šaponjić, Z.V.; Djoković, V.; Milonjić, S.K.; Nedeljković, J.M. The influence of hematite nano-crystals on the thermal stability of polystyrene. Polym. Degrad. Stab. 2006, 91, 313–316. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Myung, N.V.; Parkin, G.F.; Cwiertny, D.M. Performance comparison of hematite (α-Fe2O3)-polymer composite and core-shell nanofibers as point-of-use filtration platforms for metal sequestration. Water Res. 2019, 148, 492–503. [Google Scholar] [CrossRef]
- Mensah, E.E.; Abbas, Z.; Azis, R.S.; Ibrahim, N.A.; Khamis, A.M.; Abdalhadi, D.M. Complex permittivity and power loss characteristics of α-Fe2O3/polycaprolactone (PCL) nanocomposites: Effect of recycled α-Fe2O3 nanofiller. Heliyon 2020, 6, e05595. [Google Scholar] [CrossRef] [PubMed]
- Bora, M.A.; Adhav, P.B.; Diwate, B.B.; Pawar, D.S.; Dallavalle, S.; Chabukswar, V.V. Room temperature operating sensitive and reproducible ammonia sensor based on PANI/hematite nanocomposite. Polym. Plast. Technol. Mater. 2019, 58, 1545–1555. [Google Scholar] [CrossRef]
- Matysiak, W.; Tański, T.; Zaborowska, M. Electrospinning process and characterization of PVP/hematite nanofibers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 461, 012050. [Google Scholar] [CrossRef]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. A study on the corrosion inhibition properties of silane-modified Fe2O3 nanoparticle on mild steel and its effect on the anticorrosion properties of the polyurethane coating. J. Coat. Technol. Res. 2015, 12, 277–292. [Google Scholar] [CrossRef]
- Peršić, A.; Popov, N.; Kratofil Krehula, L.; Krehula, S. The Influence of Different Hematite (α-Fe2O3) Particles on the Thermal, Optical, Mechanical, and Barrier Properties of LDPE/Hematite Composites. Materials 2023, 16, 706. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Abel, P.R.; Heller, A.; Mullins, C.B. α-Fe2O3 Nanorods as Anode Material for Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2885–2891. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chang, Z.; Sun, X.; Li, Y. Crystal plane effect of Fe2O3 with various morphologies on CO catalytic oxidation. Catal. Commun. 2011, 12, 530–534. [Google Scholar] [CrossRef]
- Zhou, X.; Lan, J.; Liu, G.; Deng, K.; Yang, Y.; Nie, G.; Yu, J.; Zhi, L. Facet-Mediated Photodegradation of Organic Dye over Hematite Architectures by Visible Light. Angew. Chem. Int. Ed. 2012, 51, 178–182. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Composto, R.J. Functional Polymer Nanocomposites Enhanced by Nanorods. Macromolecules 2014, 47, 875–887. [Google Scholar] [CrossRef]
- Li, Z.; Lai, X.; Wang, H.; Mao, D.; Xing, C.; Wang, D. Direct hydrothermal synthesis of single-crystalline hematite nanorods assisted by 1,2-propanediamine. Nanotechnology 2009, 20, 245603. [Google Scholar] [CrossRef]
