Ruby Nanoparticles for Greenhouse Farming: Synthesis, Features and Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Characterization
2.2. Preparation of Luminescent Polymer Composite
2.3. Planting and Growing Conditions
2.4. Measuring Chlorophyll Content in Leaves
2.5. Measurement of Morphological Parameters of Plants
2.6. Measuring the Kinetics of Photoinduced Changes in Chlorophyll a Fluorescence and the Intensity of Carbon Dioxide Assimilation and Transpiration
2.7. Statistical Analysis
3. Results
3.1. Synthesis and Analysis of the Synthesized Ruby Particles
3.2. The Effect of PCCs on Plant Growth and Development
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lambin, E.F.; Meyfroidt, P. Global Land Use Change, Economic Globalization, and the Looming Land Scarcity. Proc. Natl. Acad. Sci. USA 2011, 108, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and Challenges for Nanotechnology in the Agri-Tech Revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kamrul Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning Photosynthesis to Sustainably Meet Global Food and Bioenergy Demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; da Fonseca-Pereira, P.; Martins, A.O.; Zsögön, A.; Nunes-Nesi, A.; Araújo, W.L. Engineering Improved Photosynthesis in the Era of Synthetic Biology. Plant Commun. 2020, 1, 100032. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Engelmann, T.W. Untersuchungen Über Die Quantitativen Beziehungen Zwischen Absorption Des Lichtes Und Assimilation in Pflanzenzellen. Bot. Zeit. 1884, 44, 43–52. [Google Scholar]
- Timiriazev, K.A.S.A. The Life of the Plant; Longmans, Green & Co.: London, UK; New York, NY, USA, 1912. [Google Scholar]
- Folta, K.M.; Childers, K.S. Light as a Growth Regulator: Controlling Plant Biology with Narrow-Bandwidth Solid-State Lighting Systems. HortScience 2008, 43, 1957–1964. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta BBA Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Rahman, M.M.; Field, D.L.; Ahmed, S.M.; Hasan, M.T.; Basher, M.K.; Alameh, K. LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments. Plants 2021, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10, 492746. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Dougher, T.A.O.; Bugbee, B. Evidence for Yellow Light Suppression of Lettuce Growth. Photochem. Photobiol. 2001, 73, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Kawaguchi, H.; Mizusawa, N.; Yamori, W.; Suzuki, Y.; Terashima, I. Far-Red Light Accelerates Photosynthesis in the Low-Light Phases of Fluctuating Light. Plant Cell Physiol. 2020, 61, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A.; Samuolienė, G.; Jankauskienė, J.; Kasiulevičiūtė-Bonakėrė, A.; Blizkinas, Z.; Novickovas, A.; Breive, K.; Žukauskas, A. The effect of light-emitting diodes lighting on cucumber transplants and after-effect on yield. Zemdirb. Agric. 2009, 96, 102–118. [Google Scholar]
