Metallic Iron or a Fe-Based Glassy Alloy to Reinforce Aluminum: Reactions at the Interface during Spark Plasma Sintering and Mechanical Properties of the Composites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
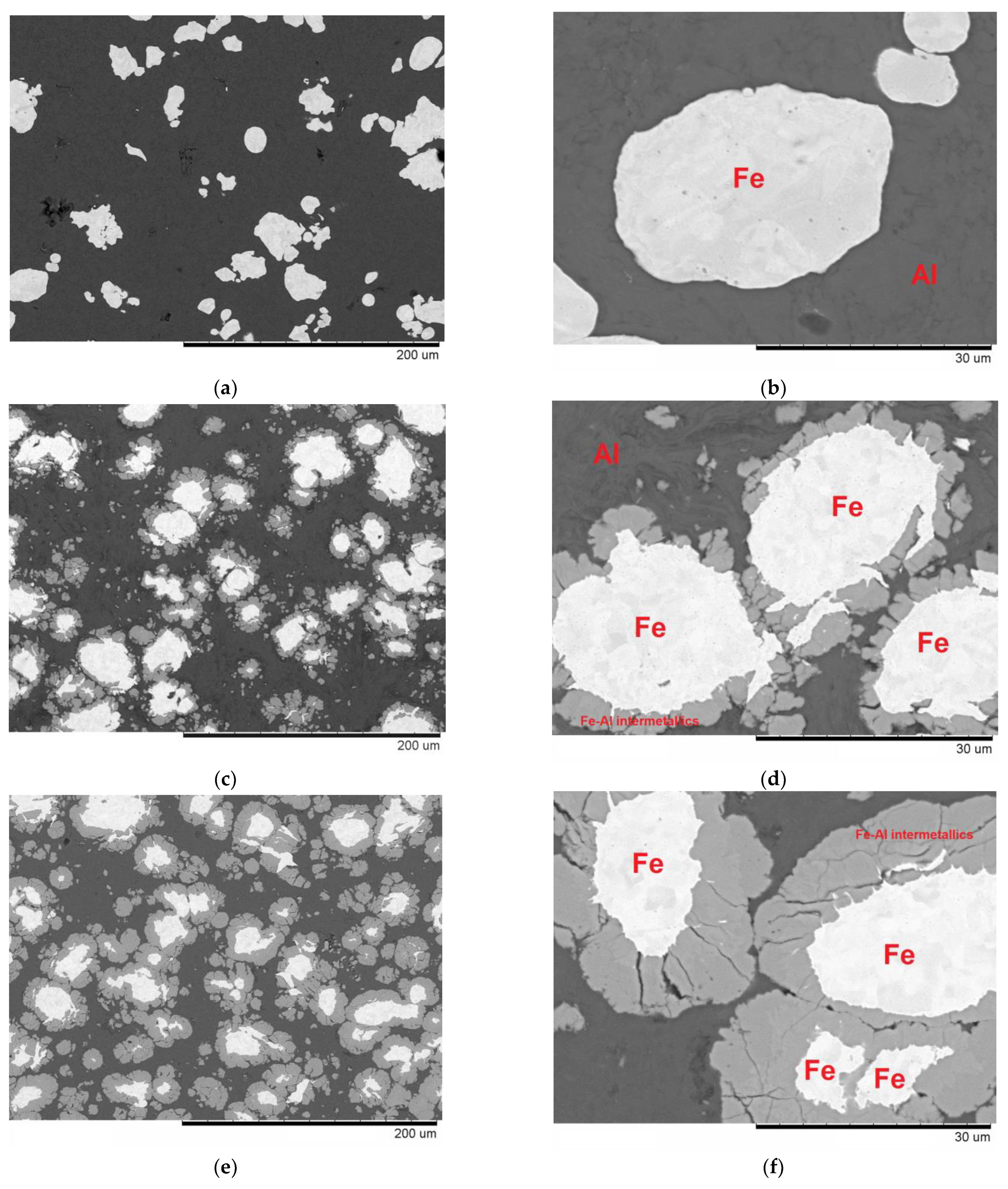
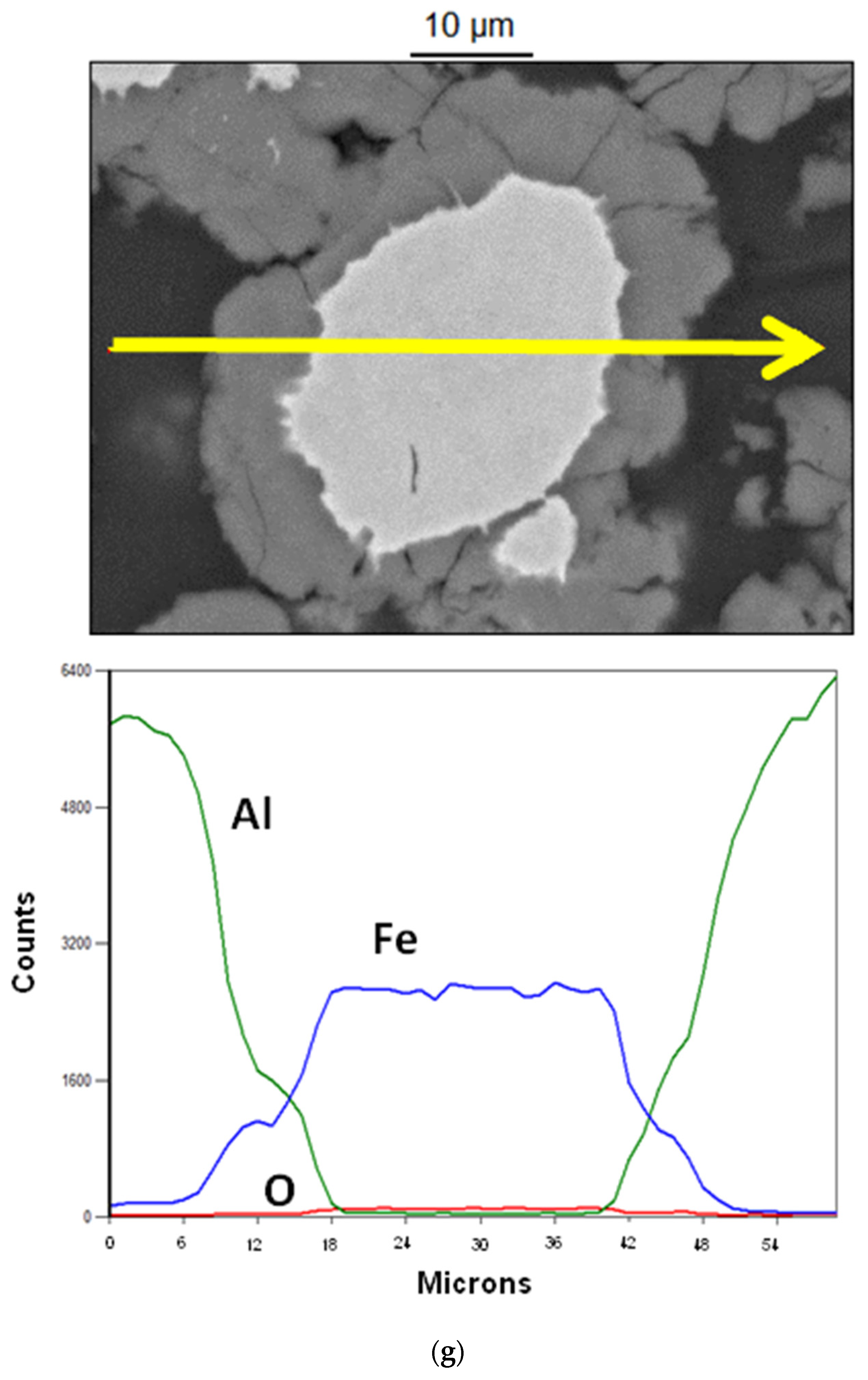
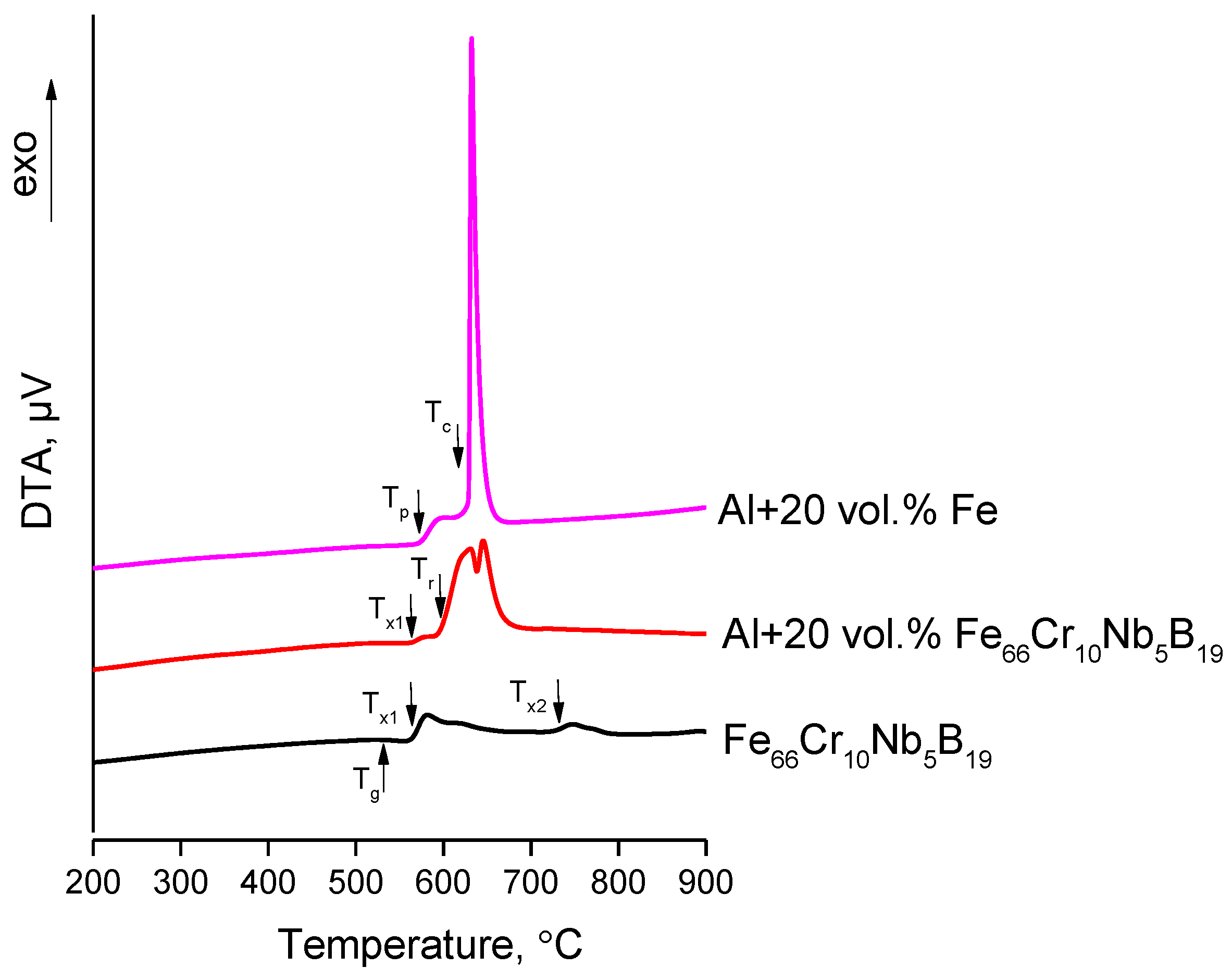
3.1. Reactions at the Al/Fe and Al/Fe66Cr10Nb5B19 Interface during Spark Plasma Sintering
3.2. Mechanical Properties of Composites Derived from Al-20 vol.% Fe and Al-20 vol.% Fe66Cr10Nb5B19—The Influence of the Nature of Added Particles on the Mechanical Properties of Composites
- The efficient load transfer to the reinforcement should be enabled in the composites, for which the product layer of a certain thickness between the matrix and the reinforcement is required.
- The intermetallic (shell) component should not be continuous.
- The glass core/intermetallic shell interface should be strong to avoid the separation of the reinforcement.
