Effect of ECAP on Microstructure, Mechanical Properties, Corrosion Behavior, and Biocompatibility of Mg-Ca Alloy Composite
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
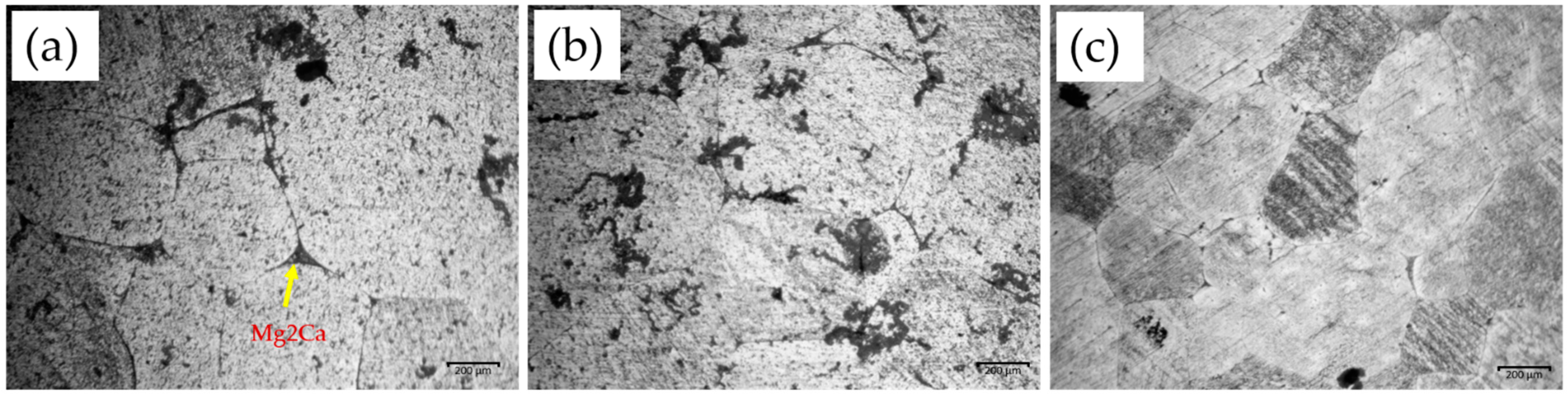