- Huché-Thélier, L.; Crespel, L.; Gourrierec, J.L.; Morel, P.; Sakr, S.; Leduc, N. Light Signaling and Plant Responses to Blue and UV Radiations—Perspectives for Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant Flavoprotein Photoreceptors. Plant Cell Physiol. 2015, 56, 401–413. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the Light Environment in Plants: Photoreceptors and Early Signaling Steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.G.; Okajima, K. Diverse Photoreceptors and Light Responses in Plants. J. Plant Res. 2016, 129, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-Mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Zhigalova, T.V. Light Quality as a Driver of Photosynthetic Apparatus Development. Biophys. Rev. 2022, 14, 779–803. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Simakin, A.V.; Paskhin, M.O.; Ivanyuk, V.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; Gudkov, S.V. Cultivation of Solanum Lycopersicum under Glass Coated with Nanosized Upconversion Luminophore. Appl. Sci. 2021, 11, 10726. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Burmistrov, D.E.; Simakin, A.V.; Ermakova, J.A.; Gudkov, S.V. Effect of Up-Converting Luminescent Nanoparticles with Increased Quantum Yield Incorporated into the Fluoropolymer Matrix on Solanum Lycopersicum Growth. Agronomy 2022, 12, 108. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Paskhin, M.O.; Simakin, A.V.; Burmistrov, D.E.; Pobedonostsev, R.V.; Vyatchinov, A.A.; Vedunova, M.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; et al. Plant Photochemistry under Glass Coated with Upconversion Luminescent Film. Appl. Sci. 2022, 12, 7480. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Popov, A.V.; Pobedonostsev, R.V.; Kazantseva, D.V.; Dorokhov, A.S.; Izmailov, A.Y.; Vyatchinov, A.A.; Orlovskaya, E.O.; Shaidulin, A.T.; et al. Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae 2023, 9, 846. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Lu, Y.; Wang, D.; Wang, C.; Li, J. One-Step Synthesis of Eu3+-Modified Cellulose Acetate Film and Light Conversion Mechanism. Polymers 2020, 13, 113. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Ai, X.; Pan, H.; Zhang, H.; Dong, L. Polylactide/Poly(Butylene Adipate-Co-Terephthalate)/Rare Earth Complexes as Biodegradable Light Conversion Agricultural Films. Polym. Adv. Technol. 2019, 30, 203–211. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Jia, S.; Pan, H.; Zhang, H.; Wang, D.; Dong, L. Exploring Polylactide/Poly(Butylene Adipate-Co-Terephthalate)/Rare Earth Complexes Biodegradable Light Conversion Agricultural Films. Int. J. Biol. Macromol. 2019, 127, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Wada, E.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. The Effect of Spectrum Conversion Covering Film on Cucumber in Soilless Culture. Acta Hortic 2012, 956, 481–487. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing Plant Productivity by Changing the Solar Spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Sánchez-Lanuza, M.B.; Menéndez-Velázquez, A.; Peñas-Sanjuan, A.; Navas-Martos, F.J.; Lillo-Bravo, I.; Delgado-Sánchez, J.M. Advanced Photonic Thin Films for Solar Irradiation Tuneability Oriented to Greenhouse Applications. Materials 2021, 14, 2357. [Google Scholar] [CrossRef] [PubMed]
