The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Presintered Zirconia Specimen Preparation
2.2. Specimen Grouping and Presintered Zirconia Surface Treatment
2.2.1. Subtractive Surface Treatment Technique
Hydrofluoric Acid Gel Left on Presintered Zirconia Surface (s.ttt. 1)
Hydrofluoric Acid Gel Washed from Presintered Zirconia Surface (s.ttt. 2)
2.2.2. Additive Surface Treatment Technique (Silica Coating)
Nanosilica Mixed with Gel-Forming Agent (s.ttt. 3)
Microsilica Mixed with Gel-Forming Agent (s.ttt. 4)
2.2.3. Combination
Coating Followed by Airborne-Particle Abrasion (s.ttt. 5)
Coating Followed by Etching
2.3. Specimen Sintering
2.4. Test Procedures
2.4.1. Surface Microstructure
2.4.2. Crystalline Phase Identification
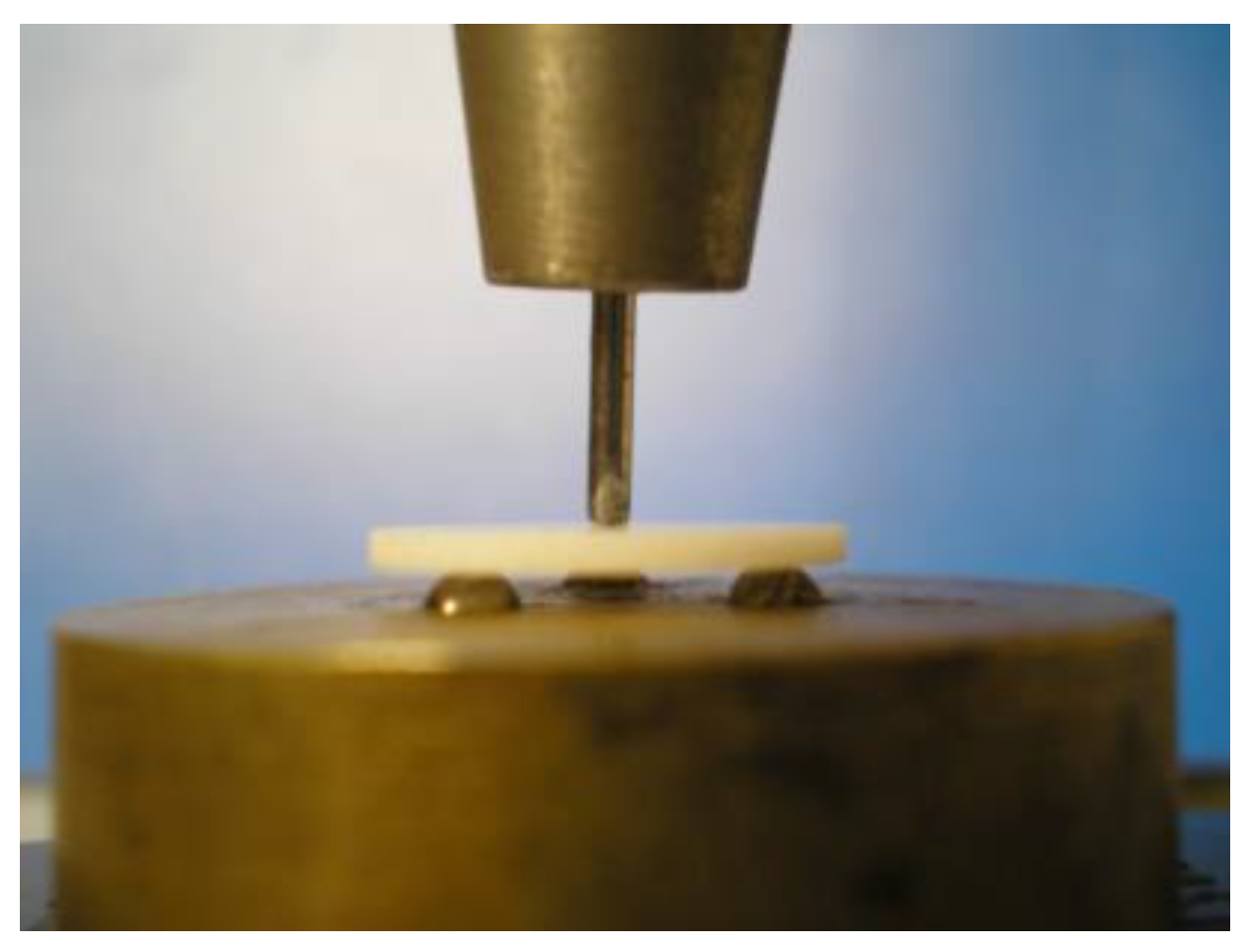
2.4.3. Flexural Strength Test
3. Results
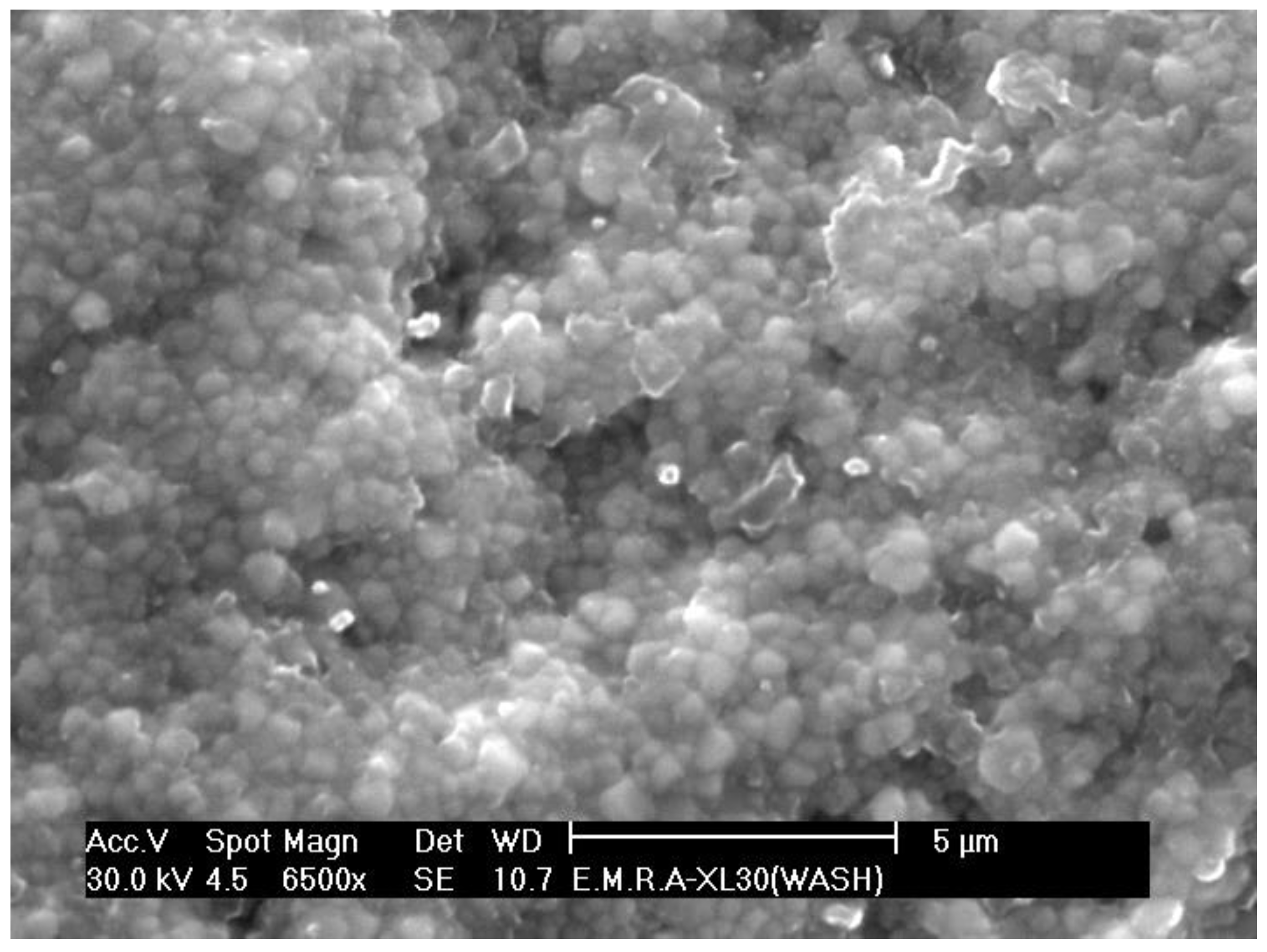
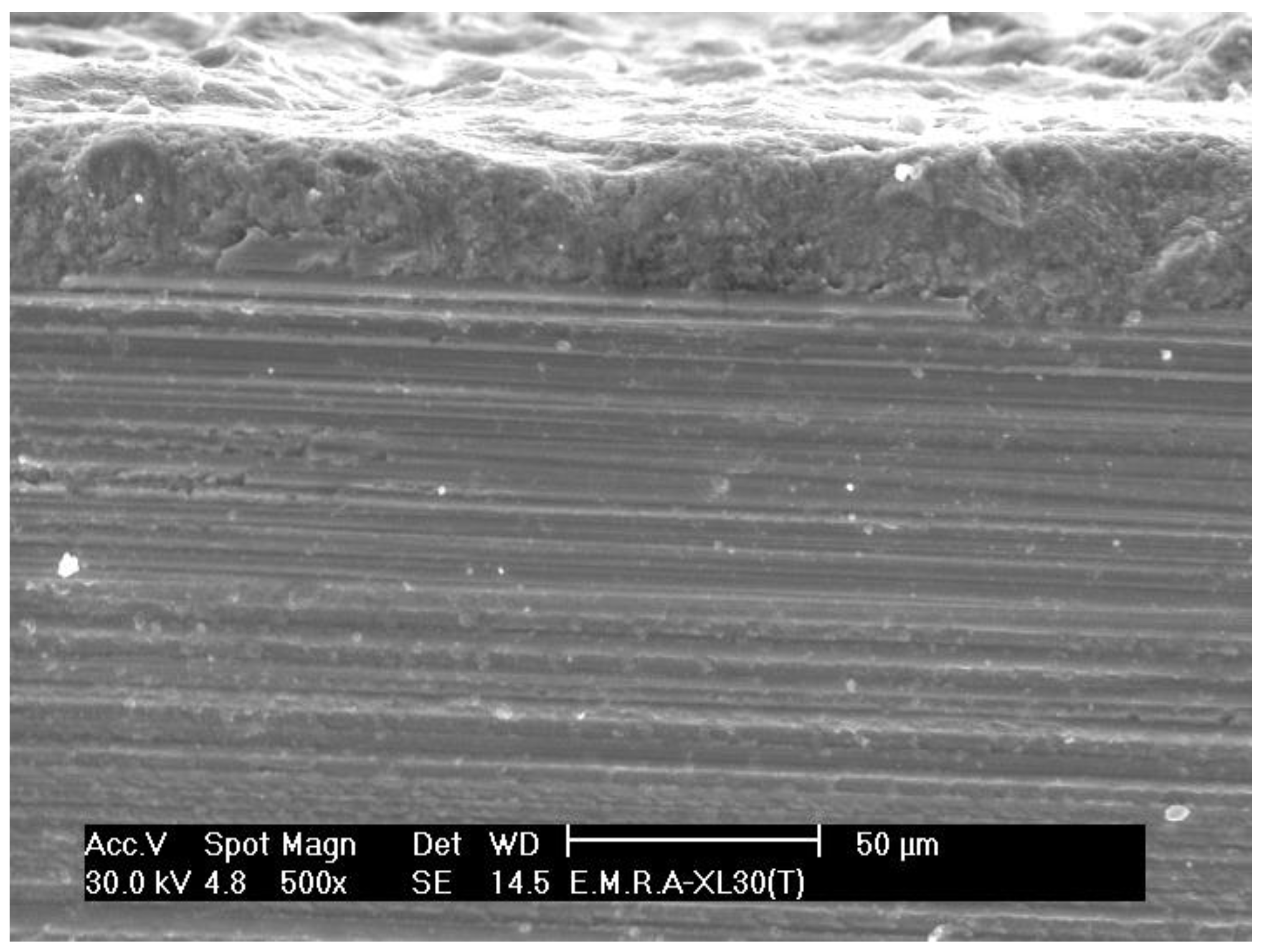
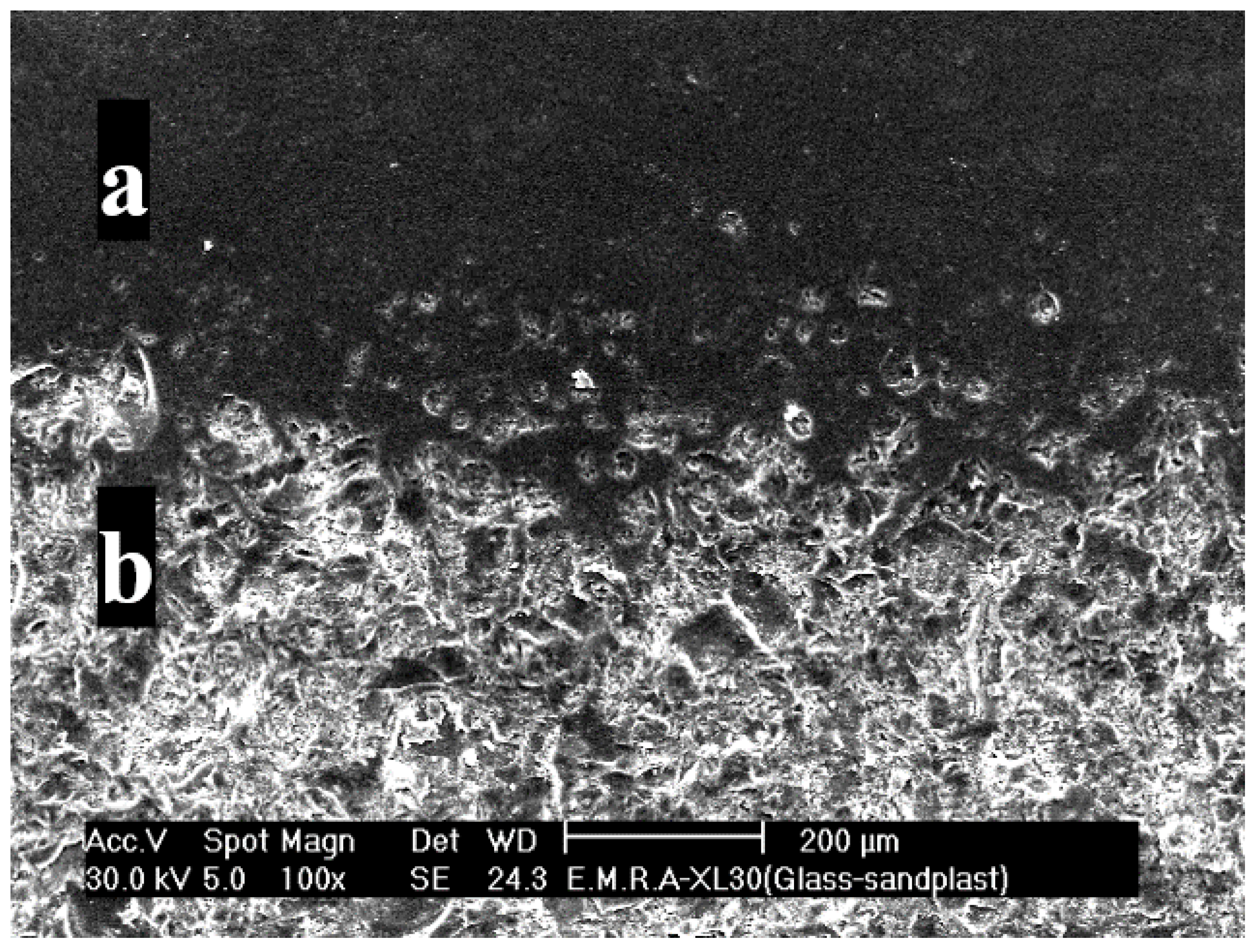
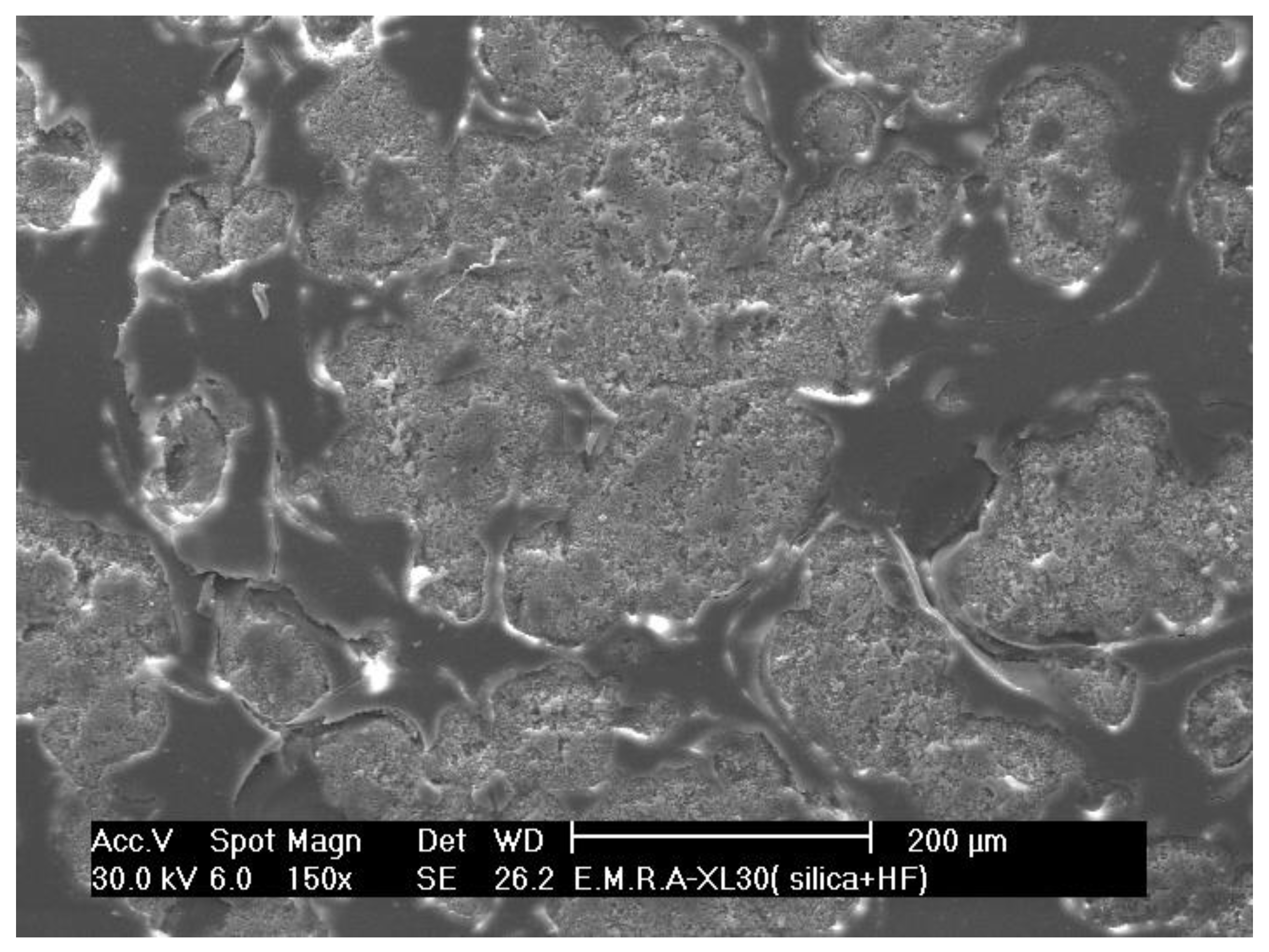
3.1. Surface Microstructure
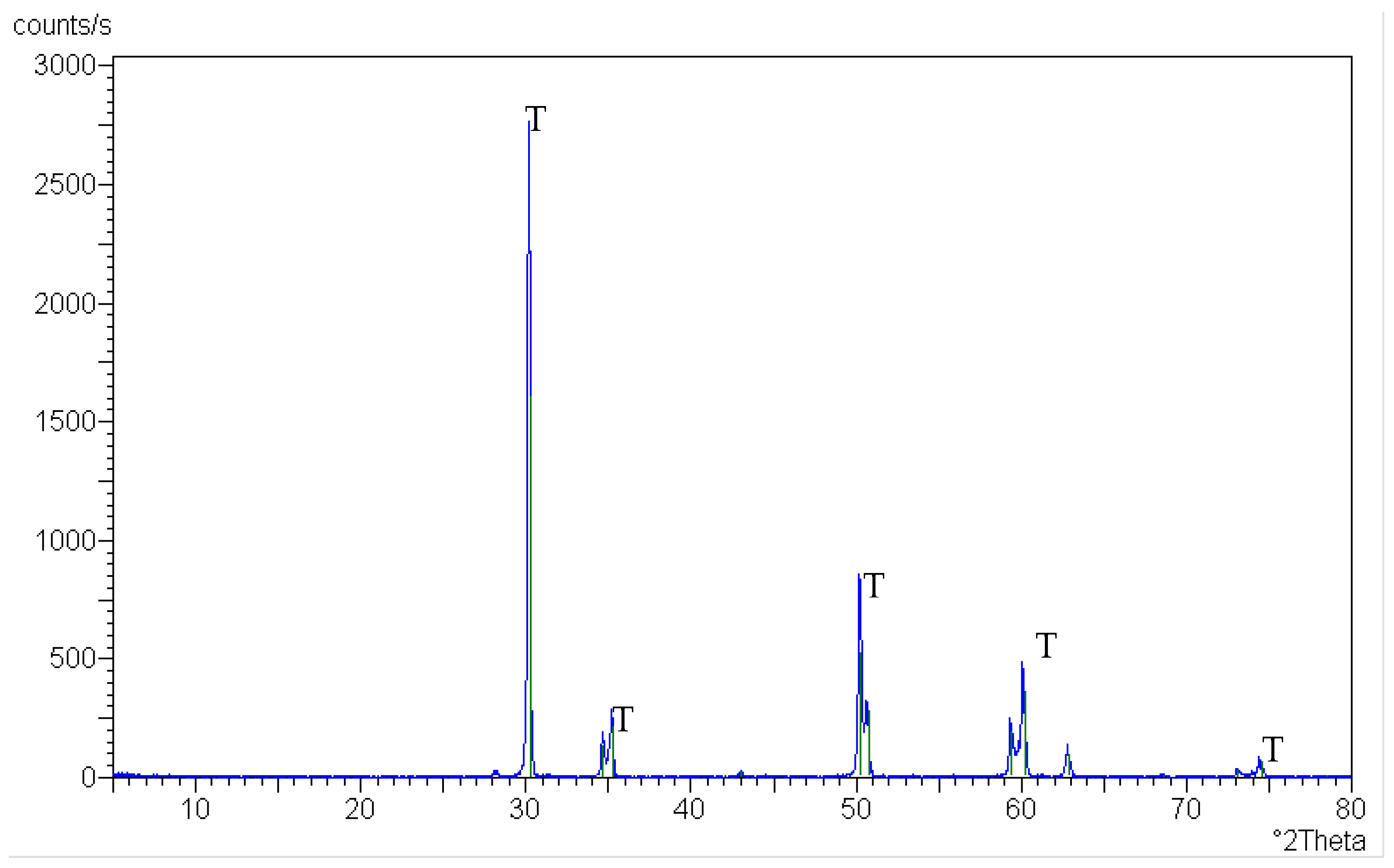
3.2. Crystalline Phase Identification
3.3. Flexural Strength
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Habib, A.W.; Aboushelib, M.N.; Habib, N.A. Effect of chemical aging on color stability and surface properties of stained all-ceramic restorations. J. Esthet. Restor. Dent. 2021, 33, 636–647. [Google Scholar] [CrossRef]