3.1. Materials Characterization
Microstructural Analysis
3.2. Mechanical Properties
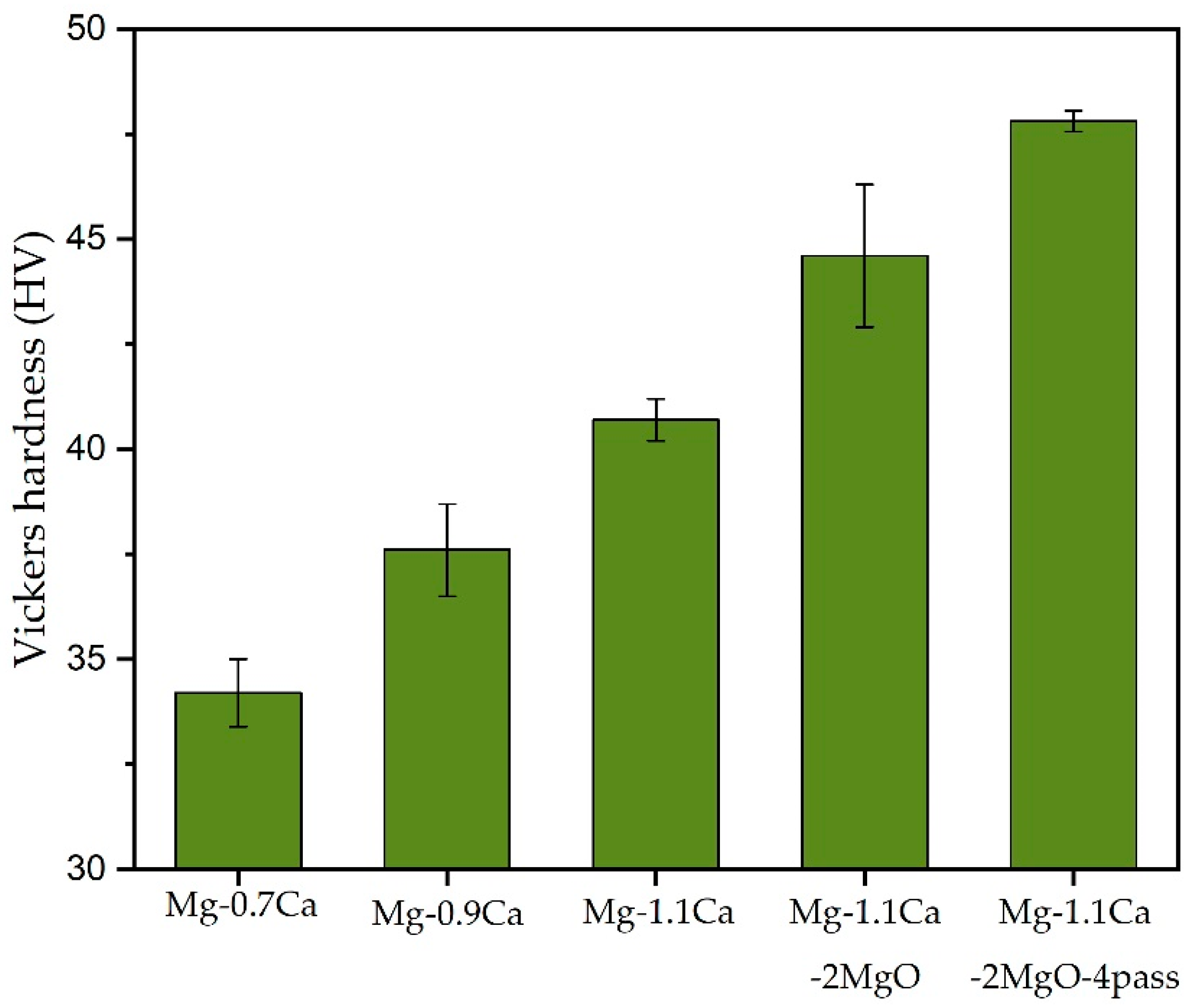
3.2.1. Hardness
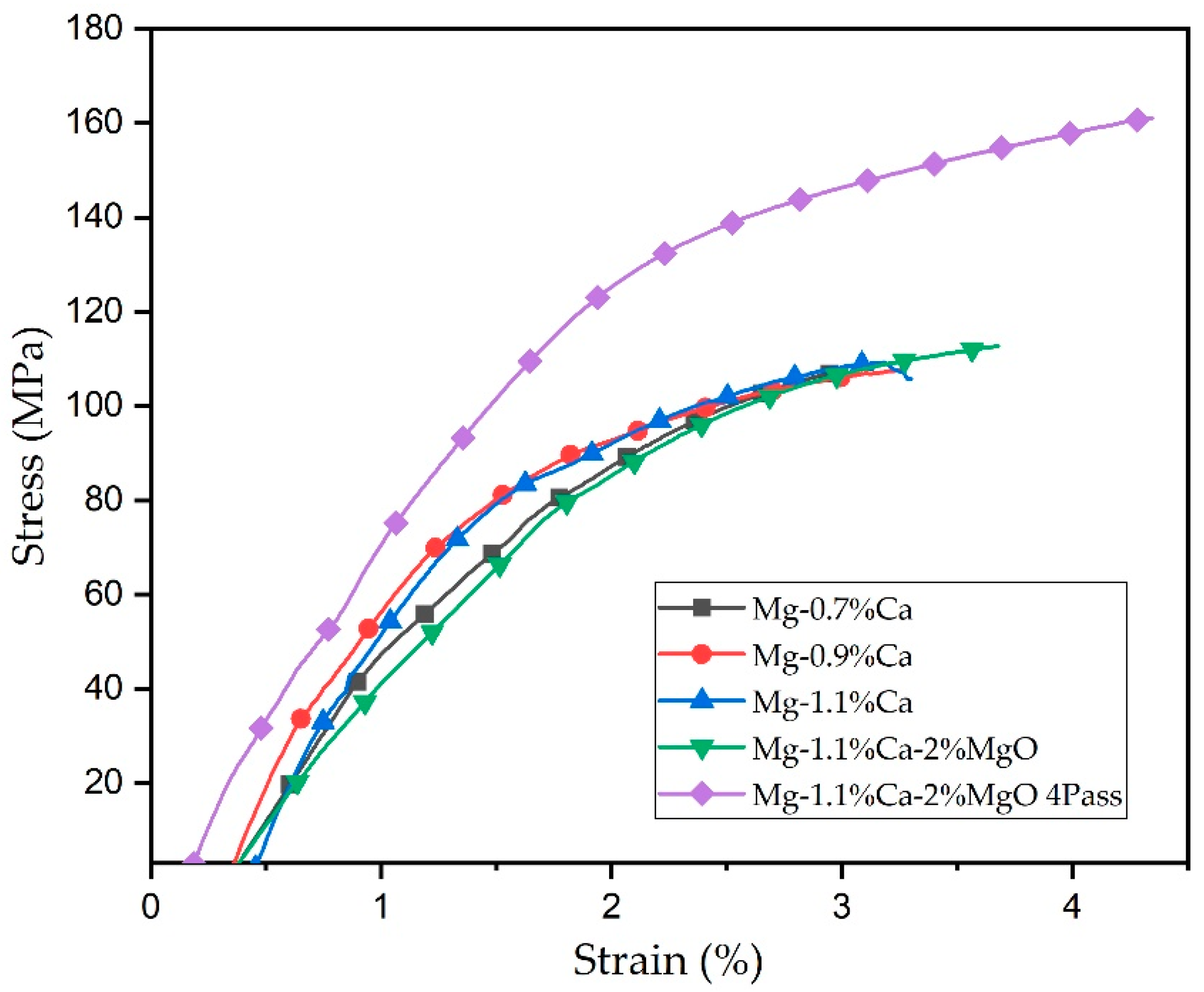
