Abstract
This work presents the extrusion-based preparation of new biocomposites from two plant fibres (namely Cannabis sativa L. and Opuntia ficus-indica Mill.) that are added to two different polymers (an ethylene–octene elastomer and polylactic acid), which act as matrices. Structural and morphological characterization (using X-ray diffraction and field emission scanning electron microscopy) have been used to correlate the interactions between the biomass and the polymers employed with the efficiency of the proposed approach. It was found that Opuntia-based composites can be easily formed in a range of biomass/polymer ratios. However, the interaction between hemp and the matrix means that only specific ratios can form tightly bound composites. The present communication thus paves the way for more complex and comprehensive studies on the formulation of biocomposites containing these matrices.
1. Introduction
In view of the urgent need to reduce the environmental impact of non-biodegradable plastic production, the emergence of bioplastics and biocomposites is a significant development in the polymer industry [1,2,3]. These sustainable materials, when produced using techno-economically viable procedures, show great promise as a potential means to limit the current reliance on conventional, fossil fuel-derived plastics, while also boosting innovation in the field of materials science and engineering [4,5,6,7]. As part of this strategy, the loading of pure polymers with plant-derived fibres is currently seen as a promising approach to reducing the use of petroleum-based polymers [8,9,10,11].
Emerging mechanochemistry applications display many advantages, including the possibility to work in solvent-free or solvent-limited protocols, and high energy efficiency (up to 1000-fold reduction/improvement), while they also provide higher reaction yields than conventional transformations, even when performed at lower temperatures. Further emerging applications of mechanochemistry in a variety of fields can be found in the literature [12,13,14,15,16]. Indeed, its use is in accordance with the 12 principles of Green Chemistry, while the field was indicated by the IUPAC as one of ten world-changing innovations in 2019. In a dry milling process, mechanochemical activation produces high-energy microenvironments due to localized high pressure and frictional heat provided by kinetic energy. Short-term consequences, including hot spots, magma–plasma regions, the rapid induction of defects (point, linear and planar) and their propagation throughout the material, can take place within a few milliseconds following the impact of two solids at high speed. Interestingly, the imparted energy is distributed differently in different solids: those with high rigidity and those in which structural movement allows the energy to be distributed over a much larger distance. Chemical bonds can be broken and formed in the early stages, while longer term events that are associated with energy dissipation and the relaxation of the system occur later [17]. Natural resources such as hemp, flax and bamboo are abundant, cost-effective, lightweight and sustainable, providing a biodegradable substrate that can be loaded into composite materials [18,19,20]. Indeed, the high content of cellulose, hemicellulose and lignin in these vegetable materials offers numerous opportunities for the preparation of advanced natural-fibre-reinforced composites with specific properties such as sound absorption and thermal insulation. Of the vegetable materials that are abundantly available, co-products related to cannabis (Cannabis sativa L.) and opuntia (Opuntia ficus-indica Mill.) are among the most prevalent sources of agricultural fibre and show promising loading potential for the preparation of novel composites with similar, or even enhanced, mechanical and thermal properties as pure polymers. For instance, industrial hemp hurds, an abundant by-product of the Cannabis-related industry, contain around 40 to 48% cellulose, 18 to 24% hemicellulose and 21 to 24% lignin [21], whilst Opuntia ficus-indica cladodes and spines have been found to contain 21.6 to 47.9% cellulose and 1.2 to 3.6% lignin, respectively [22,23].
This study reports a simple mechanochemical approach for the preparation of new materials from two different polymers and plant-derived fibres from Cannabis sativa L. and Opuntia Ficus-indica, investigating the effect of the loading of the fibres on the morphological and structural characterization of the prepared composites.
2. Materials and Methods
Micronized hemp hurds were provided by Millasensi Srl (Bari, Italy) while Opuntia cladodes were provided by O.P. La Deliziosa (Biancavilla, CT, Italy). Polylactic acid (PLA, melt flow index 23.5 g/min, density 1.08 g/cm3, molecular weight approximately 130,000 g/mol) and an ethylene–octene elastomer (QUEO 0203, Borealis, Wien, Austria) were provided by GIMAC (Castronno, VA, Italy). Calcium carbonate (CaCO3) was purchased from Merck (Merck KGaA, Darmstadt, Germany). Prior to its usage, PLA was oven-dried to constant weight at 80 °C in a laboratory oven, while hemp hurds were dried at 45 °C until 0.5% moisture content was reached and then stored in a tightly sealed container until use.