4. Conclusions
- Upon conventional heating of the Al + 20 vol.% Fe66Cr10Nb5B19 mixture, the exothermic reaction at the interface starts at 595 °C, while the reaction between Fe and Al starts at 570 °C. Conducting SPS experiments at two different pressures (40 MPa and 80 MPa) allowed us to reveal the formation features of the Fe-based glass/Al and Fe/Al interfaces. Local overheating at the Fe-based glass/Al interface during SPS governed the formation of the product layers at 40 MPa. A higher pressure hindered the reactions by lowering the interfacial resistance in the composite. The opposite behavior was observed in the Fe-Al system—a higher pressure providing a better interfacial contact and facilitating the interaction at the interface. Both SPS temperature and pressure can be used as parameters in the structural design of composites influencing the reactivity of the mixture components.
- The micrometer-sized particles of iron and metallic glass added at a concentration of 20 vol.% cannot provide significant levels of strengthening to an aluminum matrix. In composites without the reaction products or with thin layers of the products, the hardness of the composite increased by 13–38% relative to the unreinforced sintered aluminum, the added particles of metallic glass and iron producing similar outcomes.
- The influence of the nature of added particles on the mechanical properties of composites could be seen when the microstructure of the composite enabled an efficient load transfer from the matrix to the reinforcement. This was possible upon the formation of thick layers of the reaction products between Al and the added reinforcement. Upon compressive loading, the composite with the glassy component showed a much higher strength than the composite containing pure iron cores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawla, N.; Chawla, K.K. Metal Matrix Composites, 2nd ed.; Springer Science + Business Media: New York, NY, USA, 2013; 370p. [Google Scholar]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Dadkhah, M.; Saboori, A.; Fino, P. An overview of the recent developments in metal matrix nanocomposites reinforced by graphene. Materials 2019, 12, 2823. [Google Scholar] [CrossRef]
- Karthik, B.M.; Gowrishankar, M.C.; Sharma, S.; Hiremath, P.; Shettar, M.; Shetty, N. Coated and uncoated reinforcements metal matrix composites characteristics and applications—A critical review. Cogent Eng. 2020, 7, 1856758. [Google Scholar]
- Alaneme, K.K.; Okotete, E.A.; Fajemisin, A.V.; Bodunrin, M.O. Applicability of metallic reinforcements for mechanical performance enhancement in metal matrix composites: A review. Arab J. Basic Appl. Sci. 2019, 26, 311–330. [Google Scholar] [CrossRef]
- Dudina, D.V.; Georgarakis, K.; Olevsky, E.A. Progress in aluminium and magnesium matrix composites obtained by spark plasma, microwave and induction sintering. Int. Mater. Rev. 2023, 68, 225–246. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of ductile magnesium composite materials using titanium as reinforcement. J. Alloys Comp. 2002, 345, 246–251. [Google Scholar] [CrossRef]
- Leong, W.W.; Gupta, M. Enhancing thermal stability, modulus and ductility of magnesium using molybdenum as reinforcement. Adv. Eng. Mater. 2005, 7, 250–256. [Google Scholar]
- Aljerf, M.; Georgarakis, K.; Louzguine-Luzgin, D.; Le Moulec, A.; Inoue, A.; Yavari, A.R. Strong and light metal matrix composites with metallic glass particulate reinforcement. Mater. Sci. Eng. A 2012, 532, 325–330. [Google Scholar] [CrossRef]
- Bao, W.; Chen, J.; Yang, X.; Xiang, T.; Cai, Z.; Xie, G. Improved strength and conductivity of metallic-glass-reinforced nanocrystalline CuCrZr alloy. Mater. Des. 2022, 214, 110420. [Google Scholar] [CrossRef]
