CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrochemically Exfoliated Black Phosphorus (EEBP)
2.3. Preparation of CoP/EEBP
2.4. Preparation of CoP/EEBP/N-FLGS
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huangfu, Z.; Hu, H.; Xie, N.; Zhu, Y.-Q.; Chen, H.; Wang, Y. The Heterogeneous Influence of Economic Growth on Environmental Pollution: Evidence from Municipal Data of China. Pet. Sci. 2020, 17, 1180–1193. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon Quantum Dots for Advanced Electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Do, M.N.; Berezina, N.M.; Bazanov, M.I.; Gyseinov, S.S.; Berezin, M.M.; Koifman, O.I. Electrochemical Behavior of a Number of Bispyridyl-Substituted Porphyrins and Their Electrocatalytic Activity in Molecular Oxygen Reduction Reaction. J. Porphyrins Phthalocyanines 2016, 20, 615–623. [Google Scholar] [CrossRef]
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent Advances in Transition Metal Phosphide Nanomaterials: Synthesis and Applications in Hydrogen Evolution Reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Ruqia, B.; Choi, S. Catalytic Surface Specificity on Pt and Pt–Ni(OH)2 Electrodes for the Hydrogen Evolution Reaction in Alkaline Electrolytes and Their Nanoscaled Electrocatalysts. ChemSusChem 2018, 11, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Jiang, B.; Shen, W.; Chen, Y.; Li, Y.; Shen, Y.; Yin, K.; Shao, M. Ir-Au Bimetallic Nanoparticle Modified Silicon Nanowires with Ultralow Content of Ir for Hydrogen Evolution Reaction. ChemCatChem 2019, 11, 2126–2130. [Google Scholar] [CrossRef]
- Ma, F.; Xu, C.; Lyu, F.; Song, B.; Sun, S.; Li, Y.Y.; Lu, J.; Zhen, L. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef] [PubMed]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode Catalysts for Direct Methanol Fuel Cells in Acidic Media: Do We Have Any Alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in Fuel Cell Catalysis. Energy Environ. Sci. 2009, 2, 915. [Google Scholar] [CrossRef]