Biomass–polymer blends were prepared via extrusion, using a TR12/20GM (GIMAC, Castronno, VA, Italy), which is a single screw extruder with 4 independent temperature sections. Two fibre loadings, 22 and 32 wt%, were employed to produce the blends. These loadings were chosen after preliminary investigations carried out in our laboratory (unpublished data).
Additionally, CaCO3 was incorporated into the compositions at a concentration of 7 wt%, as a reinforcing material, with the objective of enhancing the materials’ compactness. The working temperatures were based on the choice to produce a solid material, and were different for the two examined polymers, as shown in Table 1.

Table 1.
Temperatures for each extruder section.
In order to prepare the biocomposites, a mixture of the polymer, biomass and CaCO3 was weighed and mixed manually, and the mix was then poured into the loading chamber. The extruder was operated at 20 rpm. The resulting samples were then cut into small pieces and extruded a second time, to improve homogenization.
X-ray diffraction (XRD) patterns were collected using a PW3050/60 X′ Pert PRO MPD diffractometer, from PANalytical (Mumbai, India), working in Bragg–Brentano geometry. The high-powered ceramic tube PW3373/10 LFF was used as the source and was equipped with a Cu anode (Cu Kα1 radiation λ = 1.5406 Å) and a Ni filter to attenuate Kβ. Scattered photons were collected by a real-time multiple strip (RTMS) X′ celerator detector. Data were collected in the 3° ≤ 2θ ≤ 50° angular range, with 0.02° 2θ steps. The samples were examined in their as-received form and posed in a spinning sample holder to minimize preferred orientations. The XRD patterns of the polymers were acquired as received and after extrusion, as references. The crystallite size of CaCO3 was determined by applying the Scherrer equation, giving a crystallite size of 56.4 nm.
Field emission scanning electron microscopy (FESEM) measurements were carried out using a TESCAN S9000G FESEM 3010 microscope (30 kV) (Brno, Czech Republic), equipped with a high-brightness Schottky emitter, and Energy-Dispersive X-ray Spectroscopy (EDS) analysis, which was provided by an Ultim Max Silicon Drift Detector (SDD, Oxford, Abingdon-on-Thames, UK). The samples were deposited on a stub that was coated with a conducting adhesive and inserted into the chamber in a fully motorized procedure. The samples were submitted to metallization with Cr (ca. 5 nm) to avoid any charging effects (Emitech K575X sputter coater (Quorum, East Sussex, UK)). Images were acquired using both secondary electron (SE) and backscattered electron (BSE) detectors. All sample surfaces, including the sides and the most representative cross-sections of the extruded samples, were analyzed. Images of the composites’ cross-sections were acquired after breaking the samples previously cooled by liquid nitrogen.
3. Results and Discussion
In order to investigate the interactions between the biomass and the polymer components, a detailed structural and morphological characterization of the produced blends was performed by XRD and FESEM. The XRD patterns of 22 wt% (light blue line) and 32 wt % hemp-loaded blends (dark blue line) are shown along with the hemp pattern (dark green line) in Figure 1A. The XRD pattern of unprocessed hemp exhibits a prominent broad and structured peak in the 15–25° 2θ range, which is consistent with both the cellulose (JCPDS Card No. 00-003-0192) and the amorphous lignin components. Nevertheless, the presence of several peaks in the 15–48° 2θ range (15.3°, 21.9°, 24.8°, 27.0°, 28.5°, 29.7°, 30.5°, 36.3°, 38.5°, 40.1° and 47.5°) indicates that hemp exhibits some degree of structural order and is not fully amorphous. The XRD patterns of both blends show two hemp-related peaks at about 30° and 37° 2θ. In addition, peaks in the 20–25° 2θ range, which are typical of the elastomer, were also clearly observed for both blends, although they were slightly shifted, most likely due to the mechanochemical synthesis. As reported in Figure 1A’, extrusion did not modify the polymer structure (the pink line for the as-received elastomer vs. the violet line for the extruded elastomer). Moreover, some peaks at higher 2θ were ascribed to the presence of nanocrystalline CaCO3 particles in both blends (see Figure S1 in the Supporting Information for the bare CaCO3 XRD pattern). Overall, this comparison highlights that no relevant structural change appears to occur upon mechanochemical extrusion, and also indicates that there are weak interactions between the hemp fibres and the polymer. It can be hypothesized that the presence of peaks related to both the biomass and elastomer, with intensity increasing with the amount of hemp, is an indication of lower dispersion between the two components, since both retain some kind of ordered structure inside the blend. This is further supported by the analysis of the FESEM representative images of the cross-sections of elastomer–hemp blends with 22 and 32 wt% hemp, which are shown in Figure 1B and 1C, respectively. Indeed, hemp appears in the form of well-defined fibres embedded in the polymeric matrix (indicated by arrows).