- Chen, T.; Gao, M.; Tong, Y. Effects of alloying elements on the formation of core-shell-structured reinforcing particles during heating of Al–Ti powder compacts. Materials 2018, 11, 138. [Google Scholar] [CrossRef]
- Dudina, D.V.; Georgarakis, K. Core–shell particle reinforcements—A new trend in the design and development of metal matrix composites. Materials 2022, 15, 2629. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Li, F.; Wang, W.; Hou, J.; Cui, W.; Li, Y. Study on in-situ synthesis process of Ti–Al intermetallic compound-reinforced Al matrix composites. Materials 2019, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shen, R.; Ni, S.; Xiao, D.; Song, M. Effects of sintering atmosphere on the mechanical properties of Al-Fe particle-reinforced Al-based composites. J. Mater. Eng. Perform. 2015, 24, 1890–1896. [Google Scholar] [CrossRef]
- Park, K.; Park, J.; Kwon, H. Fabrication and characterization of Al-SUS316L composite materials manufactured by the spark plasma sintering process. Mater. Sci. Eng. A 2017, 691, 8–15. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Ashby, M.F.; Greer, A.L. Metallic glasses as structural materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Yavari, A.R.; Lewandowski, J.J.; Eckert, J. Mechanical properties of bulk metallic glasses. MRS Bull. 2007, 32, 635–638. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Kruzic, J.J. Bulk metallic glasses as structural materials: A review. Adv. Eng. Mater. 2016, 18, 1308–1331. [Google Scholar] [CrossRef]
- Souza, C.A.C.; Ribeiro, D.V.; Kiminami, C.S. Corrosion resistance of Fe-Cr-Based amorphous alloys: An overview. J. Non Cryst. Solids 2016, 442, 56–66. [Google Scholar] [CrossRef]
- Burkov, A.A.; Chigrin, P.G. Effect of tungsten, molybdenum, nickel and cobalt on the corrosion and wear performance of Fe-based metallic glass coatings. Surf. Coat. Technol. 2018, 351, 68–77. [Google Scholar] [CrossRef]
- Ning, W.; Zhai, H.; Xiao, R.; He, D.; Liang, G.; Wu, Y.; Li, W.; Li, X. The corrosion resistance mechanism of Fe-based amorphous coatings synthesized by detonation gun spraying. J. Mater. Eng. Perform. 2020, 29, 3921–3929. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Sankaranarayanan, S.; Gupta, M. Processing and properties of aluminum and magnesium-based composites containing amorphous reinforcement: A review. Metals 2015, 5, 743–762. [Google Scholar]
- Jayalakshmi, S.; Arvind Singh, R.; Gupta, M. Metallic glasses as potential reinforcements in Al and Mg matrices: A review. Technologies 2018, 6, 40. [Google Scholar] [CrossRef]
- Georgarakis, K.; Dudina, D.V.; Kvashnin, V.I. Metallic glass-reinforced metal matrix composites: Design, interfaces and properties. Materials 2022, 15, 8278. [Google Scholar] [CrossRef]
- Qu, R.T.; Liu, Z.Q.; Wang, R.F.; Zhang, Z.F. Yield strength and yield strain of metallic glasses and their correlations with glass transition temperature. J. Alloys Compd. 2015, 637, 44–54. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; Wu, K.; Lin, H.; Wen, W.; Ruan, W.; Ren, S.; Zhang, Z.; Liang, X.; Ma, J. Metallic glue for designing composite materials with tailorable properties. Mater. Horiz. 2021, 8, 1690–1699. [Google Scholar] [CrossRef]
- Kvashnin, V.I.; Dudina, D.V.; Ukhina, A.V.; Koga, G.Y.; Georgarakis, K. The benefit of the glassy state of reinforcing particles for the densification of aluminum matrix composites. J. Compos. Sci. 2022, 6, 135. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Cahn, R.W. Atomic transport in amorphous alloys: An introduction. J. Vac. Sci. Technol. A 1986, 4, 3071. [Google Scholar] [CrossRef]