- Faber, M.S.; Jin, S. Earth-Abundant Inorganic Electrocatalysts and Their Nanostructures for Energy Conversion Applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent Advances in Heterogeneous Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon Nanotubes Decorated with CoP Nanocrystals: A Highly Active Non-Noble-Metal Nanohybrid Electrocatalyst for Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Yu, S.H.; Chua, D.H.C. Toward High-Performance and Low-Cost Hydrogen Evolution Reaction Electrocatalysts: Nanostructuring Cobalt Phosphide (CoP) Particles on Carbon Fiber Paper. ACS Appl. Mater. Interfaces 2018, 10, 14777–14785. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel-Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Yu, D.; Ilango, P.R.; Han, S.; Ye, M.; Hu, Y.; Li, L.; Peng, S. Metal-Organic Framework Derived Co@NC/CNT Hybrid as a Multifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reaction and Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2019, 44, 32054–32065. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Chen, D.; Li, J.; Klyui, N.I.; Han, W. MOF-Derived Nitrogen-Doped CoO@CoP Arrays as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Electrochim. Acta 2020, 330, 135210. [Google Scholar] [CrossRef]
- Sun, T.; Dong, J.; Huang, Y.; Ran, W.; Chen, J.; Xu, L. Highly Active and Stable Electrocatalyst of Ni 2 P Nanoparticles Supported on 3D Ordered Macro-/Mesoporous Co–N-Doped Carbon for Acidic Hydrogen Evolution Reaction. J. Mater. Chem. A 2018, 6, 12751–12758. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Ricciardulli, A.G.; Zhang, P.; Liao, Z.; Lohe, M.R.; Zschech, E.; Blom, P.W.M.; Pisula, W.; Müllen, K.; et al. A Rational Delamination Strategy towards Defect-Free, High- Mobility, Few-Layered Black Phosphorus Flakes. Angew. Chem. 2018, 130, 4767–4771. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Rui, K.; Hng, H.H.; Hippalgaonkar, K.; Xu, J.; Sun, W.; Zhu, J.; Yan, Q.; Huang, W. 2D Black Phosphorus for Energy Storage and Thermoelectric Applications. Small 2017, 13, 1700661. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Sun, Z. Opportunities and Challenges of Black Phosphorus for Electrocatalysis and Rechargeable Batteries. Adv. Sustain. Syst. 2022, 6, 2200301. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Allagui, A.; Wang, C. Single-step exfoliation of black phosphorus and deposition of phosphorene via bipolar electrochemistry for capacitive energy storage application. J. Mater. Chem. A 2019, 7, 25548–25556. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-dimensional black phosphorus nanomaterials: Emerging advances in electrochemical energy storage science. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Liu, H.; Hu, K.; Yan, D.; Chen, R.; Zou, Y.; Liu, H.; Wang, S. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater. 2018, 30, 1800295. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, Y.; Wu, X.; Shen, Q.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Black Phosphorus-Based Structures: Basic Properties, Synthesis, Stability, Photo- and Electrocatalysis-Driven Energy Conversion. Adv. Funct. Mater. 2021, 31, 2005197. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef] [PubMed]
- Kochergin, V.K.; Komarova, N.S.; Kotkin, A.S.; Manzhos, R.A.; Vasiliev, V.P.; Krivenko, A.G. Plasma Electrochemical Synthesis of Graphene-Phosphorene Composite and Its Catalytic Activity towards Hydrogen Evolution Reaction. C 2022, 8, 79. [Google Scholar] [CrossRef]