- Ke-li, Z.; Liang-jie, Y.; Mei-yun, X.; You-zu, Y.; Ju-tang, S. The Application of Lights-Conversed Polyethylene Film for Agriculture. Wuhan Univ. J. Nat. Sci. 2002, 7, 365–367. [Google Scholar] [CrossRef]
- Hassanien, R.; Hassanien, E.; Li, M. Influences of Greenhouse-Integrated Semi-Transparent Photovoltaics on Microclimate and Lettuce Growth. Int. J. Agric. Biol. Eng. 2017, 10, 11–22. [Google Scholar] [CrossRef]
- Hassanien, R.; Hassanien, E.; Li, M.; Yin, F. The Integration of Semi-Transparent Photovoltaics on Greenhouse Roof for Energy and Plant Production. Renew Energy 2018, 121, 377–388. [Google Scholar] [CrossRef]
- Aira, J.R.; Gallardo-Saavedra, S.; Eugenio-Gozalbo, M.; Alonso-Gómez, V.; Muñoz-García, M.Á.; Hernández-Callejo, L. Analysis of the Viability of a Photovoltaic Greenhouse with Semi-Transparent Amorphous Silicon (a-Si) Glass. Agronomy 2021, 11, 1097. [Google Scholar] [CrossRef]
- Aiyyzhy, K.O.; Barmina, E.V.; Shafeev, G.A. Laser Synthesis of Ruby for Photo-Conversion of Solar Spectrum. Laser Phys. Lett. 2022, 20, 046001. [Google Scholar] [CrossRef]
- Aizuddin, W.; Razali, W.; Kasim, A.; Senawi, A.; Hashim, A.; Yahya, N.; Rafaie, H.A. Fabrication and Characterization of Ruby Nanoparticles. Malays. J. Anal. Sci. 2018, 22, 458–464. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Sobczyk, A.T. Electrospraying Route to Nanotechnology: An Overview. J. Electrostat. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An Overview on the Effect of Ultrasonication Duration on Different Properties of Nanofluids. J. Therm. Anal. Calorim. 2019, 135, 393–418. [Google Scholar] [CrossRef]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlation between Microstructure and Optical Properties of ZnO Nanoparticles Synthesized by Ball Milling. J. Appl. Phys. 2007, 102, 93515. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Kazakevich, P.V.; Simakin, A.V.; Voronov, V.V.; Shafeev, G.A. Laser Induced Synthesis of Nanoparticles in Liquids. Appl. Surf. Sci. 2006, 252, 4373–4380. [Google Scholar] [CrossRef]
- Sajti, C.L.; Sattari, R.; Chichkov, B.; Barcikowski, S. Ablation Efficiency of α-Al2O3 in Liquid Phase and Ambient Air by Nanosecond Laser Irradiation. Appl. Phys. A Mater. Sci. Process 2010, 100, 203–206. [Google Scholar] [CrossRef]
- Delfour, L.; Itina, T.E. Mechanisms of Ultrashort Laser-Induced Fragmentation of Metal Nanoparticles in Liquids: Numerical Insights. J. Phys. Chem. C 2015, 119, 13893–13900. [Google Scholar] [CrossRef]
- Ziefuß, A.R.; Reichenberger, S.; Rehbock, C.; Chakraborty, I.; Gharib, M.; Parak, W.J.; Barcikowski, S. Laser Fragmentation of Colloidal Gold Nanoparticles with High-Intensity Nanosecond Pulses Is Driven by a Single-Step Fragmentation Mechanism with a Defined Educt Particle-Size Threshold. J. Phys. Chem. C 2018, 122, 22125–22136. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Controlled Size Manipulation of Free Gold Nanoparticles by Laser Irradiation and Their Facile Bioconjugation. J. Mater. Chem. 2007, 17, 4705–4710. [Google Scholar] [CrossRef]
