Medium-Temperature Glass-Composite Phosphate Materials for the Immobilization of Chloride Radioactive Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Procedures
Synthesis of the Medium-Temperature Glass-Composite Phosphate Materials
2.2. Methods
3. Results and Discussion
3.1. Geometric Parameters and the Density of Glass-Composite Phosphate Materials
3.2. Study of the Composition and Structure of Glass-Composite Phosphate Materials
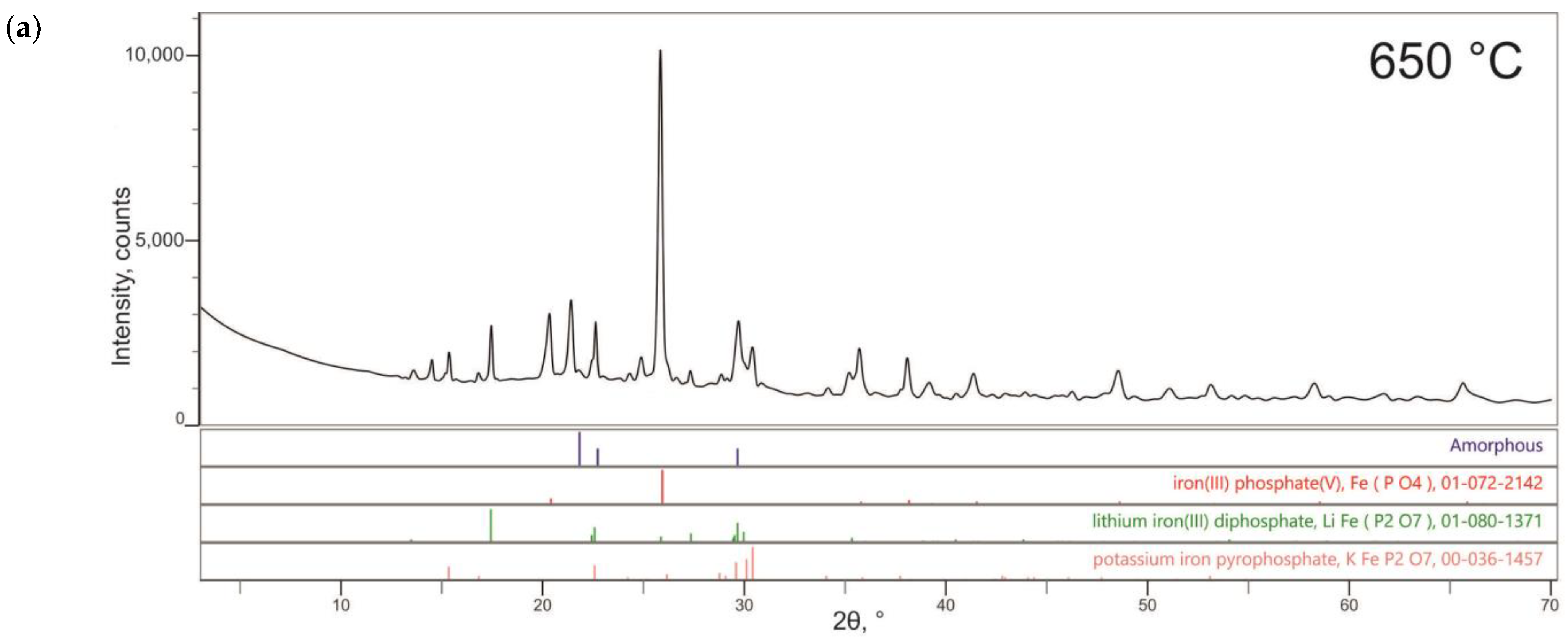
3.2.1. Characterization of the Synthesized Glass-Composite Phosphate Materials by X-ray Diffraction
3.2.2. Characterization of the Synthesized Glass-Composite Phosphate Materials by SEM
3.3. Hydrolytic Resistance of Glass-Composite Phosphate Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorov, Y.S.; Zil’berman, B.Y.; Aloi, A.S.; Puzikov, E.A.; Shadrin, A.Y.; Alyapyshev, M.Y. Problems of Modernization of Spent Nuclear Fuel Extraction Processing. Russ. J. Gen. Chem. 2011, 81, 1932–1948. [Google Scholar] [CrossRef]
- Lee, H. Oxide Electroreduction and Other Processes for Pyrochemical Processing of Spent Nuclear Fuels. In Reprocessing and Recycling of Spent Nuclear Fuel; Elsevier: Amsterdam, The Netherlands, 2015; pp. 415–436. [Google Scholar]
- Alyapyshev, M.Y.; Babain, V.A.; Ustynyuk, Y.A. Recovery of Minor Actinides from High-Level Wastes: Modern Trends. Russ. Chem. Rev. 2016, 85, 943–961. [Google Scholar] [CrossRef]
- Watson, L.C.; Aikin, A.M.; Bancroft, A.I. The Permanent Disposal of Highly Radioactive Wastes by Incorporation into Glass. In Proceedings of the Scientific Conference on the Disposal of Radioactive Wastes; International Atomic Energy Agency (IAEA), Vienna, Austria, 16–21 November 1959. [Google Scholar]
- Vuori, S. The Environmental and Ethical Basis of the Geological Disposal of Long-Lived Radioactive Waste. ATS Ydintekniikka 1995, 24, 16–17. [Google Scholar]
- Ma, B.; Charlet, L.; Fernandez-Martinez, A.; Kang, M.; Madé, B. A Review of the Retention Mechanisms of Redox-Sensitive Radionuclides in Multi-Barrier Systems. Appl. Geochem. 2019, 100, 414–431. [Google Scholar] [CrossRef]
- Gregg, D.J.; Farzana, R.; Dayal, P.; Holmes, R.; Triani, G. Synroc Technology: Perspectives and Current Status (Review). J. Am. Ceram. Soc. 2020, 103, 5424–5441. [Google Scholar] [CrossRef]
- Vance, E.R.; Chavara, D.T.; Gregg, D.J. Synroc Development—Past and Present Applications. MRS Energy Sustain. 2017, 4, 8. [Google Scholar] [CrossRef]