- Hamdy, T.M. Polymers and ceramics biomaterials in orthopedics and dentistry: A review article. Egypt. J. Chem. 2018, 61, 723–730. [Google Scholar] [CrossRef]
- Comba, A.; Paolone, G.; Baldi, A.; Vichi, A.; Goracci, C.; Bertozzi, G.; Scotti, N. Effects of substrate and cement shade on the translucency and color of CAD/CAM lithium-disilicate and zirconia ceramic materials. Polymers 2022, 14, 1778. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Ghulam, O.; Krsoum, M.; Binmahmoud, S.; Taher, H.; Elmalky, W.; Zafar, M.S. Revolution of current dental zirconia: A comprehensive review. Molecules 2022, 27, 1699. [Google Scholar] [CrossRef] [PubMed]
- Rizky, N.S.; Rikmasari, R.; Bonifacius, S. Evaluation of zirconia surface roughness after different surface treatment with sandblasting, hydrofluoric acid etching, and combination treatment. Key Eng. Mater. 2022, 932, 163–169. [Google Scholar] [CrossRef]
- Hamdy, T.M. Interfacial microscopic examination and chemical analysis of resin-dentin interface of self-adhering flowable resin composite. F1000Research 2017, 6, 1688. [Google Scholar] [CrossRef]
- Tzanakakis, E.G.C.; Tzoutzas, I.G.; Koidis, P.T. Is There a potential for durable adhesion to zirconia restorations? A systematic review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the titanium implant surface treatment on the surface roughness and chemical composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef]