- Krivenko, A.G.; Manzhos, R.A.; Kotkin, A.S.; Kochergin, V.K.; Piven, N.P.; Manzhos, A.P. Production of Few-Layer Graphene Structures in Different Modes of Electrochemical Exfoliation of Graphite by Voltage Pulses. Instrum. Sci. Technol. 2019, 47, 535–544. [Google Scholar] [CrossRef]
- Belkin, P.N.; Yerokhin, A.; Kusmanov, S.A. Plasma Electrolytic Saturation of Steels with Nitrogen and Carbon. Surf. Coat. Technol. 2016, 307, 1194–1218. [Google Scholar] [CrossRef]
- Kochergin, V.K.; Manzhos, R.A.; Khodos, I.I.; Krivenko, A.G. One-Step Synthesis of Nitrogen-Doped Few-Layer Graphene Structures Decorated with Mn1.5Co1.5O4 Nanoparticles for Highly Efficient Electrocatalysis of Oxygen Reduction Reaction. Mendeleev Commun. 2022, 32, 492–494. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P Electrocatalyst for the Hydrogen Evolution Reaction and Direct Comparison with Morphologically Equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.-F. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. 2018, 130, 2630–2634. [Google Scholar] [CrossRef]
- Ha, D.-H.; Moreau, L.M.; Bealing, C.R.; Zhang, H.; Hennig, R.G.; Robinson, R.D. The Structural Evolution and Diffusion during the Chemical Transformation from Cobalt to Cobalt Phosphide Nanoparticles. J. Mater. Chem. 2011, 21, 11498. [Google Scholar] [CrossRef]
- Kotkin, A.S.; Kochergin, V.K.; Kabachkov, E.N.; Shulga, Y.M.; Lobach, A.S.; Manzhos, R.A.; Krivenko, A.G. One-Step Plasma Electrochemical Synthesis and Oxygen Electrocatalysis of Nanocomposite of Few-Layer Graphene Structures with Cobalt Oxides. Mater. Today Energy 2020, 17, 100459. [Google Scholar] [CrossRef]
- Manivasakan, P.; Ramasamy, P.; Kim, J. Use of Urchin-like NixCo3−xO4 Hierarchical Nanostructures Based on Non-Precious Metals as Bifunctional Electrocatalysts for Anion-Exchange Membrane Alkaline Alcohol Fuel Cells. Nanoscale 2014, 6, 9665–9672. [Google Scholar] [CrossRef]