- Deokattey, S.; Bhaskar, N.; Kalyane, V.L.; Kumar, V.; Jahagirdar, P.B. Borosilicate Glass and Synroc R&D for Radioactive Waste Immobilization: An International Perspective. JOM 2003, 55, 48–51. [Google Scholar] [CrossRef]
- Ringwood, E.; Kelly, P.M. Immobilization of High-Level Waste in Ceramic Waste Forms. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1986, 319, 63–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Vance, E.R. Plutonium in Monazite and Brabantite: Diffuse Reflectance Spectroscopy Study. J. Nucl. Mater. 2008, 375, 311–314. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Bukaemskiy, A.; Neumeier, S.; Modolo, G.; Bosbach, D. Studies on Thermal and Mechanical Properties of Monazite-Type Ceramics for the Conditioning of Minor Actinides. Prog. Nucl. Energy 2014, 72, 144–148. [Google Scholar] [CrossRef]
- Crum, J.; Maio, V.; McCloy, J.; Scott, C.; Riley, B.; Benefiel, B.; Vienna, J.; Archibald, K.; Rodriguez, C.; Rutledge, V.; et al. Cold Crucible Induction Melter Studies for Making Glass Ceramic Waste Forms: A Feasibility Assessment. J. Nucl. Mater. 2014, 444, 481–492. [Google Scholar] [CrossRef]
- McCloy, J.S.; Schuller, S. Vitrification of Wastes: From Unwanted to Controlled Crystallization, a Review. Comptes Rendus Géosci. 2022, 354, 121–160. [Google Scholar] [CrossRef]
- Li, H.; Wu, L.; Wang, X.; Xu, D.; Teng, Y.; Li, Y. Crystallization Behavior and Microstructure of Barium Borosilicate Glass–Ceramics. Ceram. Int. 2015, 41, 15202–15207. [Google Scholar] [CrossRef]
- Richerson, D.W.; Lee, W.E. Modern Ceramic Engineering; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429488245. [Google Scholar]
- Jantzen, C.M.; Lee, W.E.; Ojovan, M.I. Radioactive Waste (RAW) Conditioning, Immobilization, and Encapsulation Processes and Technologies: Overview and Advances. In Radioactive Waste Management and Contaminated Site Clean-Up; Elsevier: Amsterdam, The Netherlands, 2013; pp. 171–272. [Google Scholar]
- Wu, J.; Zhou, K.; Dai, M. Impacts of the Fukushima Nuclear Accident on the China Seas: Evaluation Based on Anthropogenic Radionuclide 137Cs. Chin. Sci. Bull. 2013, 58, 552–558. [Google Scholar] [CrossRef][Green Version]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A.; Strachan, D.M.; Scheele, R.D. A Glass-Encapsulated Calcium Phosphate Wasteform for the Immobilization of Actinide-, Fluoride-, and Chloride-Containing Radioactive Wastes from the Pyrochemical Reprocessing of Plutonium Metal. J. Nucl. Mater. 2007, 361, 78–93. [Google Scholar] [CrossRef]
- Simpson, M.F.; Sachdev, P. Development of electrorefiner waste salt disposal process for the EBR- II spent fuel treatment project. Nucl. Eng. Technol. 2008, 40, 175–182. [Google Scholar] [CrossRef]