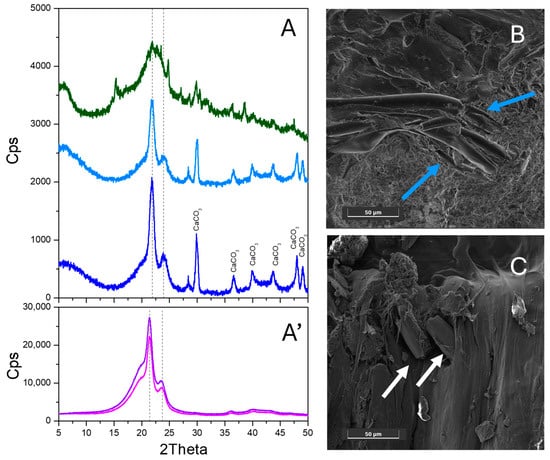
Figure 1.
(A) XRD patterns of the elastomer loaded with 22 wt% (light blue line) and 32 wt % hemp (dark blue line) along with the bare hemp XRD pattern (dark green line). The peaks related to CaCO3 in the rhombohedral calcite phase (00-002-0623) are indicated. (A’) XRD patterns of the as-received elastomer (pink line) and of the extruded elastomer (violet line). The positions of the main peaks of the elastomer are indicated using dashed lines. FESEM representative images of the cross-sections of the hemp–elastomer biocomposite obtained from the elastomer with 22 wt% (B) and 32 wt% hemp loading (C). Images taken with the SE detector at 5 keV. Instrumental magnification 1350×.
Biocomposites using the same elastomer, but with 22 and 32 wt% Opuntia ficus-indica cladodes, were also prepared and investigated. Figure 2A–C show that the biomass fibres (for the bare fibres’ FESEM image, see Figure S2 in the Supplementary Information) are clearly embedded within the polymeric matrix in a quite similar way to the elastomer–hemp blends, despite the change in loading. In particular, Opuntia stomata can be observed in Figure 2A, while the presence of fibres within the elastomer is evident in Figure 2B,C. The reference XRD pattern of Opuntia ficus-indica biomass is shown in Figure 2D (dark yellow line). A broad peak in the 3.5–9° 2θ range, due to the amorphous fraction of the material, and a second broad peak in the 16–28° 2θ range, typical of either cellulose (00-003-0192) or amorphous lignin [24], underline structural order in the biomass. Moreover, the XRD pattern is characterized by the presence of sharp peaks that can be attributed to the presence of oxalates inside the matrix, especially calcium oxalate (00-003-0090) and, probably, its hydrate form (00-002-0140) [25,26]. These features seem to indicate that the Opuntia biomass (dark yellow line in Figure 2D) possesses higher crystallinity than hemp (dark green line in Figure 1A). Interestingly, the XRD patterns reported in Figure 2D show differences in the presence and intensity of the peaks related to the elastomer. In particular, the characteristic elastomer peaks were not observed at all in the 22 wt%-loaded biocomposite (light blue line), whereas these peaks are detected at 32 wt% loading (dark blue line) with a much lower intensity than observed in both the 22 wt% and 32 wt% hemp-based blends (dark blue and light blue lines, respectively, in Figure 1A). Both biocomposites display a peak at ca. 28° 2θ, which is possibly associated with the partial formation of the blend, since this peak was not observed in the XRD patterns of bare Opuntia (dark yellow line) and the elastomer before and after extrusion (pink and violet lines in Figure 1A). Finally, the fact that the peaks related to both the biomass and elastomer are only present at the higher amount of Opuntia of 32 wt% suggests that the two components are less effectively dispersed here, and that they still retain their structure inside the blend. The absence of these peaks at 22 wt% loading indicates that the dispersion is more effective in this blend. Indeed, only a broad peak at 5–7° 2θ, related to an amorphous cellulosic component, was detected.