- Liu, T.; Yan, X.; Xi, P.; Chen, J.; Qin, D.; Shan, D.; Devaramani, S.; Lu, X. Nickel–Cobalt Phosphide Nanowires Supported on Ni Foam as a Highly Efficient Catalyst for Electrochemical Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 14124–14132. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Monodispersed Nickel Phosphide Nanocrystals with Different Phases: Synthesis, Characterization and Electrocatalytic Properties for Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Chen, Z.; Lv, C.; Meng, H.; Zhang, C. Ni12P5 Nanoparticles as an Efficient Catalyst for Hydrogen Generation via Electrolysis and Photoelectrolysis. ACS Nano 2014, 8, 8121–8129. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Wik, S.D.; Cavell, R.G.; Mar, A. Examination of the Bonding in Binary Transition-Metal Monophosphides MP (M = Cr, Mn, Fe, Co) by X-Ray Photoelectron Spectroscopy. Inorg. Chem. 2005, 44, 8988–8998. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Luo, J.; Cheng, S.; Gong, H.; Liu, J.; Xu, C.; Zhao, Z.; Sun, Y.; Song, W.; et al. Porous N, P Co-Doped Carbon-Coated Ultrafine Co2P Nanoparticles Derived from DNA: An Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction. Electrochim. Acta 2021, 393, 139051. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis: By Auger and X-Ray Photoelectron Spectroscopy, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1990; p. 674. [Google Scholar]
- Duan, D.; Feng, J.; Liu, S.; Wang, Y.; Zhou, X. MOF-Derived Cobalt Phosphide as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction. J. Electroanal. Chem. 2021, 892, 115300. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Lv, X.; Ren, J.-T.; Wang, Y.; Liu, Y.-P.; Yuan, Z.-Y. Well-Defined Phase-Controlled Cobalt Phosphide Nanoparticles Encapsulated in Nitrogen-Doped Graphitized Carbon Shell with Enhanced Electrocatalytic Activity for Hydrogen Evolution Reaction in all-pH. ACS Sustain. Chem. Eng. 2019, 7, 8993–9001. [Google Scholar] [CrossRef]