- Kvashnin, V.I.; Dudina, D.V.; Bokhonov, B.B.; Petrov, S.A.; Ukhina, A.V.; Georgarakis, K.; Coury, F.G.; Koga, G.Y. Reactivity of a glassy and a crystalline Fe66Cr10Nb5B19 alloy towards aluminum during sintering: A comparative study. Mater. Lett. 2023, 347, 134582. [Google Scholar] [CrossRef]
- Tokita, M. Trends in advanced SPS spark plasma sintering systems and technology. J. Soc. Powder Technol. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Lee, G.; Manière, C.; McKittrick, J.; Olevsky, E.A. Electric current effects in spark plasma sintering: From the evidence of physical phenomenon to constitutive equation formulation. Scr. Mater. 2019, 170, 90–94. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark Plasma Sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 2012, 983470. [Google Scholar] [CrossRef]
- Saunders, T.; Grasso, S.; Reece, M.J. Plasma formation during electric discharge (50 V) through conductive powder compacts. J. Eur. Ceram. Soc. 2015, 35, 871–877. [Google Scholar] [CrossRef]
- Weston, N.S.; Thomas, B.; Jackson, M. Processing metal powders via field assisted sintering technology (FAST): A critical review. Mater. Sci. Technol. 2019, 35, 1306–1328. [Google Scholar] [CrossRef]
- Monchoux, J.P.; Couret, A.; Durand, L.; Voisin, T.; Trzaska, Z.; Thomas, M. Elaboration of metallic materials by SPS: Processing, microstructures, properties, and shaping. Metals 2021, 11, 322. [Google Scholar] [CrossRef]
- Abedi, M.; Sovizi, S.; Azarniya, A.; Giuntini, D.; Seraji, M.E.; Hosseini, H.R.M.; Amutha, C.; Ramakrishna, S.; Mukasyan, A. An analytical review on Spark Plasma Sintering of metals and alloys: From processing window, phase transformation, and property perspective. Crit. Rev. Solid State Mater. Sci. 2023, 48, 169–214. [Google Scholar] [CrossRef]
- Garay, J.E.; Anselmi-Tamburini, U.; Munir, Z.A. Enhanced growth of intermetallic phases in the Ni–Ti system by current effects. Acta Mater. 2003, 51, 4487–4495. [Google Scholar] [CrossRef]
- Li, R.; Yuan, T.; Liu, X.; Zhou, K. Enhanced atomic diffusion of Fe–Al diffusion couple during spark plasma sintering. Scr. Mater. 2016, 110, 105–108. [Google Scholar] [CrossRef]
- Abedi, M.; Asadi, A.; Sovizi, S.; Moskovskikh, D.; Vorotilo, S.; Mukasyan, A. Influence of pulsed direct current on the growth rate of intermetallic phases in the Ni–Al system during reactive spark plasma sintering. Scr. Mater. 2022, 216, 114759. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Batraev, I.S.; Amirastanov, Y.N.; Ukhina, A.V.; Kuchumova, I.D.; Legan, M.A.; Novoselov, A.N.; Gerasimov, K.B.; Bataev, I.A.; et al. Interaction between Fe66Cr10Nb5B19 metallic glass and aluminum during spark plasma sintering. Mater. Sci. Eng. A 2021, 799, 140165. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Batraev, I.S.; Kvashnin, V.I.; Legan, M.A.; Novoselov, A.N.; Anisimov, A.G.; Esikov, M.A.; Ukhina, A.V.; Matvienko, A.A.; et al. Microstructure and mechanical properties of composites obtained by spark plasma sintering of Al–Fe66Cr10Nb5B19 metallic glass powder mixtures. Metals 2021, 11, 1457. [Google Scholar] [CrossRef]
- Crivelli, I.V.; Esposito, E.; Mele, G.; Siniscalchi, A. Formatura per Spark Sintering. Metall Ital. 1973, 65, 611–618. [Google Scholar]
- Kol’chinskii, M.Z.; Medvedenko, N.F.; Solonin, Y.M.; Gnatush, F.P. Electric-discharge sintering of mixtures of aluminum and copper powders. Sov. Powder Metall. Metal Ceram. 1977, 16, 504–507. [Google Scholar] [CrossRef]
- Ye, Y.; Li, X.; Hu, K.; Lai, Y.; Li, Y. The influence of premolding load on the electrical behavior in the initial stage of electric current activated sintering of carbonyl iron powders. J. Appl. Phys. 2013, 113, 214902. [Google Scholar] [CrossRef]