- Strasser, M.; Setoura, K.; Langbein, U.; Hashimoto, S. Computational Modeling of Pulsed Laser-Induced Heating and Evaporation of Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 25748–25755. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Zivcak, M.; Tomasz, H.; Mojski, J.; Kociel, H.; et al. A Comparison between Different Chlorophyll Content Meters under Nutrient Deficiency Conditions. J. Plant Nutr. 2017, 40, 1024–1034. [Google Scholar] [CrossRef]
- Simakin, A.V.; Ivanyuk, V.V.; Dorokhov, A.S.; Gudkov, S.V. Photoconversion Fluoropolymer Films for the Cultivation of Agricultural Plants Under Conditions of Insufficient Insolation. Appl. Sci. 2020, 10, 8025. [Google Scholar] [CrossRef]
- Aiyyzhy, K.O.; Barmina, E.V.; Voronov, V.V.; Shafeev, G.A.; Novikov, G.G.; Uvarov, O.V. Laser Ablation and Fragmentation of Boron in Liquids. Opt. Laser Technol. 2022, 155, 108393. [Google Scholar] [CrossRef]
- Fongarland, P.; Vilcocq, L.; Djakovitch, L. Catalytic Liquefaction of Kraft Lignin with Solvothermal Approach. Catalysts 2021, 11, 875. [Google Scholar] [CrossRef]
- Chang, K.L.; Lin, Y.C.; Qiu, M.Z.; Tu, C.W.; Chang, C.P.; Wu, J.L.; Lin, Y.C.; Chang, C.K. Gas-Phase Isopropanol Degradation by Nonthermal Plasma Combined with Mn-Cu/-Al2O3. Environ. Sci. Pollut. Res. Int. 2021, 28, 40693–40702. [Google Scholar] [CrossRef]
- Jo, J.O.; Mok, Y.S. Oxidation of Isopropyl Alcohol in Air by a Catalytic Plasma Reactor System. Appl. Chem. Eng. 2014, 25, 531–537. [Google Scholar] [CrossRef][Green Version]
- Czylkowski, D.; Hrycak, B.; Miotk, R.; Jasiński, M.; Mizeraczyk, J.; Dors, M. Microwave Plasma for Hydrogen Production from Liquids. Nukleonika 2016, 61, 185–190. [Google Scholar] [CrossRef]
- Mashhadani, Z.T.A. A Comparison of the Conversion of Isopropyl Alcohol by Non-Thermal Plasma and Thermally-Driven Catalysis Using In-Situ FTIR Spectroscopy. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2018. [Google Scholar]
- Loh, E. Ultraviolet absorption and excitation spectrum of ruby and sapphire. J. Chem. Phys 1965, 44, 1940–1945. [Google Scholar] [CrossRef]
- Kusuma, H.H.; Astuti, B.; Ibrahim, Z. Absorption and emission properties of ruby (Cr:Al2O3) single crystal. J. Phys. Conf. Ser. 2019, 1170, 012054. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and Applications. Mater. Biomed. Eng. Inorg. Micro-Nanostructures 2019, 7, 211–240. [Google Scholar] [CrossRef]
- Yang, X.; Maleki, A.; Lipey, N.A.; Zheng, X.; Santiago, M.; Connor, M.; Sreenivasan, V.K.A.; Dawes, J.M.; Lu, Y.; Zvyagin, A.V. Lifetime-Engineered Ruby Nanoparticles (Tau-Rubies) for Multiplexed Imaging of μ-Opioid Receptors. ACS Sensors 2021, 6, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, A.M.; Sobhan, M.A.; Sreenivasan, V.K.A.; Grebenik, E.A.; Rabeau, J.R.; Goldys, E.M.; Zvyagin, A.V. Nano-Ruby: A Promising Fluorescent Probe for Background-Free Cellular Imaging. Part. Part. Syst. Charact. 2013, 30, 506–513. [Google Scholar] [CrossRef]
