Glassy and Rubbery Epoxy Composites with Mesoporous Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SBA-15 Mesoporous Silicas
2.3. Surface Functionalization of SBA-15 Mesoporous Silicas
2.4. Preparation of Pristine Epoxy Polymers and SBA-15 Mesocomposites
2.5. Measurements
3. Results
3.1. Characterization of SBA-15 Mesoporous Silicas
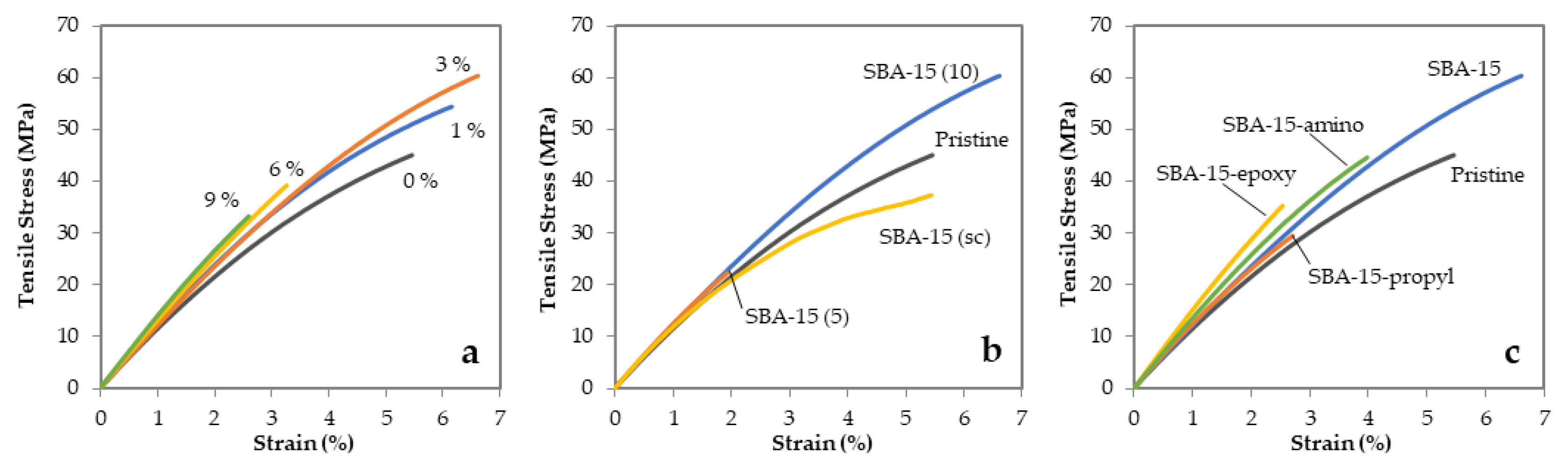
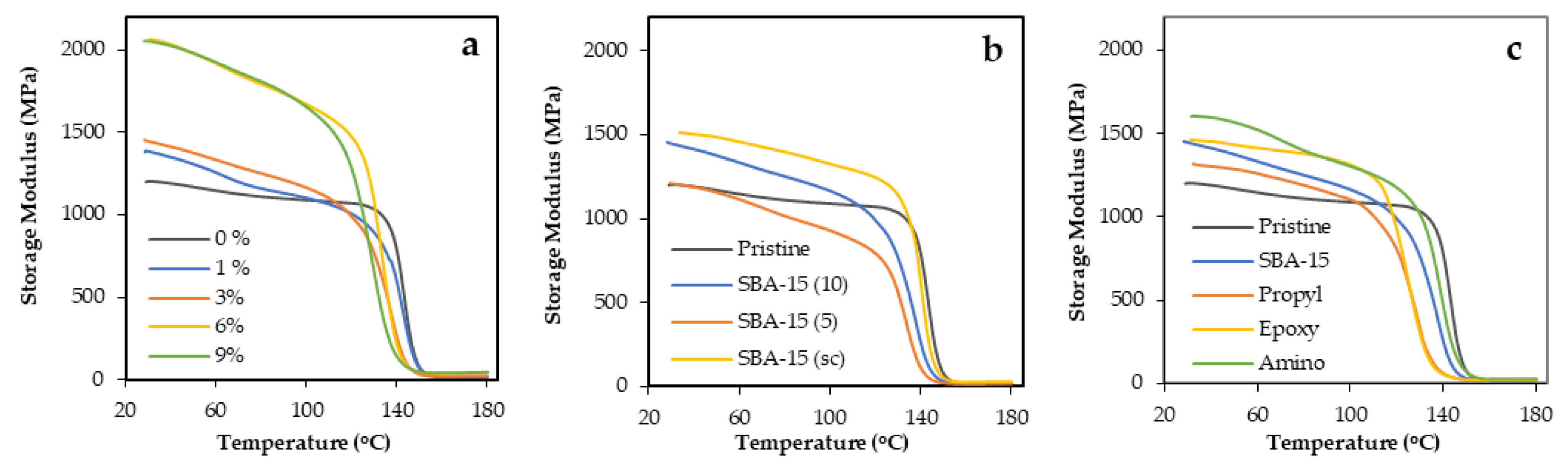
3.2. Properties of Epoxy Polymers and Mesocomposites
4. Discussion
- IPD is a cycloaliphatic molecule with a molar mass of 170.3 g·mol−1 and Jeffamine D-2000 is a long-chained poly(ether) diamine with a molar mass of 2000 g·mol−1. Thus, the curing of DGEBA with IPD results in a network with a high crosslinking density, while curing with D-2000 gives a network with a lower crosslinking density.
- The significant difference in the molar masses of the curing agents also has an impact on the mass percentages of the epoxy systems prior to curing. The percentage compositions of the masses of the rubbery and glassy systems are (i) DGEBA (26.1):D-2000 (73.9) and (ii) DGEBA (81):IPD (19), respectively. As is shown, Jeffamine D-2000 is the reagent with the higher mass fraction in the rubbery system, while DGEBA has the higher mass fraction in the glassy system.
- IPD is more reactive as a hydrogen donor than D-2000, and this may be crucial for the growth of a homogeneous crosslinked network inside the tubular SBA-15 pores.
- Considering the size of the monomers and mesoporous silicas, the molecular volume of Jeffamine D-2000 is larger by one order of magnitude than IPD and DGEBA, while a single cylindrical pore of SBA-15 is larger by four–five orders of magnitude than Jeffamine D-2000.
- For the preparation of the mesocomposites, mesoporous silicas are initially dispersed inside the epoxy resin. This means that the curing agent must penetrate inside the DGEBA-filled pores for the crosslinking network to be formed throughout the mesoporous particles.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannelis, E.P. Polymer Layered Silicate Nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem.-Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- You, X.; Zhang, Q.; Yang, J.; Dong, S. Review on 3D-printed graphene-reinforced composites for structural applications. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107420. [Google Scholar] [CrossRef]