- Delgado-Ruíz, R.A.; Calvo-Guirado, J.L.; Moreno, P.; Guardia, J.; Gomez-Moreno, G.; Mate-Sánchez, J.E.; Ramirez-Fernández, P.; Chiva, F. Femtosecond laser microstructuring of zirconia dental implants. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2011, 96, 91–100. [Google Scholar] [CrossRef]
- Tzanakakis, E.; Kontonasaki, E.; Voyiatzis, G.; Andrikopoulos, K.; Tzoutzas, I. Surface characterization of monolithic zirconia submitted to different surface treatments applying optical interferometry and raman spectrometry. Dent. Mater. J. 2020, 39, 111–117. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Reusser, E.; Hämmerle, C.H.F. Effect of Blasting Pressure, Abrasive Particle Size and Grade on Phase Transformation and Morphological Change of Dental Zirconia Surface. Surf. Coat. Technol. 2012, 206, 4293–4302. [Google Scholar] [CrossRef]
- Iinuma, Y.; Hirota, M.; Hayakawa, T.; Ohkubo, C. Surrounding tissue response to surface-treated zirconia implants. Materials 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, H.; Wang, Y.; Sun, Y. Performance of stereolithography and milling in fabricating monolithic zirconia crowns with different finish line designs. J. Mech. Behav. Biomed. Mater. 2021, 115, 104255. [Google Scholar] [CrossRef]