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I.; Veselov, S.N.; et al. PH Process as a Technology for Reprocessing Mixed Uranium–Plutonium Fuel from BREST-OD-300 Reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Pereira, C.; Hash, M.; Lewis, M.; Richmann, M. Ceramic-Composite Waste Forms from the Electrometallurgical Treatment of Spent Nuclear Fuel. JOM 1997, 49, 34–40. [Google Scholar] [CrossRef]
- Priebe, S.; Bateman, K. The Ceramic Waste Form Process at Idaho National Laboratory. Nucl. Technol. 2008, 162, 199–207. [Google Scholar] [CrossRef]
- Jena, H.; Maji, B.K.; Asuvathraman, R.; Govindan Kutty, K.V. Synthesis and Thermal Characterization of Glass Bonded Ca-Chloroapatite Matrices for Pyrochemical Chloride Waste Immobilization. J. Non-Cryst. Solids 2012, 358, 1681–1686. [Google Scholar] [CrossRef]
- Michie, E.M.; Grimes, R.W.; Boccaccini, A.R. Hot-Pressed Phosphate Glass–Ceramic Matrix Composites Containing Calcium Phosphate Particles for Nuclear Waste Encapsulation. J. Mater. Sci. 2008, 43, 4152–4156. [Google Scholar] [CrossRef]
- Stoch, P.; Ciecinska, M. Thermochemistry of Phosphate Glasses for Immobilization of Dangerous Waste. J. Therm. Anal. Calorim. 2012, 108, 705–709. [Google Scholar] [CrossRef]
- Juoi, J.M.; Ojovan, M.I.; Lee, W.E. Microstructure and Leaching Durability of Glass Composite Wasteforms for Spent Clinoptilolite Immobilisation. J. Nucl. Mater. 2008, 372, 358–366. [Google Scholar] [CrossRef]
- Danilov, S.S.; Frolova, A.V.; Kulikova, S.A.; Vinokurov, S.E.; Maslakov, K.I.; Teterin, A.Y.; Teterin, Y.A.; Myasoedov, B.F. Immobilization of Rhenium as a Technetium Surrogate in Aluminum Iron Phosphate Glass. Radiochemistry 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Danilov, S.S.; Matveenko, A.V.; Frolova, A.V.; Belova, K.Y.; Petrov, V.G.; Vinokurov, S.E.; Myasoedov, B.F. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Appl. Sci. 2021, 11, 11180. [Google Scholar] [CrossRef]
- Frolova, A.V.; Vinokurov, S.E.; Gromyak, I.N.; Danilov, S.S. Medium-Temperature Phosphate Glass Composite Material as a Matrix for the Immobilization of High-Level Waste Containing Volatile Radionuclides. Energies 2022, 15, 7506. [Google Scholar] [CrossRef]
- Brow, R.K. Review: The Structure of Simple Phosphate Glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- ASTM C1285-21; Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT). ASTM International: West Conshohocken, PA, USA, 2014.