- Grossiord, N.; Loos, J.; Regev, O.; Koning, C.E. Toolbox for Dispersing Carbon Nanotubes into Polymers To Get Conductive Nanocomposites. Chem. Mater. 2006, 18, 1089–1099. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.-J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.J.; Abot, J.L.; Daniel, I.M. Mechanical and thermal behavior of clay/epoxy nanocomposites. Compos. Sci. Technol. 2006, 66, 2415–2422. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Zhao, J.; Morgan, A.B.; Harris, J.D. Rheological characterization of polystyrene–clay nanocomposites to compare the degree of exfoliation and dispersion. Polymer 2005, 46, 8641–8660. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, Y.; Hobson, T.; Liu, J. Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett. 2010, 10, 2727–2733. [Google Scholar] [CrossRef]
- Ziolo, R.F.; Giannelis, E.P.; Weinstein, B.A.; O’Horo, M.P.; Ganguly, B.N.; Mehrotra, V.; Russell, M.W.; Huffman, D.R. Matrix-Mediated Synthesis of Nanocrystalline ggr-Fe2O3: A New Optically Transparent Magnetic Material. Science 1992, 257, 219–223. [Google Scholar] [CrossRef]
- Jiao, J.; Sun, X.; Pinnavaia, T.J. Mesostructured silica for the reinforcement and toughening of rubbery and glassy epoxy polymers. Polymer 2009, 50, 983–989. [Google Scholar] [CrossRef]
- Park, I.; Peng, H.G.; Gidley, D.W.; Xue, S.; Pinnavaia, T.J. Epoxy—Silica mesocomposites with enhanced tensile properties and oxygen permeability. Chem. Mater. 2006, 18, 650–656. [Google Scholar] [CrossRef]
- Zhang, F.A.; Lee, D.K.; Pinnavaia, T.J. PMMA-mesocellular foam silica nanocomposites prepared through batch emulsion polymerization and compression molding. Polymer 2009, 50, 4768–4774. [Google Scholar] [CrossRef]