- Collard, C.; Trzaska, Z.; Durand, L.; Chaix, J.M.; Monchoux, J.P. Theoretical and experimental investigations of local overheating at particle contacts in spark plasma sintering. Powder Technol. 2017, 321, 458–470. [Google Scholar] [CrossRef]
- Choy, C.L.; Leung, W.P.; Ng, Y.K. Thermal conductivity of metallic glasses. J. Appl. Phys. 1989, 66, 5335–5339. [Google Scholar] [CrossRef]
- Sina, H.; Corneliusson, J.; Turba, K.; Iyengar, S. A study on the formation of iron aluminide (FeAl) from elemental powders. J. Alloys Compd. 2015, 636, 261–269. [Google Scholar] [CrossRef]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of low-symmetry structures from phase equilibrium of Fe-Al system—Microstructures and mechanical properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef] [PubMed]





| Materials Designation | Composition of the Powder Mixture | SPS Temperature, C | Soaking Time, min | SPS Pressure, MPa | Sample Diameter, mm | Note |
|---|---|---|---|---|---|---|
| I | Al + 20 vol.% Fe | 500 | 0 | 40 | 12.5 | This work |
| II | Al + 20 vol.% Fe | 540 | 1 | 40 | 12.5 | This work |
| III | Al + 20 vol.% Fe | 540 | 1 | 80 | 12.5 | This work |
| IV | Al + 20 vol.% Fe | 570 | 1 | 40 | 20.0 | This work |
| V | Al + 20 vol.% Fe66Cr10Nb5B19 | 570 | 3 | 40 | 20.0 | [45,46] |
| Material Designation | Al Matrix Content, vol.% | Microstructural Features | Vickers Hardness, HV1 | Note |
|---|---|---|---|---|
| I | 80 | No reaction layer | 45 ± 5 | This work |
| II | 60 | Thin reaction layer | 55 ± 10 | This work |
| III | 45 | Thick reaction layer | 100 ± 10 | This work |
| Material Designation | Al Matrix Content, vol.% | Microstructural Features | Vickers Hardness, HV1 | Ultimate Compressive Strength, MPa | Fracture Strain, % | Note |
|---|---|---|---|---|---|---|
| IV | 40 | Thick reaction layer | 190 ± 40 | 470 ± 10 | 3 | This work |
| V | 37 | Thick reaction layer | 280 ± 40 | 780 ± 10 | 2 | [26,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudina, D.V.; Kvashnin, V.I.; Bokhonov, B.B.; Legan, M.A.; Novoselov, A.N.; Bespalko, Y.N.; Jorge, A.M., Jr.; Koga, G.Y.; Ukhina, A.V.; Shtertser, A.A.; et al. Metallic Iron or a Fe-Based Glassy Alloy to Reinforce Aluminum: Reactions at the Interface during Spark Plasma Sintering and Mechanical Properties of the Composites. J. Compos. Sci. 2023, 7, 302. https://doi.org/10.3390/jcs7070302
Dudina DV, Kvashnin VI, Bokhonov BB, Legan MA, Novoselov AN, Bespalko YN, Jorge AM Jr., Koga GY, Ukhina AV, Shtertser AA, et al. Metallic Iron or a Fe-Based Glassy Alloy to Reinforce Aluminum: Reactions at the Interface during Spark Plasma Sintering and Mechanical Properties of the Composites. Journal of Composites Science. 2023; 7(7):302. https://doi.org/10.3390/jcs7070302
Chicago/Turabian StyleDudina, Dina V., Vyacheslav I. Kvashnin, Boris B. Bokhonov, Mikhail A. Legan, Aleksey N. Novoselov, Yuliya N. Bespalko, Alberto Moreira Jorge, Jr., Guilherme Y. Koga, Arina V. Ukhina, Alexandr A. Shtertser, and et al. 2023. "Metallic Iron or a Fe-Based Glassy Alloy to Reinforce Aluminum: Reactions at the Interface during Spark Plasma Sintering and Mechanical Properties of the Composites" Journal of Composites Science 7, no. 7: 302. https://doi.org/10.3390/jcs7070302
APA StyleDudina, D. V., Kvashnin, V. I., Bokhonov, B. B., Legan, M. A., Novoselov, A. N., Bespalko, Y. N., Jorge, A. M., Jr., Koga, G. Y., Ukhina, A. V., Shtertser, A. A., Anisimov, A. G., & Georgarakis, K. (2023). Metallic Iron or a Fe-Based Glassy Alloy to Reinforce Aluminum: Reactions at the Interface during Spark Plasma Sintering and Mechanical Properties of the Composites. Journal of Composites Science, 7(7), 302. https://doi.org/10.3390/jcs7070302