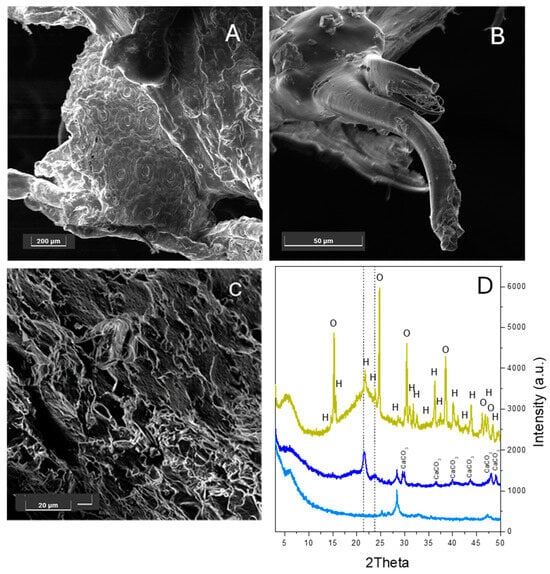
Figure 2.
FESEM representative images of the cross-sections of the Opuntia–elastomer biocomposite with 22 wt% (A,B) and 32 wt% Opuntia cladode loading (C). Images taken with the SE detector at 5 keV. Instrumental magnification 200×, 1750× and 2500×, respectively. (D) XRD patterns of the elastomer loaded with 22 wt% (light blue line) and 32 wt % Opuntia cladodes (dark blue line) along with the bare hemp XRD pattern (dark yellow line). The peaks related to CaCO3 in the rhombohedral calcite phase (00-002-0623), calcium oxalate (O, 00-003-0090) and calcium oxalate hydrate (H, 00-002-0140) are labelled. The position of the main peaks of the extruded elastomer is indicated by dashed lines.
The XRD patterns of the as-received (navy line) and extruded (cyan line) PLA are compared in Figure 3A. In this case, the XRD pattern can be observed to have undergone significant modification after the extrusion process, which reveals deep structural changes in PLA. Indeed, the peaks observed for the as-received polymer at 2θ 16.6° and 19.1° [27], which correspond to the (200/110) and (203) crystal planes (Figure 3A, navy line), increase in intensity upon extrusion, and new peaks are formed in the 9–40° 2θ range (cyan line). In the PLA–hemp blends, the characteristic PLA and hemp peaks (dark green line) can no longer be observed independently from the hemp loading (orange and red lines for 22 wt% and 32 wt% loading, respectively). This feature can be taken as an indication that PLA and the biomass interact strongly to form new materials that do not possess the crystalline structure of the precursors. Interestingly, a peak in the same position (28.4°) and with the same shape as that previously observed for the elastomer–Opuntia biocomposites is observed only in the case of PLA with 22 wt% hemp (orange line), likely indicating effective blend formation. Conversely, when hemp loading is increased, only the peaks at 16.9° and 27.6°, which are likely related to hemp embedded within the PLA matrix, are observed (red line). An FESEM analysis of the cross-sections (Figure 3B,C) reveals a morphology in which bundles of hemp fibres are coated by the polymer. This is confirmed by the XRD results, which reveal the absence of the characteristic PLA (Figure 3A) and Opuntia (Figure 4C, dark yellow line) peaks in the blends (orange line for 22 wt.% loading, red line for 32 wt.% loading). Interestingly, the peaks at 2θ = 28.4° are observed in the XRD pattern of both PLA blends, plausibly indicating the formation of the new biocomposite at increasing Opuntia loadings. Moreover, differently from hemp fibres that form bundles inside the polymer (Figure 3B,C), single Opuntia fibres can be observed within the PLA matrix fibres for both loadings (Figure 4A,B), indicating the formation of more interspersed blends fibres.