3.2.2. Tensile Test
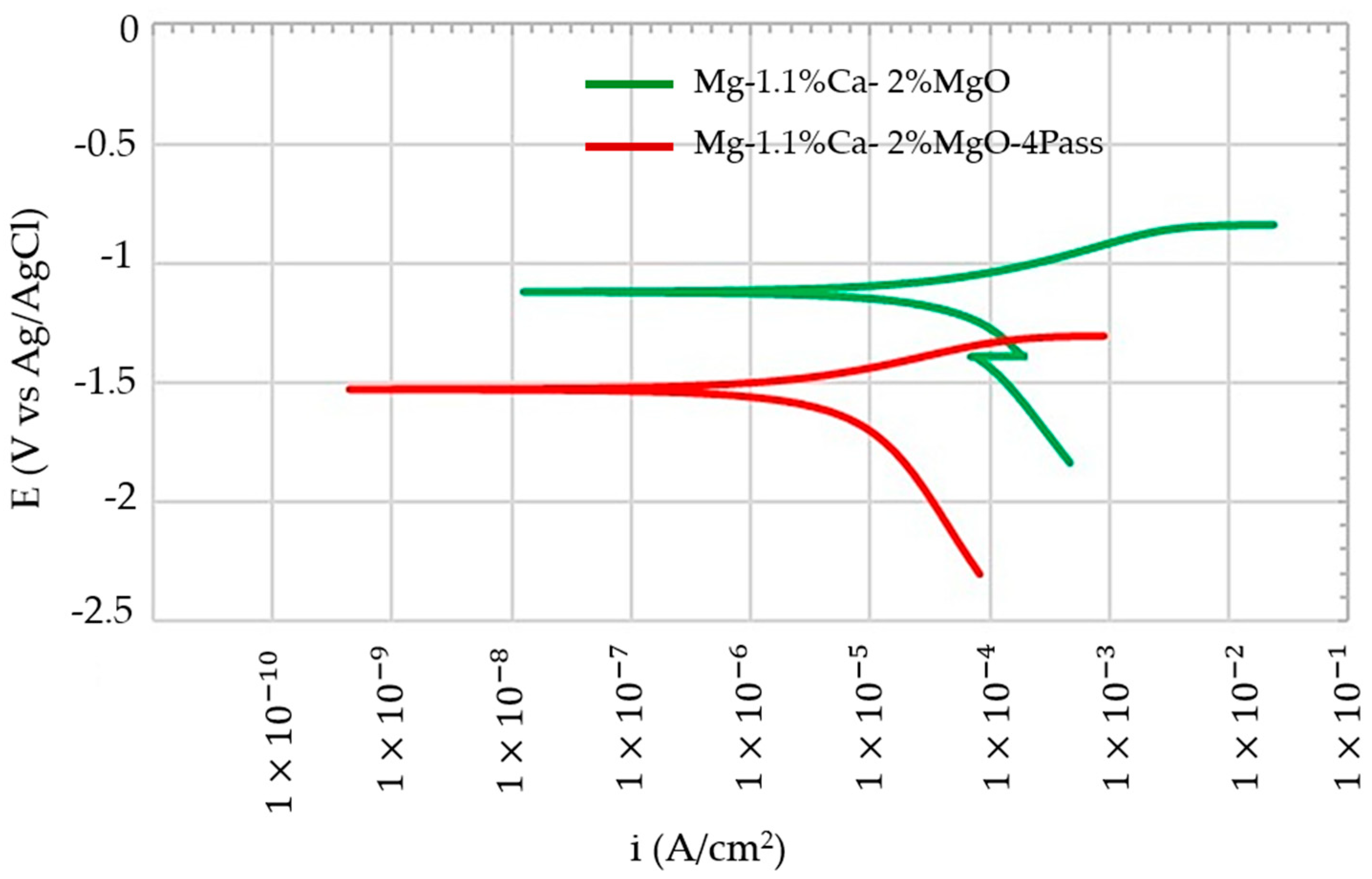
4. Electrochemical Corrosion Test
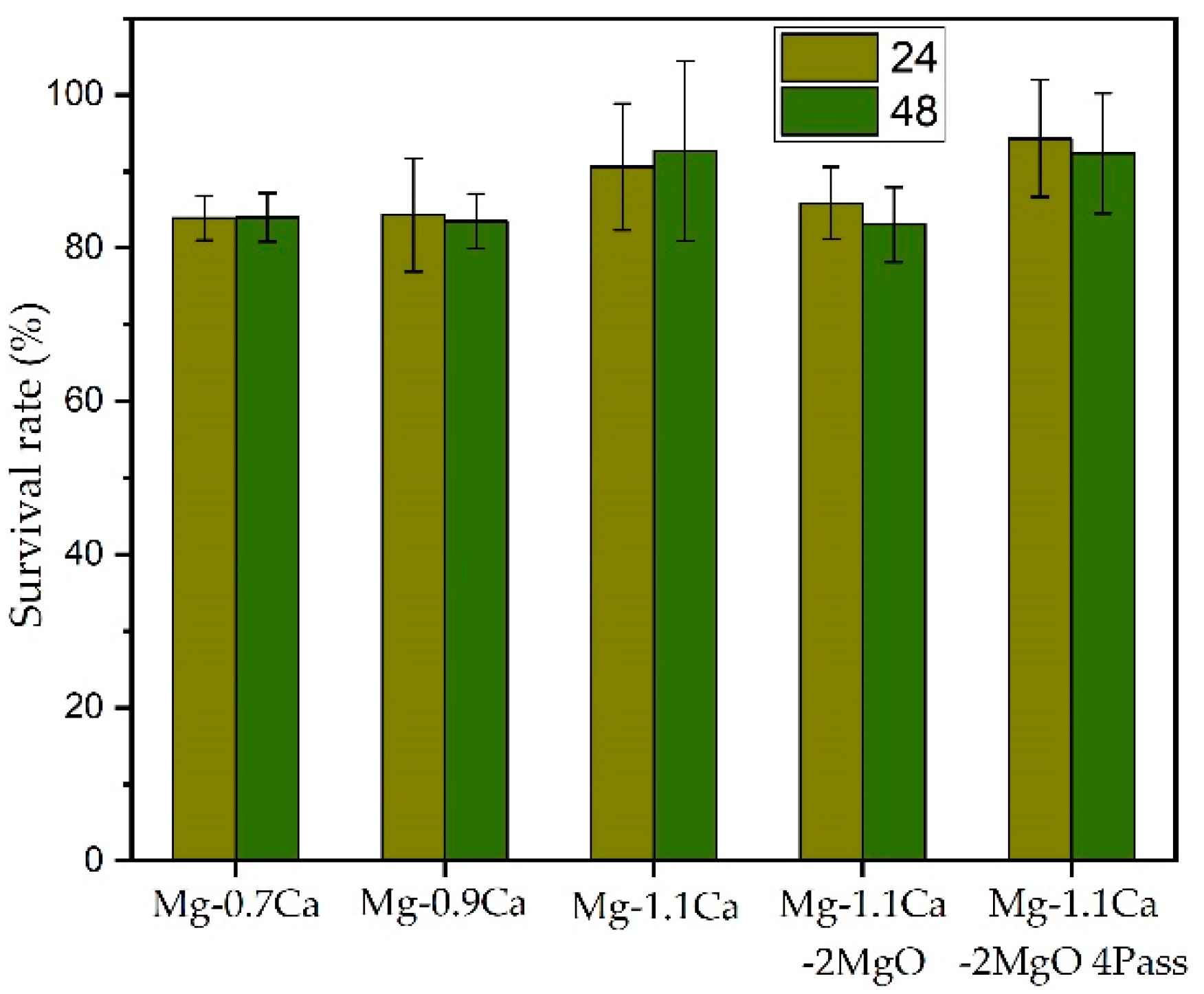
5. Biocompatibility Analysis
5.1. Cytotoxicity Test