- Yang, J.H.; Park, H.-S.; Cho, Y.-Z. Al2O3-Containing Silver Phosphate Glasses as Hosting Matrices for Radioactive Iodine. J. Nucl. Sci. Technol. 2017, 54, 1330–1337. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Wang, J.; Zhang, X.; Wang, Y.; Li, N. Simultaneous Immobilization of Radionuclides Sr and Cs by Sodium Zirconium Phosphate Type Ceramics and Its Chemical Durability. Ceram. Int. 2022, 48, 12772–12778. [Google Scholar] [CrossRef]



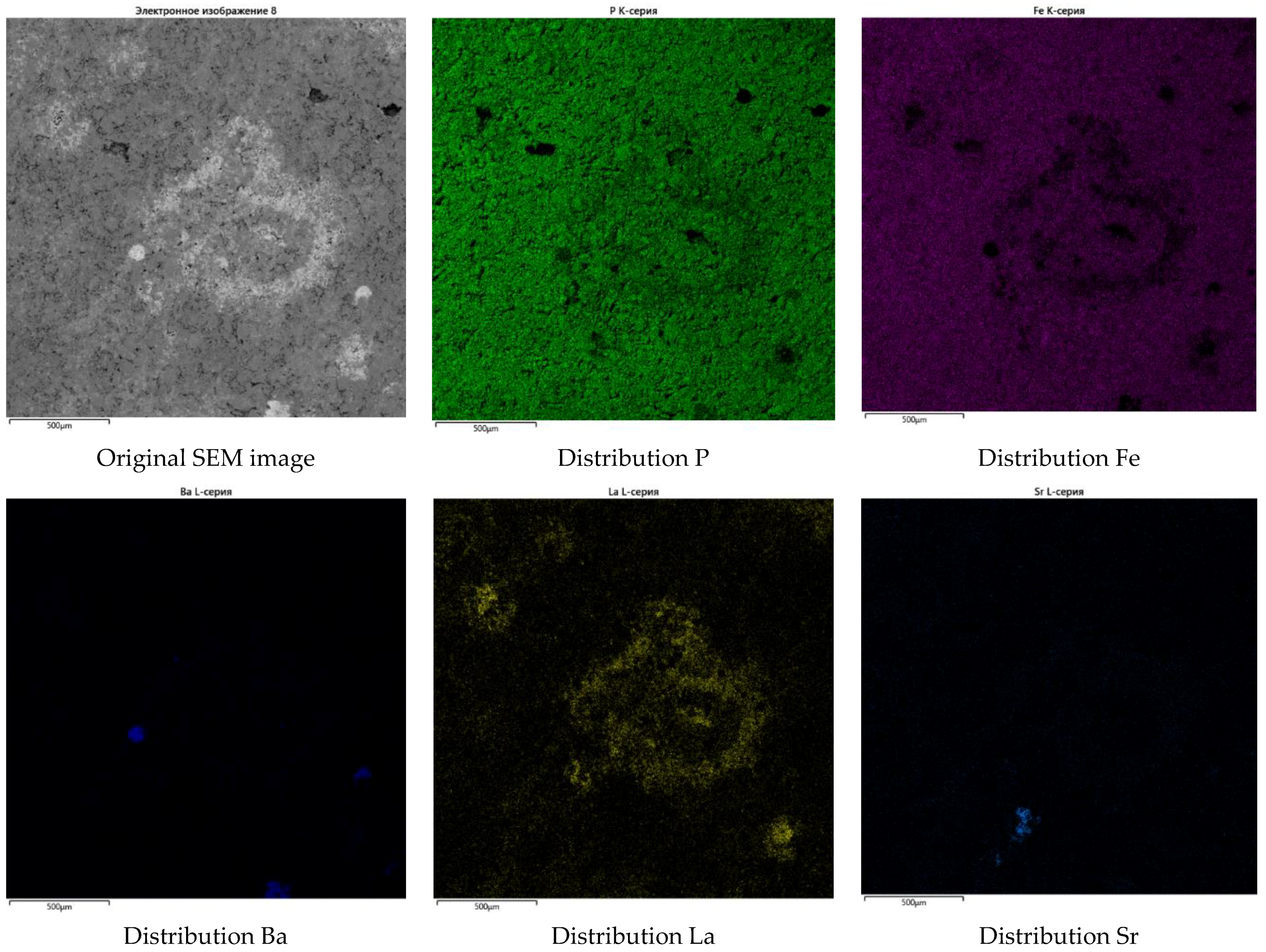
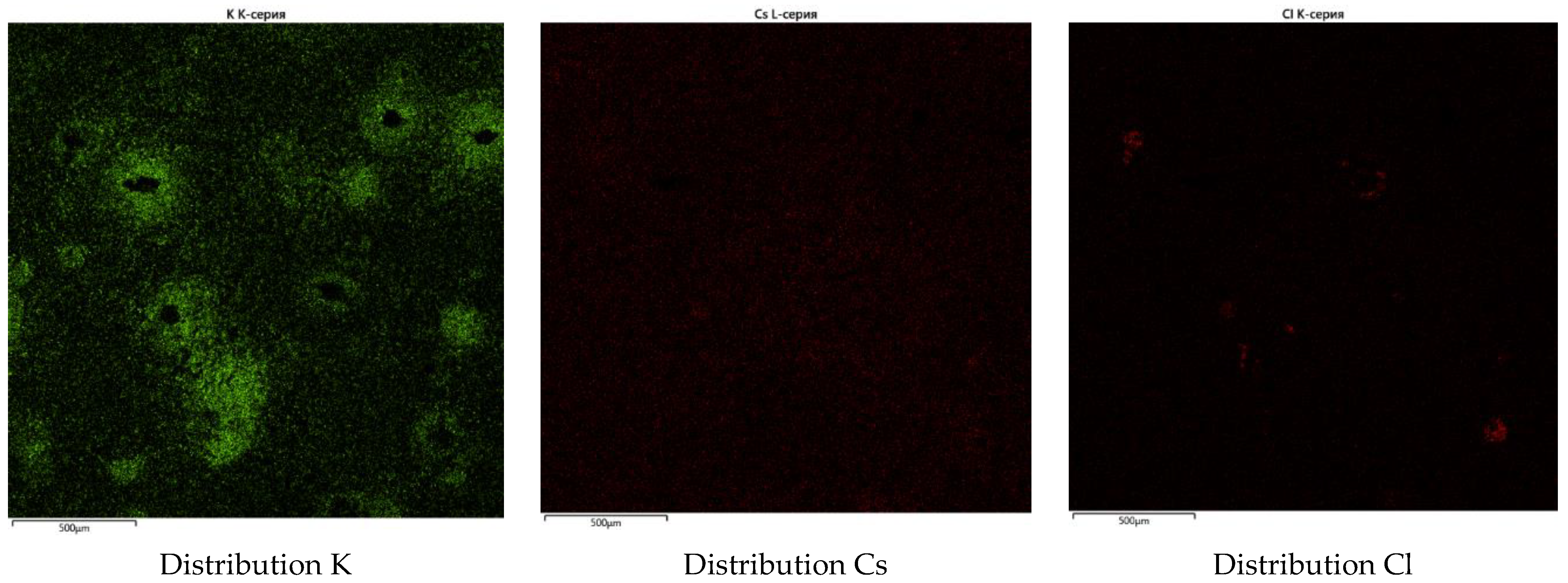

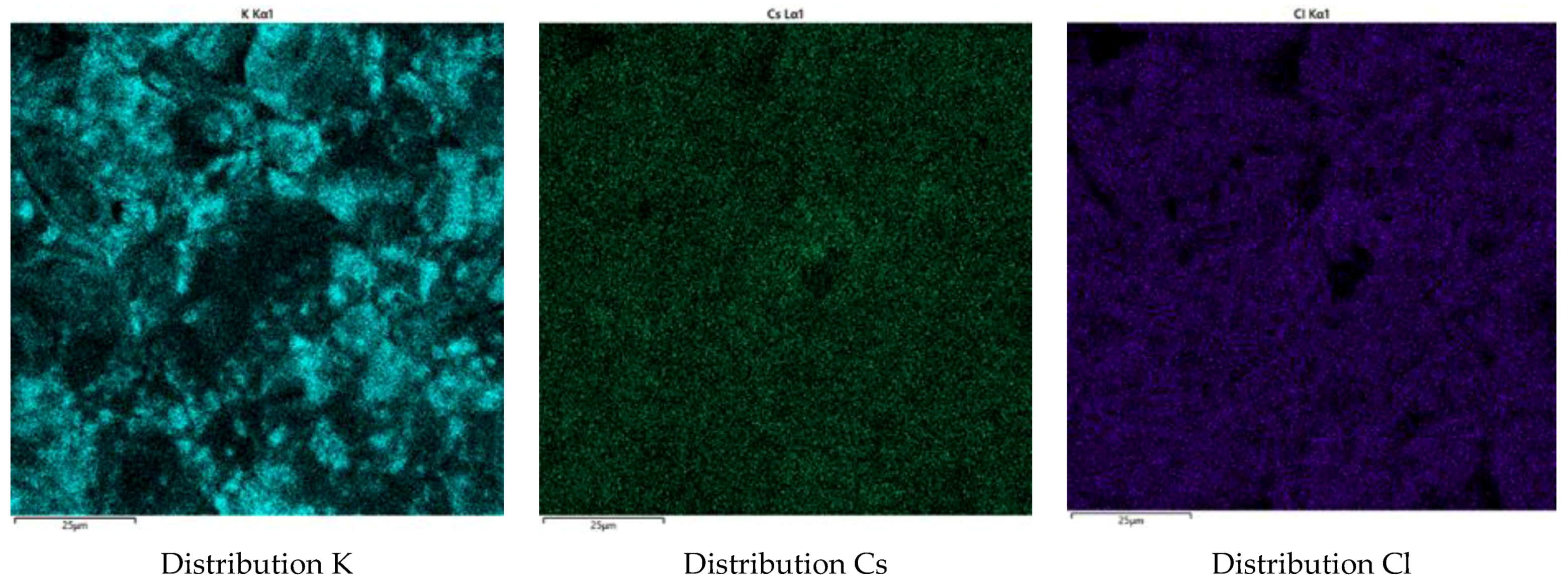

| Element | P | Fe | Cs | K | Li | La | Sr | Ba | O | Cl |
| Content | 23.6 | 25.2 | 0.2 | 1.7 | 0.4 | 1.7 | 0.2 | 0.3 | 41.3 | 5.4 |