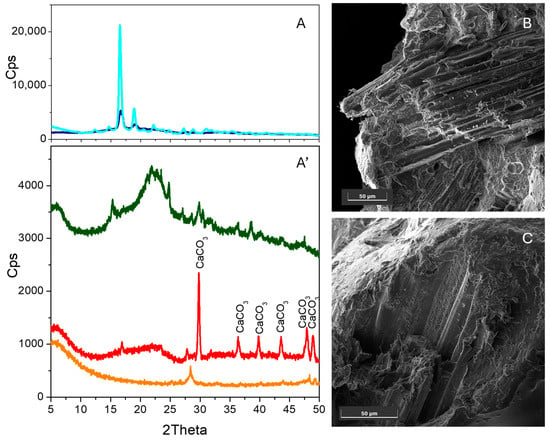
Figure 3.
(A) XRD patterns of as-received PLA (navy line) and extruded elastomer (cyan line). (A’) XRD patterns of PLA loaded with 22 wt% (orange line) and 32 wt % hemp (red line) along with the bare hemp XRD pattern (dark green line). The peaks related to CaCO3 in the rhombohedral calcite phase (00-002-0623) are labelled. FESEM representative images of the cross-sections of the hemp–elastomer biocomposite obtained from the elastomer with 22 wt% (B) and 32 wt% hemp loading (C). Images taken with the SE detector at 5 keV. Instrumental magnification 1000× and 1500×, respectively.
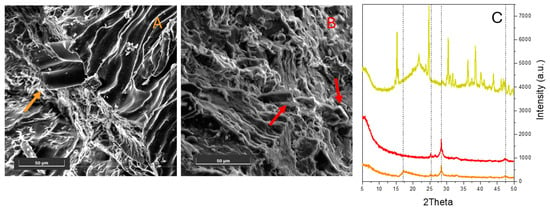
Figure 4.
FESEM representative images of the cross-sections of the Opuntia–PLA biocomposite with 22 wt% (A) and 32 wt% (B) Opuntia cladode loadings. The arrows highlight the fibres. Images taken with the SE detector at 15 keV. Instrumental magnification 1500× and 1800×, respectively. (C) XRD patterns of the elastomer loaded with 22 wt% (orange line) and 32 wt % Opuntia cladodes (red line) along with the bare hemp XRD pattern (dark yellow line). The position of the main peaks of the extruded blends is highlighted by dashed lines.
Overall, the results highlight a pattern of interactions, primarily distinguished by the considered polymer. In particular, the different interactions between the polymers and the matrices can be explained by the different polarities of the two polymer precursors, with the PLA being made up of polar monomers that still expose polar functional groups after polymerization, and the elastomer being composed of apolar monomers. The polar functionalities of PLA can thus form hydrogen bonds with the main constituents of hemp and Opuntia, e.g., cellulose and lignin, hence compromising the order of the polymeric crystalline structure.
4. Conclusions
In the present study, the effectiveness of the proposed preparation method of biocomposites was evaluated. At the same biomass-loading level, hemp–elastomer blends are found to be less homogeneously dispersed than Opuntia–elastomer biocomposites. As for the PLA–hemp biocomposites, the results obtained suggest that the optimal hemp loading to form the blend is 22 wt%, whereas the biocomposite was effectively prepared regardless of the Opuntia loading amount. Furthermore, a comparison of the PLA- and elastomer-based blends demonstrates that the two polymers clearly behave differently during the extrusion-based preparation to form the biocomposites.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs8110452/s1, Figure S1: XRD pattern of the used CaCO3; Figure S2: FESEM images of hemp fibers. Images taken with the SE detector at 5 keV.
Author Contributions
Conceptualization, L.G. and G.C.; methodology, M.M.; validation, L.G., S.C. and S.M.; formal analysis, M.M.; investigation, L.G., S.C. and S.M.; resources, G.C.; data curation, L.G. and S.C.; writing—original draft preparation, L.G. and S.C.; writing—review and editing, M.M., G.C. and S.M.; visualization, L.G.; supervision, M.M.; project administration, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The University of Turin is acknowledged for the financial support (Ricerca Locale 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Union. European Parliament and Council Directive 94/62/EC on Packaging and Packaging Waste; European Union: Brussels, Belgium, 1994.
- European Union. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Pathway to a Healthy Planet for All EU Action Plan: “Towards Zero Pollution for Air, Water and Soil”; European Union: Brussels, Belgium, 2021; Volume COM/2021/400 final.