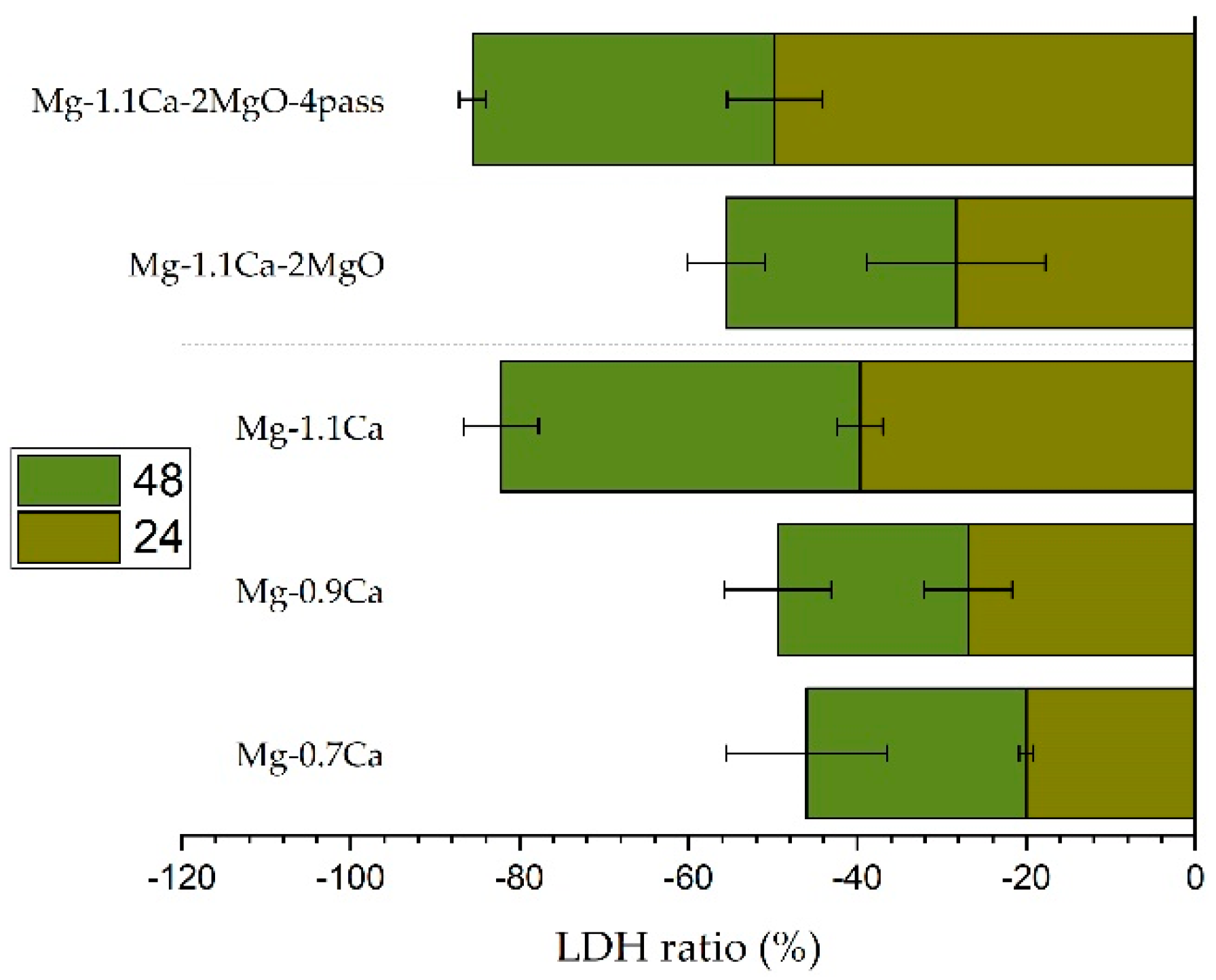
5.2. Cell Apoptosis
6. Conclusions
- The incorporation of MgO into magnesium–calcium alloy composites did not yield significant results in terms of grain refinement, improvement of tensile strength, corrosion rate reduction, and enhanced biocompatibility. However, after subjecting the Mg-Ca-MgO alloy to the ECAP process, significant improvements were observed in all these aspects.