| Synthesis Temperature | d (mm) | h (mm) | ρ (g/cm3) |
|---|---|---|---|
| Before synthesis | 10.10 | 3.33 | 2.04 |
| 650 °C | 10.08 | 3.30 | 2.07 |
| 700 °C | 10.04 | 3.28 | 2.13 |
| 750 °C | 9.95 | 3.12 | 2.24 |
| Element | Phase #1 | Phase #2 |
|---|---|---|
| P | 23.6 ± 2.5 | 22.7 ± 2.3 |
| Fe | 34.8 ± 4.1 | 33.7 ± 3.9 |
| O | 40.6 ± 4.5 | 39.6 ± 4.1 |
| K | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Cs | 0.4 ± 0.1 | - |
| La | 0.5 ± 0.2 | 3.9 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, A.V.; Belova, K.Y.; Vinokurov, S.E. Medium-Temperature Glass-Composite Phosphate Materials for the Immobilization of Chloride Radioactive Waste. J. Compos. Sci. 2023, 7, 363. https://doi.org/10.3390/jcs7090363
Frolova AV, Belova KY, Vinokurov SE. Medium-Temperature Glass-Composite Phosphate Materials for the Immobilization of Chloride Radioactive Waste. Journal of Composites Science. 2023; 7(9):363. https://doi.org/10.3390/jcs7090363
Chicago/Turabian StyleFrolova, Anna V., Ksenia Y. Belova, and Sergey E. Vinokurov. 2023. "Medium-Temperature Glass-Composite Phosphate Materials for the Immobilization of Chloride Radioactive Waste" Journal of Composites Science 7, no. 9: 363. https://doi.org/10.3390/jcs7090363
APA StyleFrolova, A. V., Belova, K. Y., & Vinokurov, S. E. (2023). Medium-Temperature Glass-Composite Phosphate Materials for the Immobilization of Chloride Radioactive Waste. Journal of Composites Science, 7(9), 363. https://doi.org/10.3390/jcs7090363