- Sugimoto, T.; Khan, M.M.; Muramatsu, A. Preparation of monodisperse peanut-type α-Fe2O3 particles from condensed ferric hydroxide gel. Colloids Surf. A Physicochem. Eng. 1993, 70, 167–169. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures; John Wiley & Sons: New York, NY, USA, 1974; p. 687. [Google Scholar]
- Gulmine, J.V.; Janissek, J.V.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Chao, Y.; Jianming, Z.; Deyan, S.; Shouke, Y. Structure characterization of melt drawn polyethylene ultrathin films. Chin. Sci. Bull. 2006, 51, 2844–2850. [Google Scholar]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Fernando, S.S.; Christensen, P.A.; Egerton, T.A.; White, J.R. Carbon dioxide evolution and carbonyl group development during photodegradation of polyethylene and polypropylene. Polym. Degrad. Stab. 2007, 92, 2163–2172. [Google Scholar] [CrossRef]
- Dudić, D.; Marinović-Cincović, M.; Nedeljković, J.M.; Djokovic, V. Electrical properties of a composite comprising epoxy resin and α-hematite nanorods. Polymer 2008, 49, 4000–4008. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Bogdanović, G.; Kovač, T.S.; Džunuzović, E.S.; Špírková, M.; Ahrenkiel, P.S.; Nedeljković, J.M. Influence of hematite nanorods on the mechanical properties of epoxy resin. J. Serbian Chem. Soc. 2017, 82, 437–447. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Ma, Z.; Quan, Q. Effect of Natural Nanostructured Rods and Platelets on Mechanical and Water Resistance Properties of Alginate-Based Nanocomposites. Front. Chem. 2018, 6, 635. [Google Scholar] [CrossRef] [PubMed]
- Caseri, W.R. Nanocomposites of polymers and inorganic particles: Preparation, structure and properties. Mater. Sci. Technol. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Pracella, M.; Rolla, L.; Chionna, D.; Galeski, A. Compatibilization and properties of poly(ethylene terephthalate)/polyethylene blends based on recycled materials. Macromol. Chem. Phys. 2002, 203, 1473–1485. [Google Scholar] [CrossRef]
- Kaya, G.G.; Deveci, H. Design and synthesis of metal oxide–polymer composites. In Renewable Polymers and Polymer-Metal Oxide Composites: Synthesis, Properties, and Applications, 1st ed.; Haider, S., Haider, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–128. [Google Scholar]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Hrnjak Murgić, Z.; Rešček, A.; Ptiček Siročić, A.; Kratofil Krehula, L.; Katančić, Z. Nanoparticles in Active Polymer Food Packaging; Smithers Pira Technology Ltd.: Shawbury, UK, 2015. [Google Scholar]