- The ECAP process, which involves severe plastic deformation, resulted in notable grain refinement, leading to smaller grain sizes within the alloy. This refinement likely contributed to the enhanced mechanical properties. The maximum YTS (124.5 MPa), UTS (161.1 MPa), and elongation (4.3%) were obtained by the four-pass Mg-1.1%Ca-2%MgO composite.
- Additionally, the ECAP-treated alloy exhibited a decrease in corrosion rate, indicating improved corrosion resistance. The corrosion current decreased to 43.2 μA/cm2 and 10.1 μA/cm2 with the ECAP passes on the Mg-1.1%Ca-2%MgO composite, which clearly shows that the corrosion resistance improved.
- Moreover, the biocompatibility of the composite also improved after the ECAP process. The survival rate after 24 h of cell incubation reached a maximum of 94.3% after 4 passes of ECAP of Mg-1.1%Ca-2%MgO, confirming that the improvement in grain refinement based on the number of ECAP passes improved the performance of the cell survival rate.
- The Mg-1.1%Ca-2%MgO composite showed a significant decrease in apoptotic performance after four ECAP cycles, with the highest apoptotic rate (−49.8%) at 24 h and −35.8% at 48 h, confirming that the improvement in grain refinement following ECAP prevented apoptotic performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atrens, A.; Johnston, S.; Shi, Z.; Dargusch, M.S. Viewpoint-Understanding Mg corrosion in the body for biodegradable medical implants. Scr. Mater. 2018, 154, 92–100. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. 2012, 100, 1691–1701. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity β-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2021, 10, 356–386. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Switzer, H.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhag, H. In vivo corrosion of four magnesium alloys and the associ-ated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D.; Zartner, P.; Michel-Behnke, I.; Akinturk, H. Bioabsorbable metalstents for percutaneous treatment of critical recoarctation of the aorta ina newborn. Catheter. Cardiovasc. Interv. 2006, 67, 671–673. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Guerrero-Urbaneja, P.; García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glycerol valorization by etherification to polyglycerols by using oxides derived from MgFe hydrotalcites. Appl. Catal. A Gen. 2014, 470, 199–207. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of acid-base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic flycerol etherification to short-chain polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erne, B.H.; Weckhuysen, B.M. Glycerol etherification over highly active CaO-based materials: New mechanistic aspects and related colloidal particle formation. Chem.-A Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.H.; Wang, F.; Wang, Y.; Ramasse, Q.M.; Fan, Z. Segregation of Ca at the Mg/MgO interface and its effect on grain refinement of Mg alloys, Materials Science and Engineering. Nano Mater. Sci. 2019, 529, 012048. [Google Scholar]
- Bellucci, D.; Sola, A.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. Role of magnesium oxide and strontium oxide as modifiers in silicate-based bioactive glasses: Effect on thermal behavior, mechanical properties and in-vitro bioactivity. Mater. Sci. Eng. C 2017, 72, 566–575. [Google Scholar] [CrossRef]
- Singh, A.K.; Pramanik, K.; Biswas, A. MgO enables enhanced bioactivity and antimicrobial activity of nano bioglass for bone tissue engineering application. Mater. Technol. 2019, 34, 818–826. [Google Scholar] [CrossRef]
- Huang, S.J.; Subramani, M.; Ali, A.N.; Alemayehu, D.B.; Aoh, J.N.; Lin, P.C. The effect of micro-SiCp content on the tensile and fatigue behavior of AZ61 magnesium alloy matrix composites. Int. J. Met. 2021, 15, 780–793. [Google Scholar] [CrossRef]