- Cortes-Vega, F.D.; Yang, W.; Zarate-Medina, J.; Brankovic, S.R.; Calderon, H.A.; Robles Hernandez, F.C. Mechanochemical Synthesis of α-Al2O3-Cr3+ (Ruby) and χ-Al2O3. J. Am. Ceram. Soc. 2019, 102, 976–980. [Google Scholar] [CrossRef]
- Inogamov, N.A.; Zhakhovsky, V.V.; Faenov, A.Y.; Khokhlov, V.A.; Shepelev, V.V.; Skobelev, I.Y.; Kato, Y.; Tanaka, M.; Pikuz, T.A.; Kishimoto, M.; et al. Spallative Ablation of Dielectrics by X-Ray Laser. Appl. Phys. A Mater. Sci. Process 2010, 101, 87–96. [Google Scholar] [CrossRef][Green Version]
- Ayyyzhy, K.O.; Voronov, V.V.; Gudkov, S.V.; Rakov, I.I.; Simakin, A.V.; Shafeev, G.A. Laser Fabrication and Fragmentation of Selenium Nanoparticles in Aqueous Media. Phys. Wave Phenom. 2019, 27, 113–118. [Google Scholar] [CrossRef]
- Bojarski, C.; Domsta, J. Tyeoriya Vliyaniya Kontsyentratsii Na Lyuminyestsyentsiyu Tvyerdykh Rastvorov. Acta Phys. Acad. Sci. Hung. 1971, 30, 145–166. [Google Scholar] [CrossRef]
- Oh, H.E.; Yoon, A.; Park, Y.G. Red Light Enhances the Antioxidant Properties and Growth of Rubus Hongnoensis. Plants 2021, 10, 2589. [Google Scholar] [CrossRef]
- Rehman, M.; Fahad, S.; Saleem, M.; Hafeez, M.; Rahman, M.U.; Liu, F.; Deng, G. Red Light Optimized Physiological Traits and Enhanced the Growth of Ramie (Boehmeria nivea L.). Photosynthetica 2020, 58, 922–931. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The Combination of Blue and Red LED Light Improves Growth and Phenolic Acid Contents in Salvia Miltiorrhiza Bunge. Ind. Crop. Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
- Petroutsos, D.; Tokutsu, R.; Maruyama, S.; Flori, S.; Greiner, A.; Magneschi, L.; Cusant, L.; Kottke, T.; Mittag, M.; Hegemann, P.; et al. A Blue-Light Photoreceptor Mediates the Feedback Regulation of Photosynthesis. Nature 2016, 537, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J. Light Quality Affects Water Use of Sweet Basil by Changing Its Stomatal Development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K.I. Phot1 and Phot2 Mediate Blue Light Regulation of Stomatal Opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Ma, X.; Grodzinski, B. The Effect of Spectral Quality on Daily Patterns of Gas Exchange, Biomass Gain, and Water-Use-Efficiency in Tomatoes and Lisianthus: An Assessment of Whole Plant Measurements. Front. Plant Sci. 2017, 8, 266308. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, R.; Liu, X.; Zhou, L.; Dong, M.; Rehman, M.; Fahad, S.; Liu, L.; Deng, G. Effects of Light Spectra on Morphology, Gaseous Exchange, and Antioxidant Capacity of Industrial Hemp. Front. Plant Sci. 2022, 13, 937436. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves. Front. Plant Sci. 2018, 9, 370722. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The Impact of the Phytochromes on Photosynthetic Processes. Biochim. Biophys. Acta BBA Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to Lower Red to Far-Red Light Ratios Improve Tomato Tolerance to Salt Stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Sharrock, R.A. The Phytochrome Red/Far-Red Photoreceptor Superfamily. Genome Biol. 2008, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S. Phytochrome-Mediated Development in Land Plants: Red Light Sensing Evolves to Meet the Challenges of Changing Light Environments. Mol. Ecol. 2006, 15, 3483–3503. [Google Scholar] [CrossRef] [PubMed]