- Song, J.; Zhu, C.; Xu, B.Z.; Fu, S.; Engelhard, M.H.; Ye, R.; Du, D.; Beckman, S.P.; Lin, Y. Bimetallic Cobalt-Based Phosphide Zeolitic Imidazolate Framework: CoPx Phase-Dependent Electrical Conductivity and Hydrogen Atom Adsorption Energy for Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1601555. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.-Y.; Paik, U. Nickel Cobalt Phosphides Quasi-Hollow Nanocubes as an Efficient Electrocatalyst for Hydrogen Evolution in Alkaline Solution. Chem. Commun. 2016, 52, 1633–1636. [Google Scholar] [CrossRef]
- Yang, M.; Feng, F.; Wang, K.; Li, S.; Huang, X.; Gong, L.; Ma, L.; Li, R. Synthesis of Metal Phosphide Nanoparticles Supported on Porous N-Doped Carbon Derived from Spirulina for Universal-pH Hydrogen Evolution. ChemSusChem 2020, 13, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Guo, B.-Y.; Chen, Q.-W.; Dong, B.; Zhang, J.-Q.; Qin, J.-F.; Xie, J.-Y.; Yang, M.; Wang, L.; Chai, Y.-M.; et al. Ultrafine and Highly-Dispersed Bimetal Ni2P/Co2P Encapsulated by Hollow N-Doped Carbon Nanospheres for Efficient Hydrogen Evolution. Int. J. Hydrogen Energy 2019, 44, 14908–14917. [Google Scholar] [CrossRef]
- Chen, A.; Fu, L.; Xiang, W.; Wei, W.; Liu, D.; Liu, C. Facile Synthesis of Ni5P4 Nanosheets/Nanoparticles for Highly Active and Durable Hydrogen Evolution. Int. J. Hydrogen Energy 2021, 46, 11701–11710. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Wei, W.; Lv, X.; Xie, J. CoP Nanoparticles Encapsulated in Three-Dimensional N-Doped Porous Carbon for Efficient Hydrogen Evolution Reaction in a Broad pH Range. Appl. Surf. Sci. 2019, 476, 749–756. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.; Zou, R. Metal-Organic Frameworks Derived Cobalt Phosphide Architecture Encapsulated into B/N Co-Doped Graphene Nanotubes for All pH Value Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1601671. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Stamenkovic, V.; Markovic, N. Electrocatalysis of the HER in Acid and Alkaline Media. J. Serb. Chem. Soc. 2013, 78, 2007–2015. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Catalysts for Electrochemical Water Splitting: Current State and Prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]

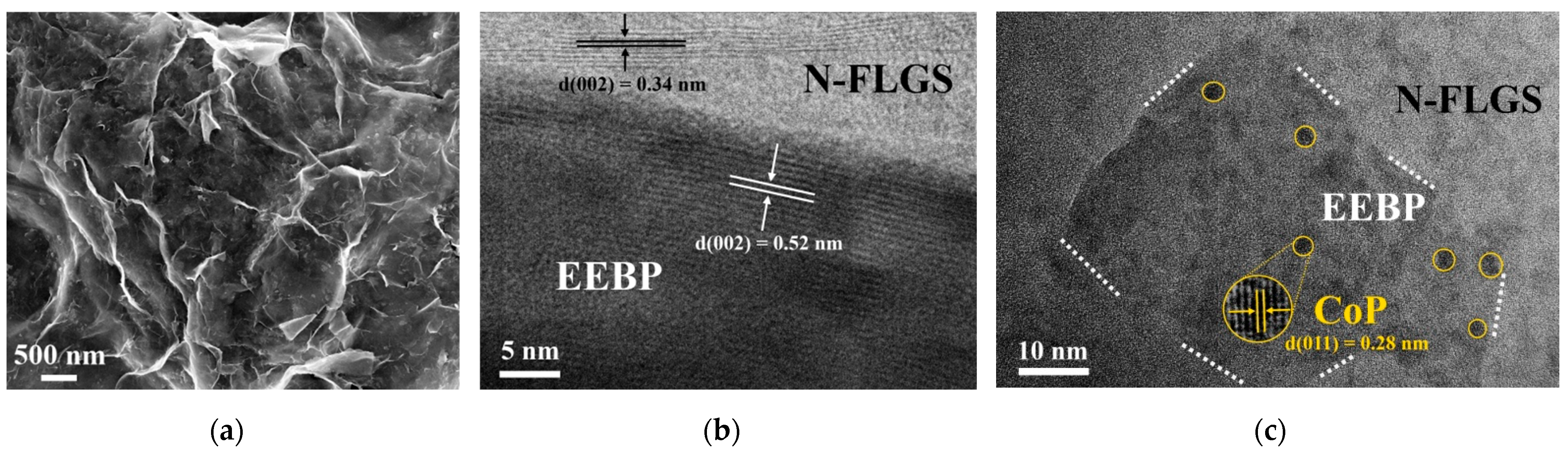
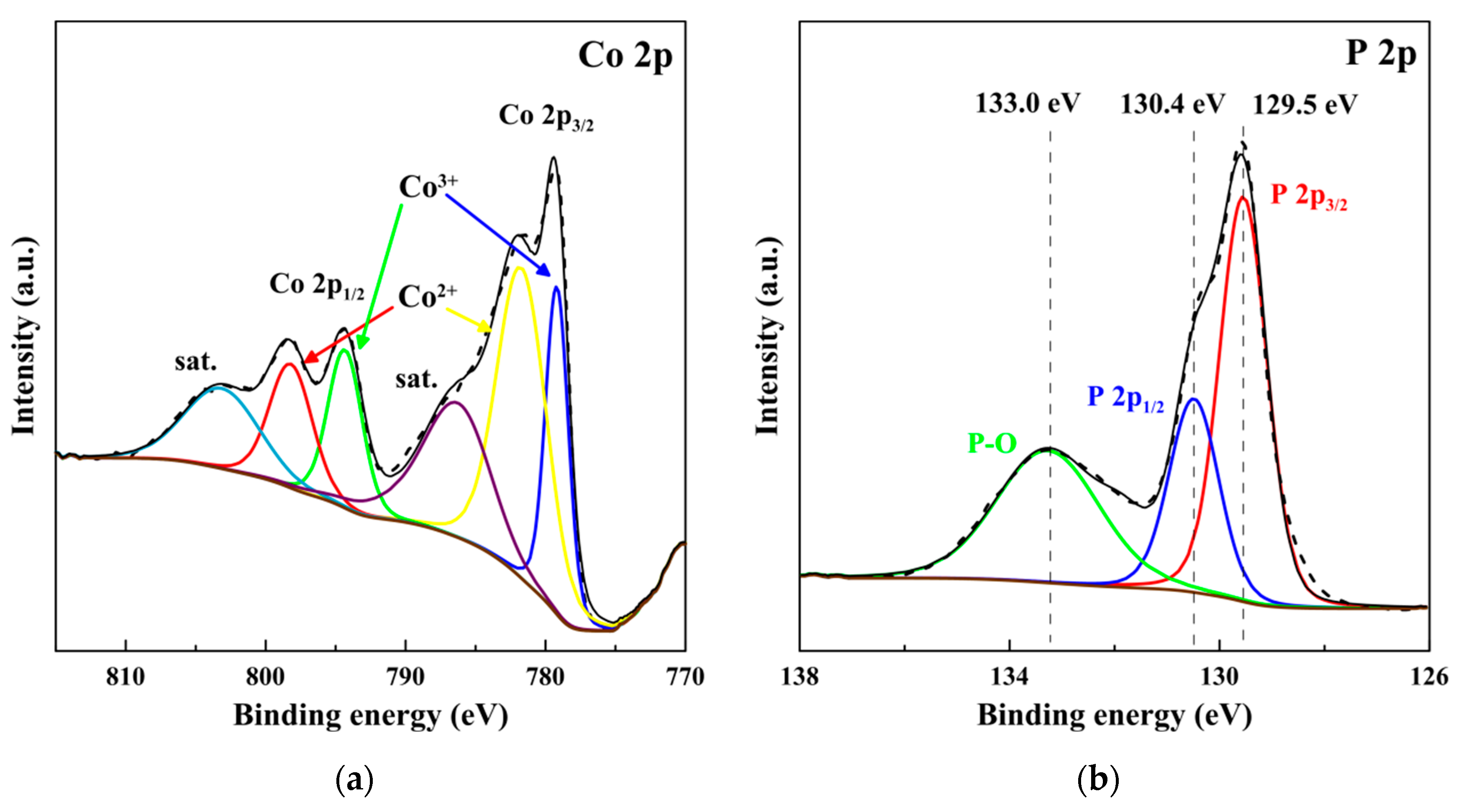
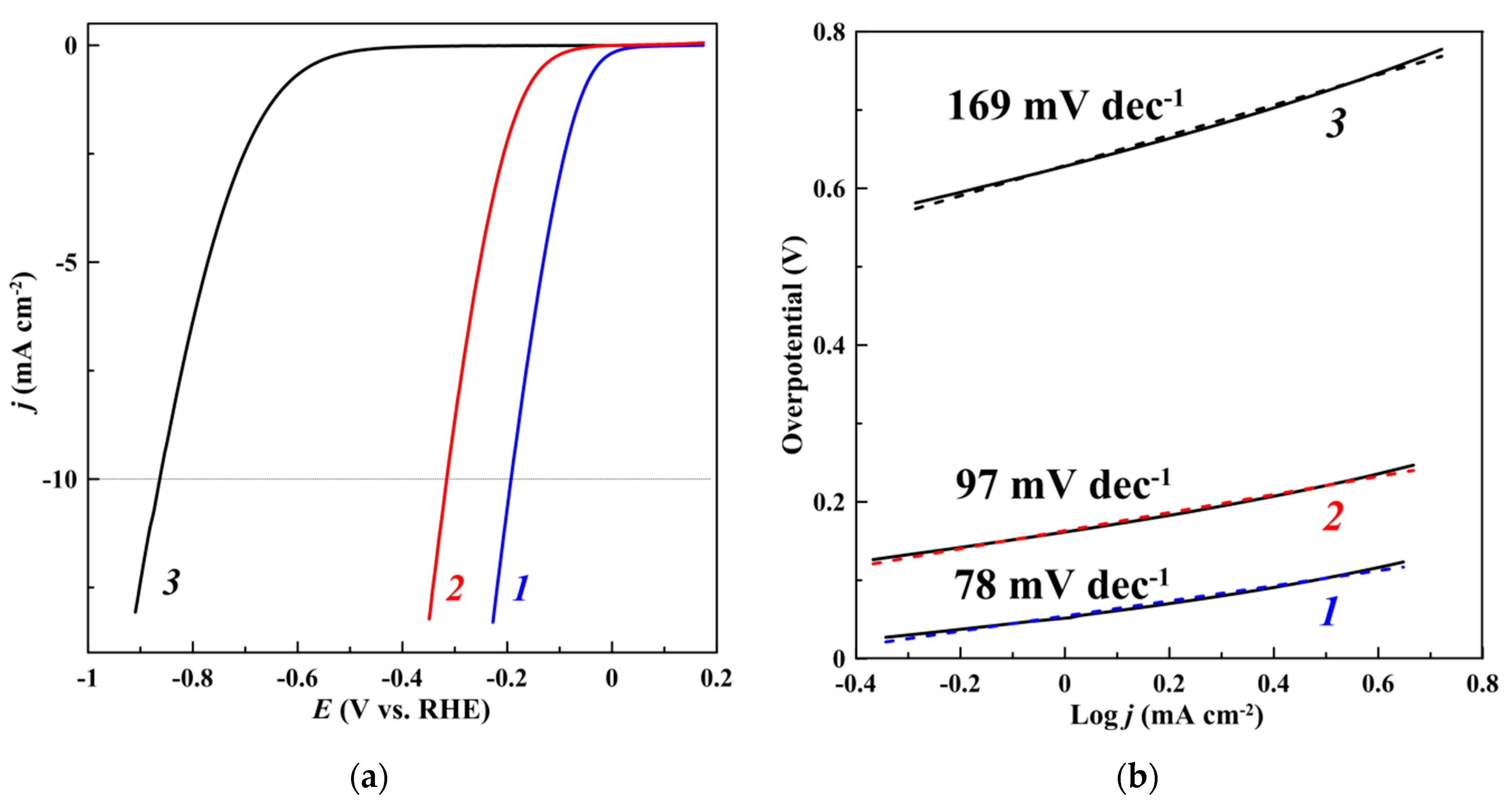
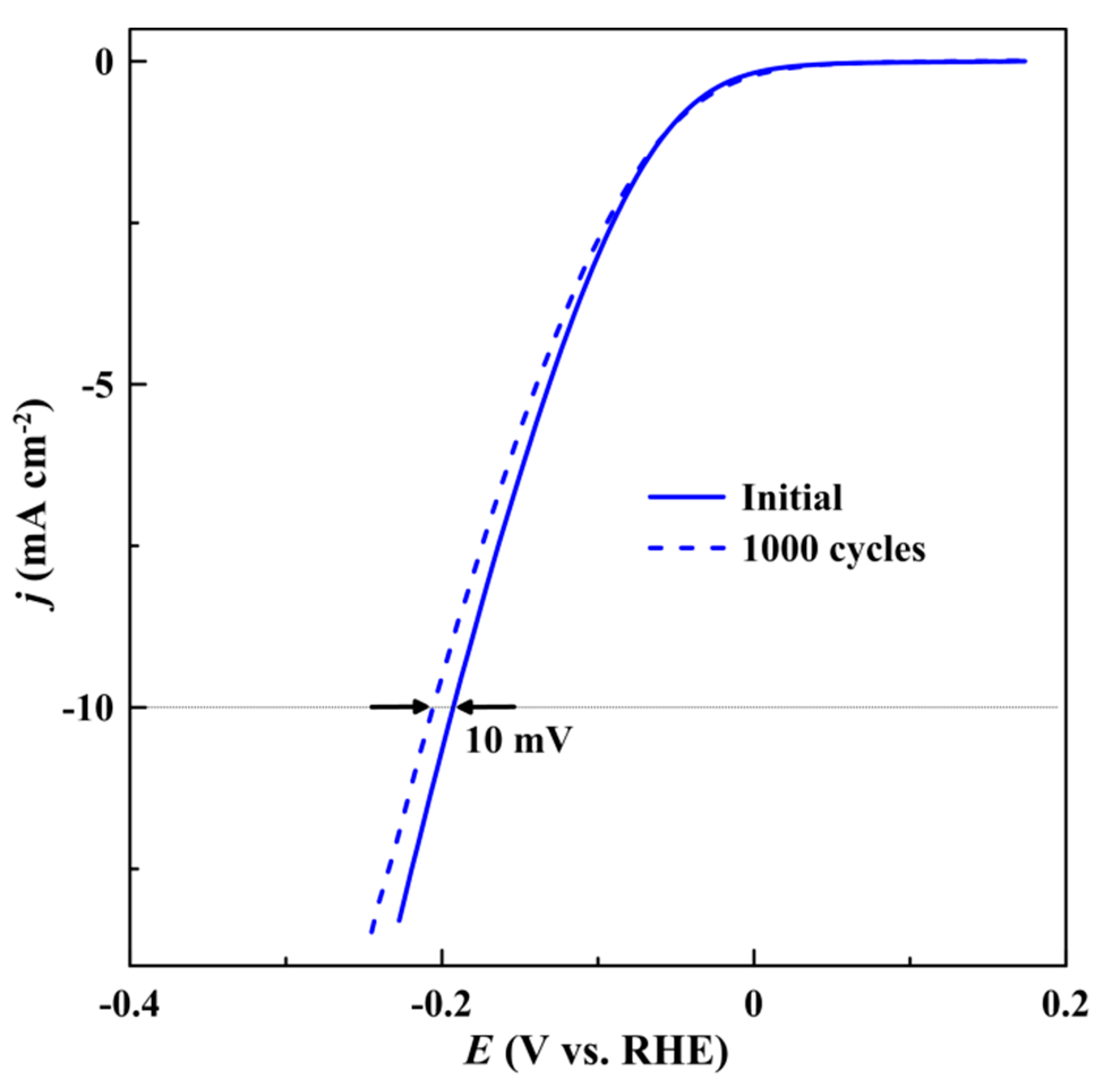
| Catalyst | η10 1, mV | Tafel slope, mV dec−1 |
|---|---|---|
| EEBP | 865 | 169 |
| CoP/EEBP | 315 | 97 |
| CoP/EEBP/N-FLGS | 190 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochergin, V.K.; Kotkin, A.S.; Manzhos, R.A.; Krivenko, A.G.; Khodos, I.I.; Kabachkov, E.N. CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media. J. Compos. Sci. 2023, 7, 328. https://doi.org/10.3390/jcs7080328
Kochergin VK, Kotkin AS, Manzhos RA, Krivenko AG, Khodos II, Kabachkov EN. CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media. Journal of Composites Science. 2023; 7(8):328. https://doi.org/10.3390/jcs7080328
Chicago/Turabian StyleKochergin, Valerii K., Alexander S. Kotkin, Roman A. Manzhos, Alexander G. Krivenko, Igor I. Khodos, and Eugene N. Kabachkov. 2023. "CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media" Journal of Composites Science 7, no. 8: 328. https://doi.org/10.3390/jcs7080328
APA StyleKochergin, V. K., Kotkin, A. S., Manzhos, R. A., Krivenko, A. G., Khodos, I. I., & Kabachkov, E. N. (2023). CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media. Journal of Composites Science, 7(8), 328. https://doi.org/10.3390/jcs7080328