- European Union. Directive (EU) 2019/of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment; European Union: Brussels, Belgium, 2019.
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo De Medeiros, G.; Do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A Review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Müssig, J.; Amaducci, S.; Bourmaud, A.; Beaugrand, J.; Shah, D.U. Transdisciplinary Top-down Review of Hemp Fibre Composites: From an Advanced Product Design to Crop Variety Selection. Compos. Part C Open Access 2020, 2, 100010. [Google Scholar] [CrossRef]
- Moscariello, C.; Matassa, S.; Esposito, G.; Papirio, S. From Residue to Resource: The Multifaceted Environmental and Bioeconomy Potential of Industrial Hemp (Cannabis sativa L.). Resour. Conserv. Recycl. 2021, 175, 105864. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Pappu, A.; Patil, V.; Jain, S.; Mahindrakar, A.; Haque, R.; Thakur, V.K. Advances in Industrial Prospective of Cellulosic Macromolecules Enriched Banana Biofibre Resources: A Review. Int. J. Biol. Macromol. 2015, 79, 449–458. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27, 449. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Bucciol, F.; Manzoli, M.; Cravotto, G. Comparative Studies of Mechanochemically Synthesized Insoluble Beta-Cyclodextrin Polymers. Curr. Org. Chem. 2021, 25, 1923–1936. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef] [PubMed]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The Mechanochemical Synthesis of Polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.; He, X.; Martini, A.; Kim, S.H. Mechanochemistry at Solid Surfaces: Polymerization of Adsorbed Molecules by Mechanical Shear at Tribological Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and Mechanochemical Technologies in the Catalytic Conversion of Biomass. Chem. Soc. Rev. 2021, 50, 1785–1812. [Google Scholar] [CrossRef]
- Gunti, R.; Ratna Prasad, A.V.; Gupta, A.V.S.S.K.S. Mechanical and Degradation Properties of Natural Fiber-reinforced PLA Composites: Jute, Sisal, and Elephant Grass. Polym. Compos. 2018, 39, 1125–1136. [Google Scholar] [CrossRef]
- Greco, A.; Gennaro, R.; Timo, A.; Bonfantini, F.; Maffezzoli, A. A Comparative Study Between Bio-Composites Obtained with Opuntia Ficus Indica Cladodes and Flax Fibres. J. Polym. Environ. 2013, 21, 910–916. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Benítez-Jiménez, J.J.; Ortega-Gudiño, P.; Cisneros-López, E.O.; Hernández-López, M. Biodegradability Assessment of Prickly Pear Waste–Polymer Fibres under Soil Composting. Polymers 2023, 15, 4164. [Google Scholar] [CrossRef]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Sahu, C.C.; Biswas, S.; Hommelsheim, R.; Bolm, C. Synthesis of α-Ketothioamides with Elemental Sulfur under Solvent-Free Conditions in a Mixer Mill. RSC Mechanochemistry 2024, 1, 38–42. [Google Scholar] [CrossRef]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and Morphology of Cladodes and Spines of Opuntia Ficus-Indica. Cellulose Extraction and Characterisation. Carbohydr. Polym. 2003, 51, 77–83. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; De Oliveira, A.C.S.; Rodrigues, D.A.C.; De Oliveira, C.R.; De Assis, O.B.G.; Dias, M.V.; Borges, S.V. Development and Characterization of Lignin Microparticles for Physical and Antioxidant Enhancement of Biodegradable Polymers. J. Polym. Environ. 2020, 28, 1326–1334. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Urbiola, M.I.; Hernández-Quevedo, G.; Del Real, A.; Rivera-Muñoz, E.M.; Rodríguez-García, M.E. Evaluation of Oxalates and Calcium in Nopal Pads (Opuntia ficus-indica Var. Redonda) at Different Maturity Stages. J. Food Compos. Anal. 2011, 24, 38–43. [Google Scholar] [CrossRef]
- Cao, M.; Cui, T.; Yue, Y.; Li, C.; Guo, X.; Jia, X.; Wang, B. Preparation and Characterization for the Thermal Stability and Mechanical Property of PLA and PLA/CF Samples Built by FFF Approach. Materials 2023, 16, 5023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).