- Casson, S.A.; Hetherington, A.M. Environmental Regulation of Stomatal Development. Curr. Opin. Plant Biol. 2010, 13, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mccree, K.J. An Equation for the Rate of Respiration of White Clover Grown under Controlled Conditions. In Prediction and Measurement of Photosynthetic Productivity, Proceedings of the IBP/PP Technical Meeting, Trebon, Czech Republic, 14–21 September 1969; PUDOC: Wageningen, The Netherlands, 1970. [Google Scholar]
- Amthor, J.S. The Role of Maintenance Respiration in Plant Growth. Plant Cell Environ. 1984, 7, 561–569. [Google Scholar] [CrossRef]
- Lötscher, M.; Klumpp, K.; Schnyder, H. Growth and Maintenance Respiration for Individual Plants in Hierarchically Structured Canopies of Medicago Sativa and Helianthus Annuus: The Contribution of Current and Old Assimilates. New Phytol. 2004, 164, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.H.; Hebert, D.; Jackson, A.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Optimizing Spectral Quality with Quantum Dots to Enhance Crop Yield in Controlled Environments. Commun. Biol. 2021, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and Application of Photoconversion Fluoropolymer Films for Greenhouses Located at High or Polar Latitudes. J. Photochem. Photobiol. B 2020, 213, 112056. [Google Scholar] [CrossRef] [PubMed]
- Ivanyuk, V.V.; Shkirin, A.V.; Belosludtsev, K.N.; Dubinin, M.V.; Kozlov, V.A.; Bunkin, N.F.; Dorokhov, A.S.; Gudkov, S.V. Influence of Fluoropolymer Film Modified with Nanoscale Photoluminophor on Growth and Development of Plants. Front. Phys. 2020, 8, 616040. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Dong, R.; Xie, G.; Zhou, J.; Wu, K.; Zhang, H.; Cai, Q.; Lei, B. Characterization and Properties of a Sr2Si5N8:Eu2+-Based Light-Conversion Agricultural Film. J. Rare Earths 2020, 38, 539–545. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, J.H.; Park, K.S.; Namgoong, J.W.; Hwang, T.G.; Kim, J.P.; Son, J.E. Quantitative Methods for Evaluating the Conversion Performance of Spectrum Conversion Films and Testing Plant Responses under Simulated Solar Conditions. Hortic Environ. Biotechnol. 2020, 61, 999–1009. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kang, J.H.; Kang, W.H.; Son, J.E. Subtle Changes in Solar Radiation under a Green-to-Red Conversion Film Affect the Photosynthetic Performance and Chlorophyll Fluorescence of Sweet Pepper. Photosynthetica 2020, 58, 1107–1115. [Google Scholar] [CrossRef]
- Schettini, E.; de Salvador, F.R.; Scarascia-Mugnozza, G.; Vox, G. Radiometric Properties of Photoselective and Photoluminescent Greenhouse Plastic Films and Their Effects on Peach and Cherry Tree Growth. J. Hortic Sci. Biotechnol. 2011, 86, 79–83. [Google Scholar] [CrossRef]