- Maridurai, T.; Balaji, D.; Sagadevan, S. Synthesis and characterization of yttrium stabilized zirconia nanoparticles. Mater. Res. 2016, 19, 812–816. [Google Scholar] [CrossRef]
- Bona, A.D.; Pecho, O.E.; Alessandretti, R. Zirconia as a Dental Biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J. Prosthet. Dent. 2007, 98, 379–388. [Google Scholar] [CrossRef]
- Jo, Y.-B.; Ahn, J.-J.; Lee, S.-H.; Park, T.; Huh, J.-B. The effect of ZrO2 slurry application to the pre-sintered zirconia surface on bonding strength. Korean Acad. Oral Maxillofac. Implantol. 2020, 24, 76–82. [Google Scholar] [CrossRef]
- Peçanha, M.; Amaral, M.; Baroudi, K.; Frizzera, F.; Vitti, R.; Silva-Concilio, L. Improving the bonding stability between resin cements and zirconia-based ceramic using different surface treatments. Int. J. Prosthodont. 2022, 35, 414–419. [Google Scholar] [CrossRef]
- Melo, R.M.; Souza, R.O.A.; Dursun, E.; Monteiro, E.B.C.; Valandro, L.F.; Bottino, M.A. Surface treatments of zirconia to enhance bonding durability. Oper. Dent. 2015, 40, 636–643. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, S.C.; Lee, M.H.; Kim, H.J.; Oh, N.S. Effect of 9% hydrofluoric acid gel hot-etching surface treatment on shear bond strength of resin cements to zirconia ceramics. Medicine 2022, 58, 1469. [Google Scholar] [CrossRef] [PubMed]