- Zhang, F.A.; Lee, D.K.; Pinnavaia, T.J. PMMA/mesoporous silica nanocomposites: Effect of framework structure and pore size on thermomechanical properties. Polym. Chem. 2010, 1, 107–113. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Saravanan, M.; Sudalai, S.; Dharaneesh, A.B.; Prahaaladhan, V.; Srinivasan, G.; Arumugam, A. An extensive review on mesoporous silica from inexpensive resources: Properties, synthesis, and application toward modern technologies. J. Sol-Gel Sci. Technol. 2023, 105, 1–29. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Lin, V.S.Y.; Trewyn, B.G. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Florensa, M.; Llenas, M.; Medina-Gutiérrez, E.; Sandoval, S.; Tobías-Rossell, G. Key Parameters for the Rational Design, Synthesis, and Functionalization of Biocompatible Mesoporous Silica Nanoparticles. Pharmaceutics 2022, 14, 2703. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Dudhipala, N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023, 7, 70–89. [Google Scholar] [CrossRef]
- Lu, S.; Chun, W.; Yu, J.; Yang, X. Preparation and characterization of the mesoporous SiO2–TiO2/epoxy resin hybrid materials. J. Appl. Polym. Sci. 2008, 109, 2095–2102. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Yamauchi, Y. Fabrication of mesoporous silica/polymer composites through solvent evaporation process and investigation of their excellent low thermal expansion property. Phys. Chem. Chem. Phys. 2011, 13, 4957–4962. [Google Scholar] [CrossRef]
- Ji, X.; Hampsey, J.E.; Hu, Q.; He, J.; Yang, Z.; Lu, Y. Mesoporous Silica-Reinforced Polymer Nanocomposites. Chem. Mater. 2003, 15, 3656–3662. [Google Scholar] [CrossRef]
- Zhang, F.-A.; Song, C.; Yu, C.-L. Effects of preparation methods on the property of PMMA/SBA-15 mesoporous silica composites. J. Polym. Res. 2011, 18, 1757–1764. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.; Zhang, J. Polymer-filled porous MCM-41: An effective means to design polymer-based nanocomposite. Mater. Lett. 2005, 59, 2685–2688. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Palani, A.; Gilliopoulos, D.; Triantafyllidis, K.; Bikiaris, D.N. Mechanical properties and crystallization of high-density polyethylene composites with mesostructured cellular silica foam. J. Therm. Anal. Calorim. 2013, 113, 1651–1665. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Cheng, H.-H.; Cheng, P.-W.; Lee, Y.-J. Effect of Reactive Channel Functional Groups and Nanoporosity of Nanoscale Mesoporous Silica on Properties of Polyimide Composite. Macromolecules 2006, 39, 7583–7590. [Google Scholar] [CrossRef]