- Hamada, K.; Shimasaki, K.; Ogata, T.; Nishimura, Y.; Nakamura, K.; Oyama-Egawa, H.; Yoshida, K. Effects of Spectral Composition Conversion Film and Plant Growth Regulators on Proliferation of Cymbidium Protocorm Like Body (PLB) Cultured In Vitro. Environ. Control. Biol. 2010, 48, 127–132. [Google Scholar] [CrossRef][Green Version]
- Liu, X.Y.; Chang, T.T.; Guo, S.R.; Xu, Z.G.; Li, J. Effect of Different Light Quality of LED on Growth and Photosynthetic Character in Cherry Tomato Seedling. Acta Hortic 2011, 907, 325–330. [Google Scholar] [CrossRef]
- Edser, C. Light Manipulating Additives Extend Opportunities for Agricultural Plastic Films. Plast. Addit. Compd. 2002, 4, 20–24. [Google Scholar] [CrossRef]
- González, A.; Rodríguez, R.; Bañón, S.; Franco, J.A.; Fernández, J.A.; Salmerón, A.; Espí, E. Strawberry and Cucumber Cultivation under Fluorescent Photoselective Plastic Films Cover. Acta Hortic 2003, 614, 407–413. [Google Scholar] [CrossRef]
- De Salvador, F.R.; Mugnozza, G.S.; Vox, G.; Schettini, E.; Mastrorilli, M.; Bou Jaoudé, M. Innovative Photoselective and Photoluminescent Plastic Films for Protected Cultivation. Acta Hortic 2008, 801, 115–121. [Google Scholar] [CrossRef]
- Hidaka, K.; Yoshida, K.; Shimasaki, K.; Murakami, K.; Yasutake, D.; Kitano, M. Spectrum Conversion Film for Regulation of Plant Growth. J. Fac. Agric. Kyushu Univ. 2008, 53, 549–552. [Google Scholar] [CrossRef]
- In Yoon, H.; Hyeun Kang, J.; Kim, D.; Eek Son, J. Seedling Quality and Photosynthetic Characteristic of Vegetables Grown Under a Spectrum Conversion Film. J. Bio-Environ. Control. 2021, 30, 110–117. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Paskhin, M.O.; Nagaev, E.V.; Efimov, A.D.; Kaziev, A.V.; Ageychenkov, D.G.; Gudkov, S.V. Additive Production of a Material Based on an Acrylic Polymer with a Nanoscale Layer of Zno Nanorods Deposited Using a Direct Current Magnetron Discharge: Morphology, Photoconversion Properties, and Biosafety. Materials 2021, 14, 6586. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral-Conversion Film Potential for Greenhouses: Utility of Green-to-Red Photons Conversion and Far-Red Filtration for Plant Growth. PLoS ONE 2023, 18, e0281996. [Google Scholar] [CrossRef] [PubMed]
- Paskhin, M.O.; Pobedonostsev, R.V.; Kazantseva, D.V.; Simakin, A.V.; Gorudko, I.V.; Yanykin, D.V.; Gudkov, S.V. The Influence of Composite Luminescent Materials Based on Graphene Oxide on the Growth and Development of Solanum Lycopersicum in Greenhouses. J. Compos. Sci. 2023, 7, 474. [Google Scholar] [CrossRef]

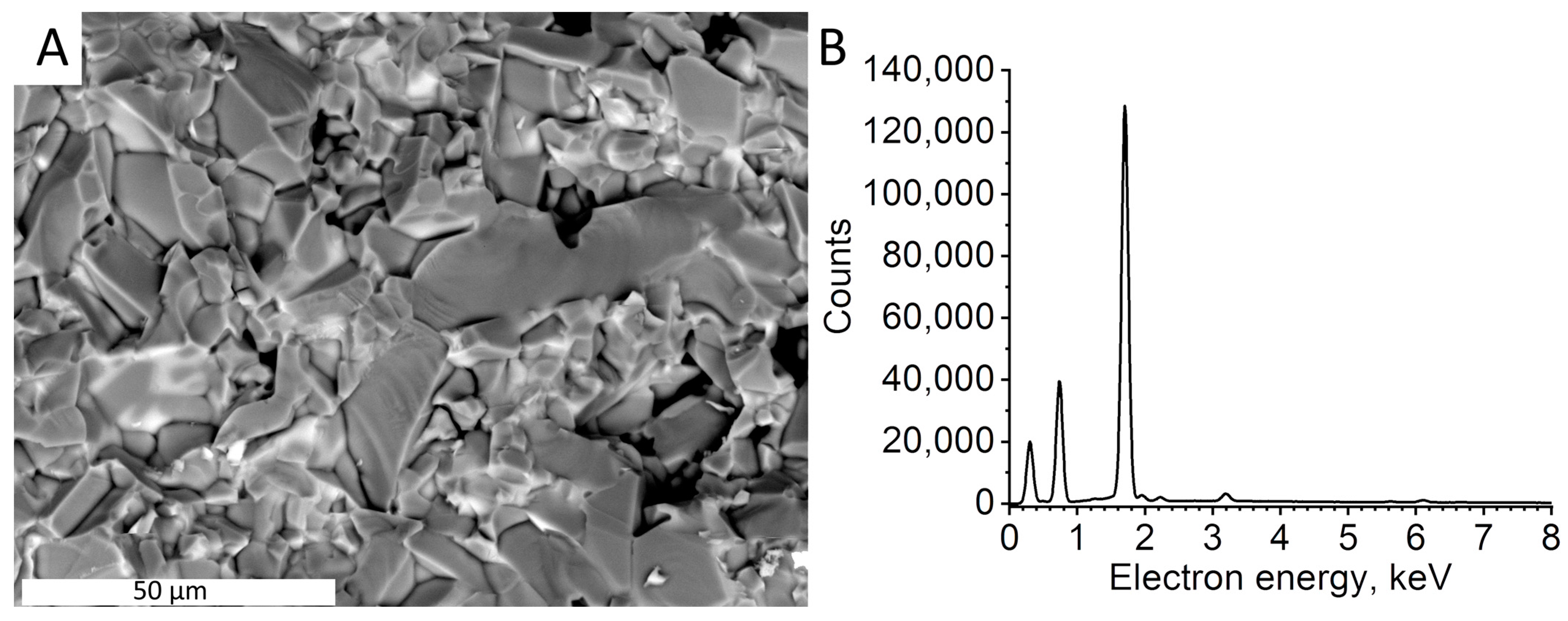

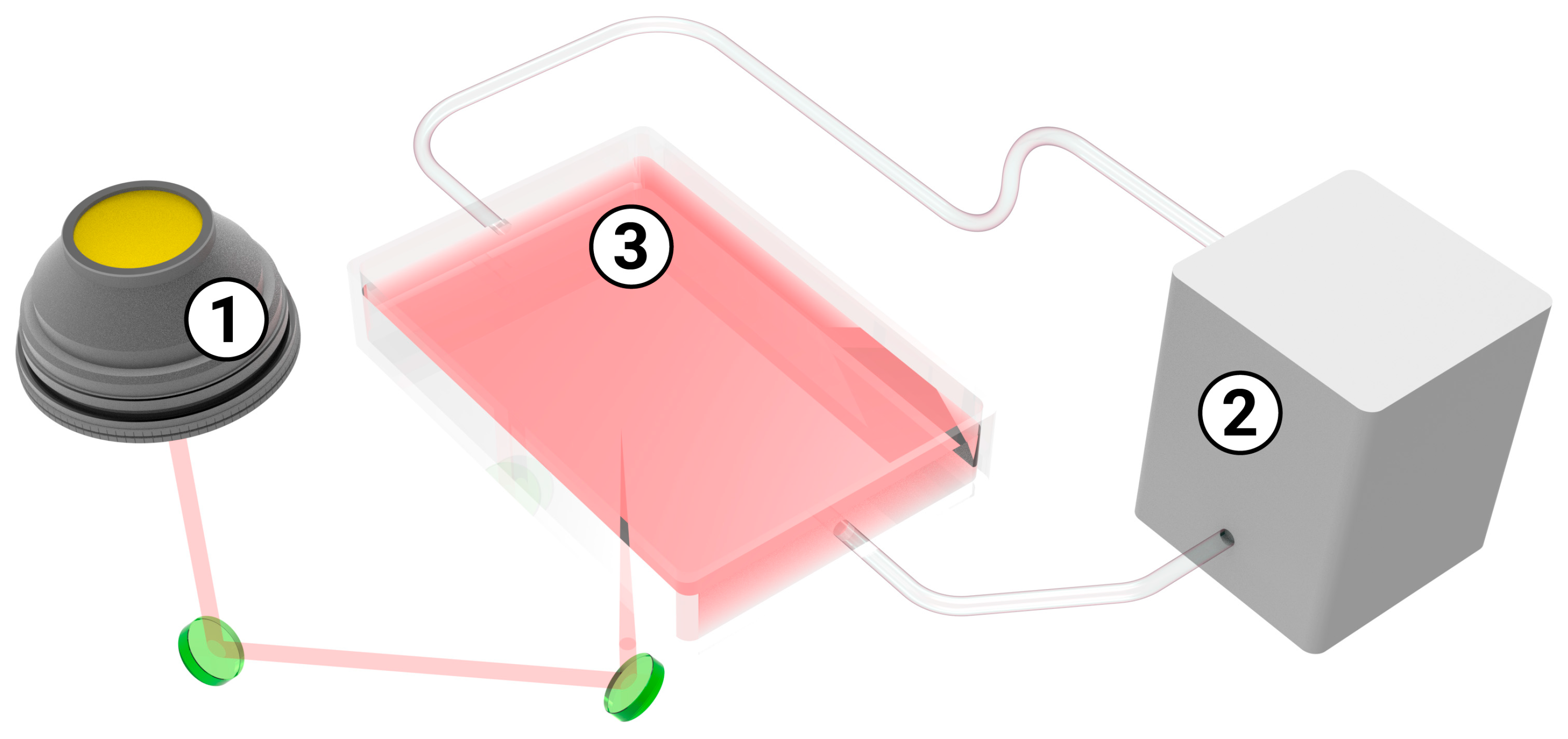
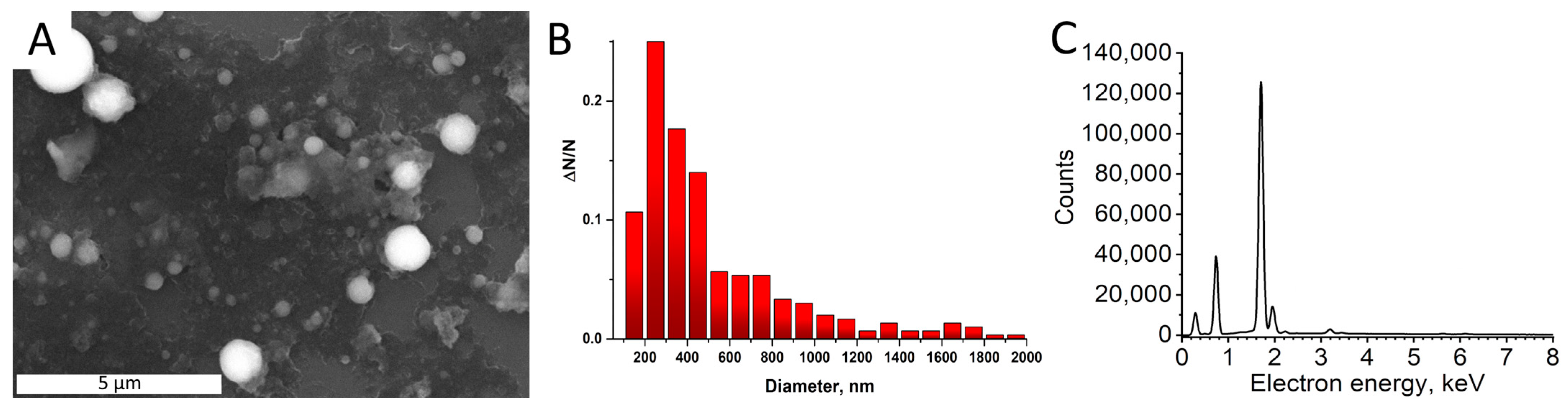
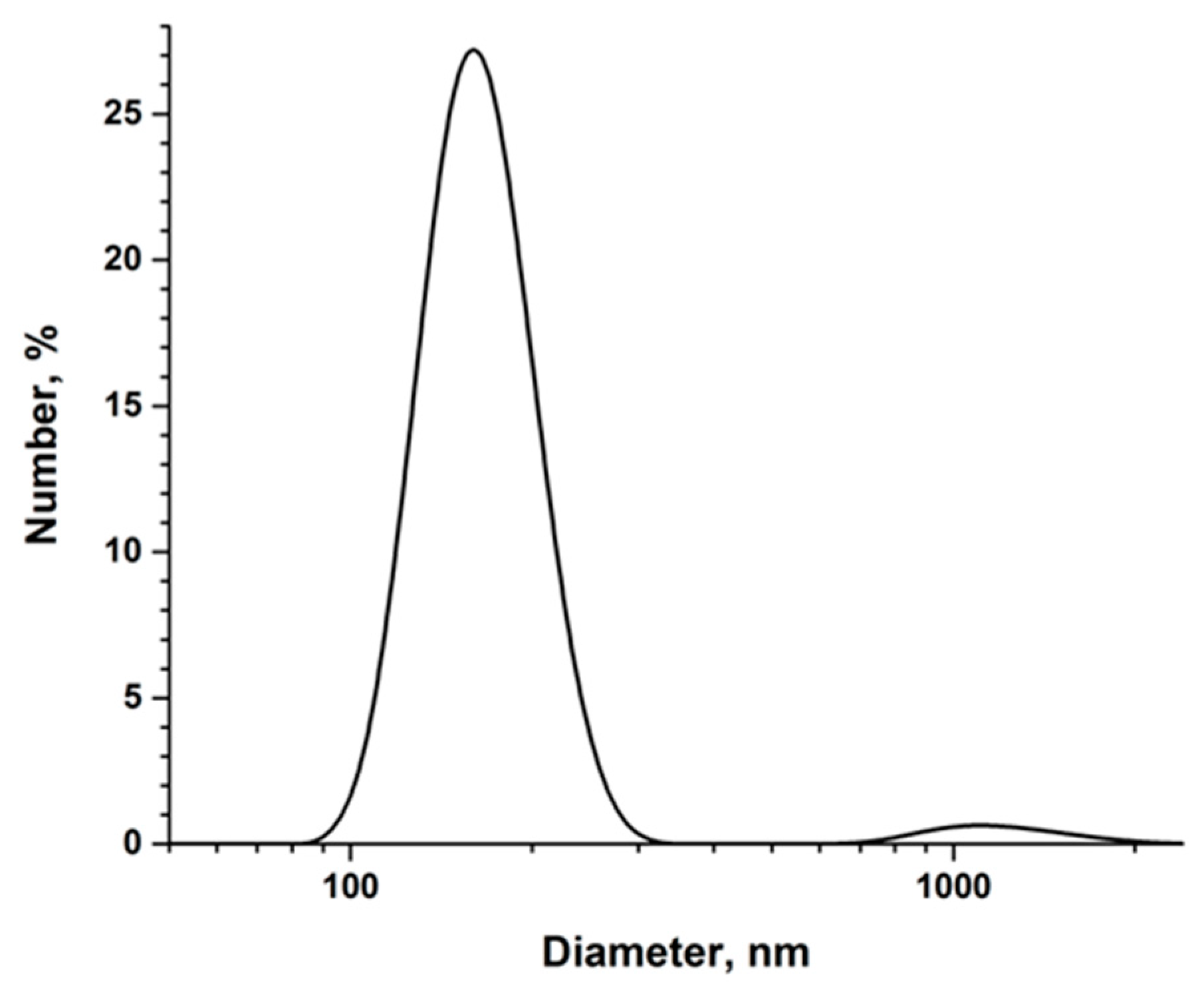



| Sample | Proportion of the Atoms, % | Chromium to Aluminum Ratio | ||||
|---|---|---|---|---|---|---|
| O | Al | Si | Cr | Mn | ||
| VK94-1 | 60.1 ± 0.1 | 39.4 ± 0.4 | 0.26 ± 0.01 | 0.13 ± 0.01 | 0.20 ± 0.06 | 3.3 × 10−3 |
| Primary ruby particles | 61.1 ± 0.1 | 33.2 ± 0.8 | 5.43 ± 0.64 | 0.11 ± 0.01 | 0.15 ± 0.01 | 3.3 × 10−3 |