- Tsalouchou, E.; Cattell, M.; Knowles, J.; Pittayachawan, P.; Mcdonald, A. Fatigue and fracture properties of yttria partially stabilized zirconia crown systems. Dent. Mater. 2008, 24, 308–318. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Lee, J.; Ha, S.; Choi, Y. The effect of preparation order on the crystal structure of yttria-stabilized tetragonal zirconia polycrystal and the shear bond strength of dental resin cements. Dent. Mater. 2011, 27, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, E.I.; Vajeeston, N.; Knarvang, T.; Kvam, K. A Novel etching technique for surface treatment of zirconia ceramics to improve adhesion of resin-based luting cements. Acta Biomater. Odontol. Scand. 2017, 3, 36–46. [Google Scholar] [CrossRef]
- Borouni, M.; Niroumand, B.; Maleki, A. A Study on crystallization of amorphous nano silica particles by mechanical activation at the presence of pure aluminum. J. Solid State Chem. 2018, 263, 208–215. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Irie, M.; Teixeira, E.C.N.; Jurado, C.A.; Maruo, Y.; Nishigawa, G.; Matsumoto, T.; Garcia-Godoy, F. Relationships between flexural and bonding properties, marginal adaptation, and polymerization shrinkage in flowable composite restorations for dental application. Polymers 2021, 13, 2613. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Depth of cure, flexural properties and volumetric shrinkage of low and high viscosity bulk-fill giomers and resin composites. Dent. Mater. J. 2017, 36, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Itinoche, K.M.; Özcan, M.; Bottino, M.A.; Oyafuso, D. Effect of mechanical cycling on the flexural strength of densely sintered ceramics. Dent. Mater. 2006, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Miura, D.; Ishida, Y.; Miyasaka, T.; Shinya, A.; Aoki, H. Reliability of different bending test methods for dental press ceramics. Materials 2020, 13, 5162. [Google Scholar] [CrossRef]
- Liu, R.; Sun, T.; Zhang, Y.; Zhang, Y.; Jiang, D.; Shao, L. The effect of graded glass-zirconia structure on the bond between core and veneer in layered zirconia restorations. J. Mech. Behav. Biomed. Mater. 2015, 46, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, A.; Ramdev, P.; Shruthi, C.S. Evaluation of effect of zirconia surface treatment, using plasma of argon and silane, on the shear bond strength of two composite resin cements. J. Clin. Diagn. Res. 2017, 11, ZC39–ZC43. [Google Scholar] [CrossRef] [PubMed]
- Daou, E.E. The zirconia ceramic: Strengths and weaknesses. Open Dent. J. 2014, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kurtulmus-Yilmaz, S.; Aktore, H. Effect of the application of surface treatments before and after sintering on the flexural strength, phase transformation and surface topography of zirconia. J. Dent. 2018, 72, 29–38. [Google Scholar] [CrossRef]
- Gąsiorek, J.; Mazur-Nowacka, A.; Szczurek, A.; Babiarczuk, B.; Tic, W.J.; Guziałowska-Tic, J.; Kaleta, J.; Krzak, J. Influence of zirconia and organic additives on mechanical and ectrochemical properties of silica sol-gel coatings. Materials 2021, 14, 2389. [Google Scholar] [CrossRef] [PubMed]


















| Material | Used in S.ttt. | Commercial Names | Main Composition (wt %) | Manufacturer |
|---|---|---|---|---|
| Yttrium, tetragonal zirconia, polycrystalline ceramic blocks (Y-TZP) | All (Substructure) | In-Ceram 2000 YZ 20/19 | ZrO2, Y2O3 5%, HfO2 < 3%, Al2O3, and SiO2 < 1%. | Vita, Zahnfabrik, Germany |
| Hydrofluoric acid gel | S.ttt. 1 and 2 | Porcelain Etch | Buffered 9% hydrofluoric acid. | Ultradent Products, South Jorda, UT, USA |
| Silicon dioxide | S.ttt. 3 and 4 | Dentex | Nanosilica dispersed in water, SiO2 40%, Na2O 0.34%, and H2O 59.66%. | Bee Chems, India |
| Neutral porcelain | S.ttt. 5, 6, and 7 | Vita VM13 Kit | SiO2, Na2O K2O, MgO, CaO, BaO, B2O3, Al2O3, Fe2O3, TiO2, P2O5, ZrO2, and SnO2. | Vita, Zahnfabrik, Germany |