- Huang, S.J.; Subramani, M.; Chiang, C.C. Effect of hybrid reinforcement on microstructure and mechanical properties of AZ61 magnesium alloy processed by stir casting method. Compos. Commun. 2021, 25, 100772. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Muralidhar, A.; Narendranath, S.; Nayaka, H.S. Effect of equal channel angular pressing on AZ31 wrought magnesium alloys. J. Magnes. Alloys 2013, 1, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Hosaka, T.; Yoshihara, S.; Amanina, I.; MacDonald, B.J. Influence of Grain Refinement and Residual Stress on Corrosion Behavior of AZ31 Magnesium Alloy Processed by ECAP in RPMI-1640 Medium. Procedia Eng. 2017, 184, 432–441. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Lowe, T.C. Observations and issues on mechanisms of grain refinement duringECAP process. Mater. Sci. Eng. A 2000, 291, 46–53. [Google Scholar] [CrossRef]
- Xu, M.F.; Horita, Z.; Langdon, T.G. The evolution of homogeneity and grain refinement during equal-channelangular pressing: A model for grain refinement in ECAP. Mater. Sci. Eng. A 2005, 398, 66–76. [Google Scholar] [CrossRef]
- Fu, J.; Du, W.; Liu, K.; Du, X.; Zhao, C.; Liang, H.; Mansoor, A.; Li, S.; Wang, Z. Effect of the Ca2Mg6Zn3 Phase on the Corrosion Behavior of Biodegradable Mg-4.0Zn-0.2Mn-xCa Alloys in Hank’s Solution. Materials 2022, 15, 2079. [Google Scholar] [CrossRef] [PubMed]
- Makkar, P.; Sarkar, S.K.; Padalhin, A.R.; Moon, B.G.; Lee, Y.S.; Lee, B.T. In vitro and in vivo assessment of biomedical Mg–Ca alloys for bone implant applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Idris, M.H.; Kadir, M.R.A. Saeed Farahany aMicrostructure analysis and corrosion behavior of biodegradable Mg–Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Shen, J.; Shen, D.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Guan, S.; Kulyasova, O.B.; Valiev, R.Z. In vitro and in vivo studies on pure Mg, Mg-1Ca and Mg-2Sr alloys processed by equal channel angular pressing. Nano Mater. Sci. 2020, 2, 96–108. [Google Scholar] [CrossRef]



| Chemicals | g/L |
|---|---|
| NaCl | 8.0 |
| KCl | 0.4 |
| CaCl2 | 0.14 |
| MgSO4·7H2O | 0.06 |
| MgCl2·6H2O | 0.1 |
| Na2HPO4·12H2O | 0.06 |
| KH2PO4 | 0.06 |
| C6H12O6 | 1.0 |
| NaHCO3 | 0.35 |
| Materials | Tensile Strength | Hardness | ||
|---|---|---|---|---|
| YTS (MPa) | UTS (MPa) | E (%) | HV | |
| Mg-0.7%Ca | 69.8 | 106.8 | 2.9 | 34.2 ± 0.8 |
| Mg-0.9%Ca | 79.3 | 107.6 | 3.2 | 37.6 ± 1.1 |
| Mg-1.1%Ca | 82.6 | 109.2 | 3.3 | 40.7 ± 0.5 |
| Mg-1.1%Ca-2%MgO | 90.3 | 112.7 | 3.7 | 44.6 ± 1.7 |
| Mg-1.1%Ca-2%MgO 4Pass | 124.5 | 161.1 | 4.3 | 47.8 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-J.; Wang, C.-F.; Subramani, M.; Fan, F.-Y. Effect of ECAP on Microstructure, Mechanical Properties, Corrosion Behavior, and Biocompatibility of Mg-Ca Alloy Composite. J. Compos. Sci. 2023, 7, 292. https://doi.org/10.3390/jcs7070292
Huang S-J, Wang C-F, Subramani M, Fan F-Y. Effect of ECAP on Microstructure, Mechanical Properties, Corrosion Behavior, and Biocompatibility of Mg-Ca Alloy Composite. Journal of Composites Science. 2023; 7(7):292. https://doi.org/10.3390/jcs7070292
Chicago/Turabian StyleHuang, Song-Jeng, Chih-Feng Wang, Murugan Subramani, and Fang-Yu Fan. 2023. "Effect of ECAP on Microstructure, Mechanical Properties, Corrosion Behavior, and Biocompatibility of Mg-Ca Alloy Composite" Journal of Composites Science 7, no. 7: 292. https://doi.org/10.3390/jcs7070292
APA StyleHuang, S.-J., Wang, C.-F., Subramani, M., & Fan, F.-Y. (2023). Effect of ECAP on Microstructure, Mechanical Properties, Corrosion Behavior, and Biocompatibility of Mg-Ca Alloy Composite. Journal of Composites Science, 7(7), 292. https://doi.org/10.3390/jcs7070292