- Mathias, L.J.; Davis, R.D.; Jarrett, W.L. Observation of α and γ Crystal Forms and Amorphous Regions of Nylon 6−Clay Nanocomposites Using Solid-State 15N Nuclear Magnetic Resonance. Macromolecules 1999, 32, 7958–7960. [Google Scholar] [CrossRef]
- Chen, Z.; Song, C.; Bai, R.; Wei, Z.; Zhang, F. Effects of mesoporous SBA-15 contents on the properties of polystyrene composites via in-situ emulsion polymerization. J. Polym. Res. 2012, 19, 9846. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Kamachi, Y.; Miyamoto, N.; Yamauchi, Y. Mesoporous silica as smart inorganic filler: Preparation of robust silicone rubber with low thermal expansion property. J. Mater. Chem. 2011, 21, 5338–5344. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Park, I.; Pinnavaia, T.J. Mesocellular Silica Foam as an Epoxy Polymer Reinforcing Agent. Adv. Funct. Mater. 2007, 17, 2835–2841. [Google Scholar] [CrossRef]
- Sun, L.; Gibson, R.F.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A review. Compos. Sci. Technol. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Wu, S. A generalized criterion for rubber toughening: The critical matrix ligament thickness. J. Appl. Polym. Sci. 1988, 35, 549–561. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, Q.X.; Bannister, M.; Mai, Y.W. Investigation of the mechanical properties of DGEBA-based epoxy resin with nanoclay additives. Compos. Struct. 2006, 75, 514–519. [Google Scholar] [CrossRef]
- Miyagawa, H.; Drzal, L.T. Thermo-physical and impact properties of epoxy nanocomposites reinforced by single-wall carbon nanotubes. Polymer 2004, 45, 5163–5170. [Google Scholar] [CrossRef]
- Chatterjee, A.; Islam, M.S. Fabrication and characterization of TiO2–epoxy nanocomposite. Mater. Sci. Eng. A 2008, 487, 574–585. [Google Scholar] [CrossRef]


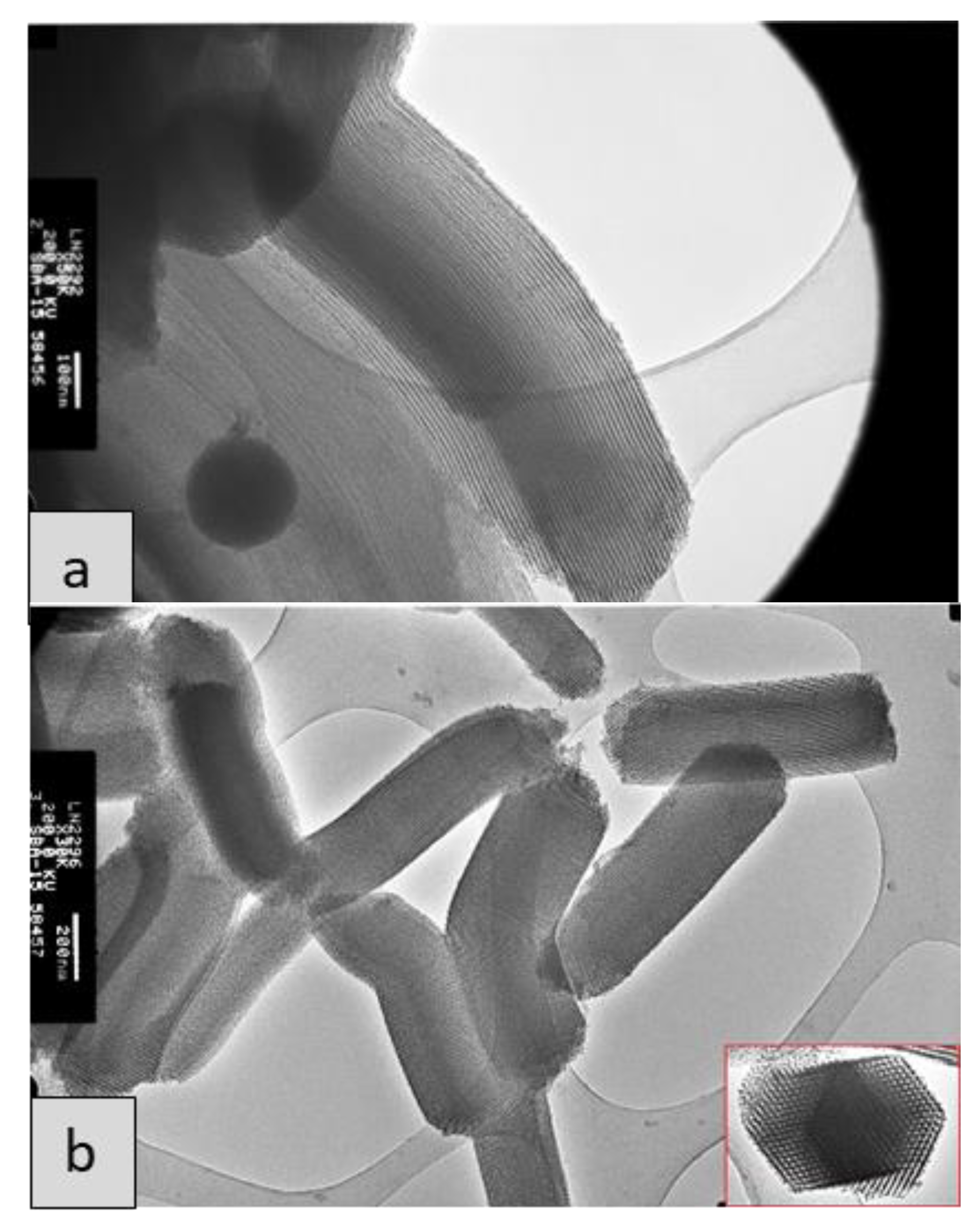




| Synthesis Step | SBA-15 (10) | SBA-15 (5) | SBA-15 (sc) |
|---|---|---|---|