| S.ttt. | Control (No ttt) | s.ttt. 1 | s.ttt. 2 | s.ttt. 3 | s.ttt. 4 | s.ttt. 5 | s.ttt. 6 | s.ttt. 7 |
|---|---|---|---|---|---|---|---|---|
| Flexural strength (MPa) | 846.3 c ± 3.6 | 830 c ± 7.4 | 835 c ± 7.1 | 634.1 b ± 2.4 | 982.3 e ± 3.5 | 863.1 d ± 5.4 | 872.2 d ± 6.4 | 386.6 a ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelraouf, R.M.; Tsujimoto, A.; Hamdy, T.M.; Alhotan, A.; Jurado, C.A.; Abadir, M.; Habib, N.A. The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces. J. Compos. Sci. 2023, 7, 396. https://doi.org/10.3390/jcs7090396
Abdelraouf RM, Tsujimoto A, Hamdy TM, Alhotan A, Jurado CA, Abadir M, Habib NA. The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces. Journal of Composites Science. 2023; 7(9):396. https://doi.org/10.3390/jcs7090396
Chicago/Turabian StyleAbdelraouf, Rasha M., Akimasa Tsujimoto, Tamer M. Hamdy, Abdulaziz Alhotan, Carlos A. Jurado, Magdi Abadir, and Nour A. Habib. 2023. "The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces" Journal of Composites Science 7, no. 9: 396. https://doi.org/10.3390/jcs7090396
APA StyleAbdelraouf, R. M., Tsujimoto, A., Hamdy, T. M., Alhotan, A., Jurado, C. A., Abadir, M., & Habib, N. A. (2023). The Effect of Surface Treatments of Presintered Zirconia on Sintered Surfaces. Journal of Composites Science, 7(9), 396. https://doi.org/10.3390/jcs7090396