- Kausar, A. A review of high-performance polymer nanocomposites for packaging applications in electronics and food industries. J. Plast. Film Sheeting 2020, 36, 94–112. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- He, J.; Shao, W.; Zhang, L.; Deng, C.; Li, C. Crystallization behavior and UV-protection property of PET-ZnO nanocomposites prepared by in situ polymerization. J. Appl. Polym. Sci. 2009, 114, 1303–1311. [Google Scholar] [CrossRef]
- Truffault, L.; Choquenet, B.; Konstantinov, K.; Devers, T.; Couteau, C.; Coiffard, L.J.M. Synthesis of nano-hematite for possible use in sunscreens. J. Nanosci. Nanotechnol. 2011, 11, 2413–2420. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Ponnurangam, S.; Somasundaran, P. On the origin of an unusual dependence of (bio)chemical reactivity of ferric hydroxides on nanoparticle size. Phys. Chem. Chem. Phys. 2010, 12, 14045–14056. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Surya, R.; Filser, S.; Wimmer, A.; Weigl, F.; Fraga-García, P.; Berensmeier, S. Formation of iron oxide nanoparticles for the photooxidation of water: Alteration of finite size effects from ferrihydrite to hematite. Sci. Rep. 2017, 7, 12609. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Duan, X.; Ma, J.; Peng, P.; Kim, T.; Zheng, W. Hematite (α-Fe2O3) with Various Morphologies: Ionic Liquid-Assisted Synthesis, Formation Mechanism, and Properties. ACS Nano 2009, 3, 3749–3761. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Li, Y.; Hou, X.; Wang, Y.; Bai, R.; Zhao, J. Synthesis, optical and magnetic properties of α-Fe2O3 nanoparticles with various shapes. Mater. Lett. 2013, 99, 111–114. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, S.; Zhang, C.; Lian, J.; Liang, Y.; Yuan, W. Facile synthesis of hematite nanoparticles and nanocubes and their shape-dependent optical properties. New J. Chem. 2014, 38, 46–49. [Google Scholar] [CrossRef]
- Popov, N.; Krehula, S.; Ristić, M.; Kuzmann, E.; Homonnay, Z.; Bošković, M.; Stanković, D.; Kubuki, S.; Musić, S. Influence of Cr doping on the structural, magnetic, optical and photocatalytic properties of α-Fe2O3 nanorods. J. Phys. Chem. Solids 2021, 148, 109699. [Google Scholar] [CrossRef]
- Popov, N.; Bošković, M.; Perović, M.; Nemeth, Z.; Wang, J.; Kuang, Z.; Reissner, M.; Kuzmann, E.; Homonnay, Z.; Kubuki, S.; et al. Influence of low-spin Co3+ for high-spin Fe3+ substitution on the structural, magnetic, optical and catalytic properties of hematite (α-Fe2O3) nanorods. J. Phys. Chem. Solids 2021, 152, 109929. [Google Scholar] [CrossRef]
- Popov, N.; Bošković, M.; Perović, M.; Zadro, K.; Gilja, V.; Kratofil Krehula, L.; Robić, M.; Marciuš, M.; Ristić, M.; Musić, S.; et al. Effect of Ru3+ ions on the formation, structural, magnetic and optical properties of hematite (α-Fe2O3) nanorods. J. Magn. Magn. Mater. 2021, 538, 16831. [Google Scholar] [CrossRef]
- Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Marciuš, M.; Stanković, D.; Krehula, S. Influence of Sn doping on the structural, magnetic, optical and photocatalytic properties of hematite (α-Fe2O3) nanoparticles. J. Phys. Chem. Solids 2022, 161, 110372. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides-Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]

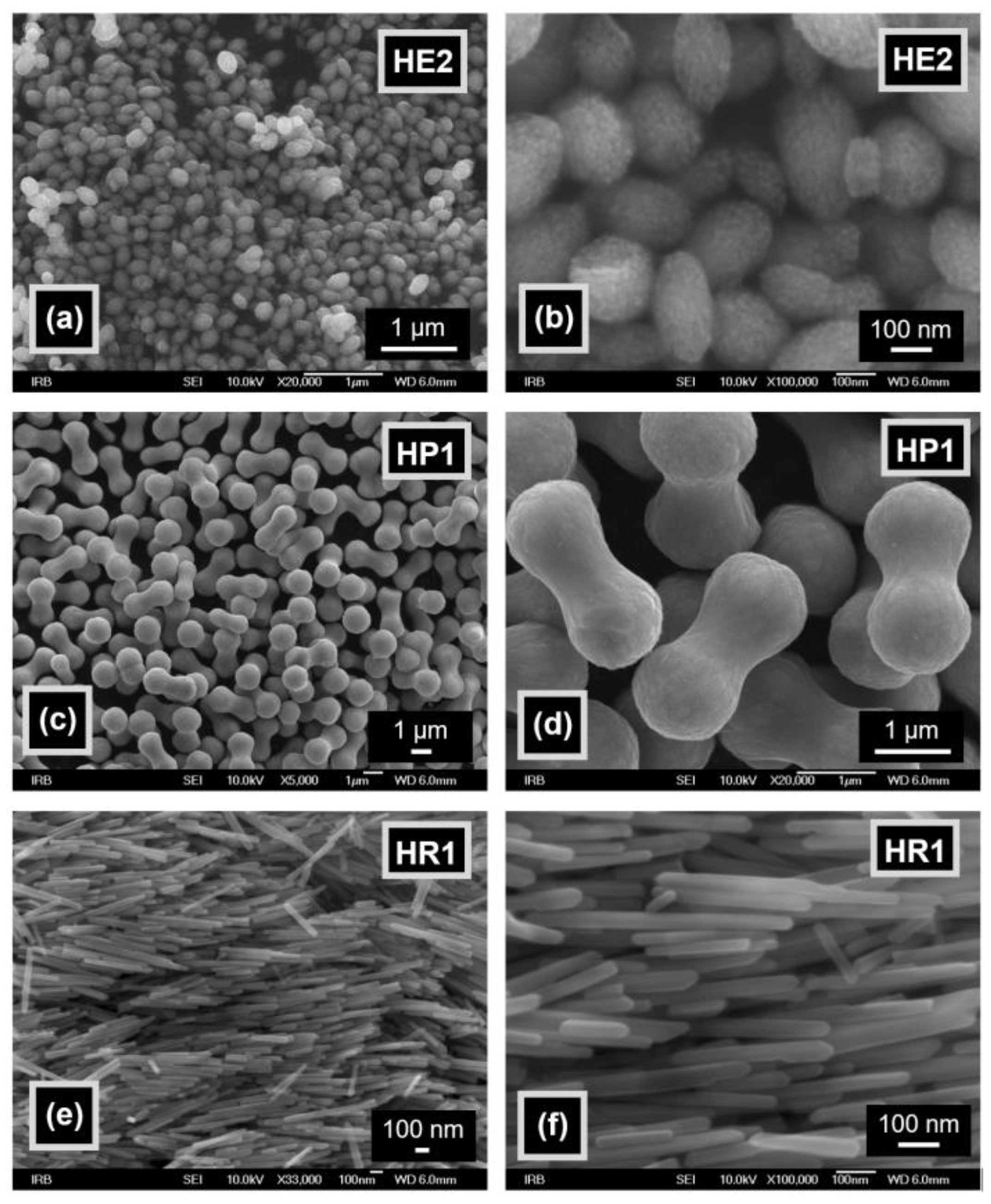





| Sample | LDPE (wt.%) | Hematite (wt.%) |
|---|---|---|
| LDPE | 100 | 0.00 |
| LDPE/0.25%HE2 | 99.75 | 0.25 |
| LDPE/0.5%HE2 | 99.50 | 0.50 |
| LDPE/1%HE2 | 99.00 | 1.00 |
| LDPE/0.25%HP1 | 99.75 | 0.25 |
| LDPE/0.5%HP1 | 99.50 | 0.50 |
| LDPE/1%HP1 | 99.00 | 1.00 |
| LDPE/0.25%HR1 | 99.75 | 0.25 |
| LDPE/0.5%HR1 | 99.50 | 0.50 |
| LDPE/1%HR1 | 99.00 | 1.00 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| LDPE | 6.98 | 230.5 |
| LDPE/0.25%HE2 | 8.56 | 437.84 |
| LDPE/0.5%HE2 | 9.58 | 443.97 |
| LDPE/1%HE2 | 7.96 | 435.90 |
| LDPE/0.25%HP1 | 8.92 | 461.29 |
| LDPE/0.5%HP1 | 9.66 | 461.12 |
| LDPE/1%HP1 | 9.72 | 459.15 |
| LDPE/0.25%HR1 | 9.28 | 461.56 |
| LDPE/0.5%HR1 | 7.31 | 145.01 |
| LDPE/1%HR1 | 8.05 | 171.33 |
| Sample | T95% (°C) | Tmax (°C) |
|---|---|---|
| LDPE | 422.60 | 467.07 |
| LDPE/0.25%HE2 | 416.23 | 486.07 |
| LDPE/0.5%HE2 | 440.95 | 490.29 |
| LDPE/1%HE2 | 438.83 | 483.01 |
| LDPE/0.25%HP1 | 433.54 | 481.04 |
| LDPE/0.5%HP1 | 434.99 | 479.67 |
| LDPE/1%HP1 | 432.58 | 482.85 |
| LDPE/0.25%HR1 | 428.93 | 475.92 |
| LDPE/0.5%HR1 | 428.45 | 474.43 |
| LDPE/1%HR1 | 429.60 | 474.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratofil Krehula, L.; Peršić, A.; Popov, N.; Krehula, S. Polymer Composites of Low-Density Polyethylene (LDPE) with Elongated Hematite (α-Fe2O3) Particles of Different Shapes. J. Compos. Sci. 2024, 8, 73. https://doi.org/10.3390/jcs8020073
Kratofil Krehula L, Peršić A, Popov N, Krehula S. Polymer Composites of Low-Density Polyethylene (LDPE) with Elongated Hematite (α-Fe2O3) Particles of Different Shapes. Journal of Composites Science. 2024; 8(2):73. https://doi.org/10.3390/jcs8020073
Chicago/Turabian StyleKratofil Krehula, Ljerka, Ana Peršić, Nina Popov, and Stjepko Krehula. 2024. "Polymer Composites of Low-Density Polyethylene (LDPE) with Elongated Hematite (α-Fe2O3) Particles of Different Shapes" Journal of Composites Science 8, no. 2: 73. https://doi.org/10.3390/jcs8020073
APA StyleKratofil Krehula, L., Peršić, A., Popov, N., & Krehula, S. (2024). Polymer Composites of Low-Density Polyethylene (LDPE) with Elongated Hematite (α-Fe2O3) Particles of Different Shapes. Journal of Composites Science, 8(2), 73. https://doi.org/10.3390/jcs8020073