| Secondary ruby particles | 61.1 ± 0.2 | 33.1 ± 0.3 | 5.52 ± 0.48 | 0.13 ± 0.01 | 0.21 ± 0.02 | 3.3 × 10−3 |
| PCC-R Version | Control | PCC-R7 | PCC-R8 | PCC-R9 | |
|---|---|---|---|---|---|
| Parameter | |||||
| A, µmol CO2 m−2 s−1 | 0 min | −0.31 ± 0.02 a | −0.34 ± 0.03 a | −0.39 ± 0.01 b | −0.36 ± 0.01 a |
| 20 min | 0.71 ± 0.07 a | 0.92 ± 0.23 a | 0.81 ± 0.03 a | 0.85 ± 0.13 a | |
| E, mmol H2O m−2 s−1 | 0 min | 0.30 ± 0.04 a | 0.36 ± 0.05 a | 0.09 ± 0.01 b | 0.36 ± 0.10 a |
| 20 min | 0.65 ± 0.10 a | 0.68 ± 0.09 a | 0.46 ± 0.06 b | 0.65 ± 0.15 a | |
| WUEleaf, µmol CO2 mmol H2O−1 | 0 min | −0.97 ± 0.02 a | −0.93 ± 0.03 a | −3.94 ± 0.30 b | −1.04 ± 0.03 a |
| 8 min | 1.06 ± 0.22 a | 1.72 ± 0.14 a | 2.80 ± 0.17 b | 1.42 ± 0.09 a | |
| 20 min | 1.22 ± 0.18 a | 1.36 ± 0.19 a | 1.82 ± 0.20 b | 1.34 ± 0.21 a | |
| WUEi, nmol CO2 mmol H2O−1 | 0 min | −9 ± 2 a | −5 ± 1 a | −21 ± 4 b | −6 ± 2 a |
| 8 min | 7 ± 1 a | 9 ± 1 a | 21 ± 1 b | 8 ± 1 a | |
| 20 min | 9 ± 1 a | 8 ± 2 a | 10 ± 1 a | 9 ± 1 a |
| Control | PCC-R7 | PCC-R8 | PCC-R9 | |
|---|---|---|---|---|
| Fv/Fm | 0.80 ± 0.002 a | 0.80 ± 0.02 a | 0.79 ± 0.02 a | 0.80 ± 0.01 a |
| Y(II) | 0.37 ± 0.02 a | 0.39 ± 0.06 a | 0.35 ± 0.01 a | 0.37 ± 0.01 a |
| ETR(II), µmol electrons (PSII s)−1 | 23.9 ± 1.4 a | 25.5 ± 3.9 a | 22.6 ± 0.6 a | 24.1 ± 0.7 a |
| qN | 0.57 ± 0.02 a | 0.56 ± 0.03 a | 0.58 ± 0.01 a | 0.58 ± 0.02 a |
| qP | 0.55 ± 0.03 a | 0.58 ± 0.07 a | 0.53 ± 0.01 a | 0.56 ± 0.01 a |
| Y(I) | 0.80 ± 0.05 a | 0.78 ± 0.03 a | 0.81 ± 0.03 a | 0.81 ± 0.01 a |
| ETR(I), µmol electrons (PSI s)−1 | 51.4 ± 3.3 a | 50.5 ± 2.2 a | 52.5 ± 2.0 a | 52.1 ± 0.6 a |
| Control | PCC-R7 | PCC-R8 | PCC-R9 | |
|---|---|---|---|---|
| B:G ratio | 0.74 ± 0.003 a | 0.73 ± 0.02 a | 0.69 ± 0.001 b | 0.71 ± 0.002 a |
| R:B ratio | 1.24 ± 0.01 a | 1.27 ± 0.01 a | 1.34 ± 0.004 b | 1.31 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskhin, M.O.; Aiyyzhy, K.O.; Pobedonostsev, R.V.; Kazantseva, D.V.; Rakov, I.I.; Barmina, E.V.; Yanykin, D.V.; Gudkov, S.V. Ruby Nanoparticles for Greenhouse Farming: Synthesis, Features and Application. J. Compos. Sci. 2024, 8, 7. https://doi.org/10.3390/jcs8010007
Paskhin MO, Aiyyzhy KO, Pobedonostsev RV, Kazantseva DV, Rakov II, Barmina EV, Yanykin DV, Gudkov SV. Ruby Nanoparticles for Greenhouse Farming: Synthesis, Features and Application. Journal of Composites Science. 2024; 8(1):7. https://doi.org/10.3390/jcs8010007
Chicago/Turabian StylePaskhin, Mark O., Kuder O. Aiyyzhy, Roman V. Pobedonostsev, Dina V. Kazantseva, Ignat I. Rakov, Ekaterina V. Barmina, Denis V. Yanykin, and Sergey V. Gudkov. 2024. "Ruby Nanoparticles for Greenhouse Farming: Synthesis, Features and Application" Journal of Composites Science 8, no. 1: 7. https://doi.org/10.3390/jcs8010007
APA StylePaskhin, M. O., Aiyyzhy, K. O., Pobedonostsev, R. V., Kazantseva, D. V., Rakov, I. I., Barmina, E. V., Yanykin, D. V., & Gudkov, S. V. (2024). Ruby Nanoparticles for Greenhouse Farming: Synthesis, Features and Application. Journal of Composites Science, 8(1), 7. https://doi.org/10.3390/jcs8010007