| Pluronic® P123 solubilization in HCl(aq) 1.6 M | Stirring at 38 °C until dissolved | Stirring at 38 °C until dissolved | Stirring at 38 °C until dissolved |
| TEOS hydrolysis and polymerization | Stirring at 38 °C for 24 h | Stirring at 35 °C for 1 h | Stirring at 40 °C for 8 min |
| Hydrothermal treatment | 100 °C, 72 h | 35 °C, 48 h | 40 °C, 24 h |
| Product recovery | Filtration. Wash with deionized water and ethanol. Drying at Troom | Filtration. Wash with deionized water and ethanol. Drying at Troom | Filtration. Wash with deionized water and ethanol. Drying at Troom |
| Organic content removal | Calcination at 550 °C for 6 h with 1 °C·min−1 | Calcination at 550 °C for 6 h with 1 °C·min−1 | Calcination at 550 °C for 6 h with 1 °C·min−1 |
| Sample | SEM-TEM | N2 Physisorption | Elemental Analysis | ||||
|---|---|---|---|---|---|---|---|
| Average Particle Length | Average Particle Width | Specific Surface Area | Total Pore Volume | Pore Diameter | Carbon Concentration | Organic Group Content | |
| (μm) | (μm) | (m2/g) | (cc/g) | (nm) | (wt. %) | (mol/g) | |
| SBA-15 (10) | 1.33 | 0.62 | 815 | 1.363 | 10.0 | 0 | 0 |
| SBA-15 (5) | 0.68 | 0.44 | 651 | 0.615 | 5.0 | 0 | 0 |
| SBA-15 (sc) | 0.84 | 0.30 | 780 | 1.140 | 10.0 | 0 | 0 |
| SBA-15-propyl | - | - | 735 | 1.358 | 8.9 | 1.97 | 5.5·10−4 |
| SBA-15-epoxy | - | - | 600 | 1.251 | 8.9 | 3.47 | 4.8·10−4 |
| SBA-15-amino | - | - | 382 | 0.758 | 8.5 | 9.54 | 26.5·10−4 |
| Mesoporous Silica Content | Tensile Strength | DMA | ||||
|---|---|---|---|---|---|---|
| Stress at Break | Elongation at Break | Modulus | Toughness | Storage Modulus at Glassy State 1 | Tg 2 | |
| (MPa) | (%) | (MPa) | (kJ/m3) | (MPa) | (°C) | |
| ±0.1 | ±3 | ±0.5 | ±15 | ±250 | ±2 | |
| - | 0.47 | 25.9 | 4.7 | 69.9 | 2566 | −27.7 |
| 1% SBA-15 (10) | 0.55 | 33.1 | 4.4 | 102.3 | 2808 | −31.9 |
| 3% SBA-15 (10) | 0.76 | 29.4 | 6.2 | 121.8 | 3038 | −29.1 |
| 6% SBA-15 (10) | 0.97 | 31.3 | 7.9 | 166.5 | 3704 | −26.4 |
| 9% SBA-15 (10) | 1.19 | 36.6 | 8.4 | 242.6 | 3813 | −25.8 |
| 3% SBA-15 (5) | 0.72 | 43.0 | 4.7 | 176.9 | 3447 | −24.2 |
| 3% SBA-15 (sc) | 0.89 | 47.5 | 5.4 | 241.9 | 3548 | −24.6 |
| 3% SBA-15-propyl | 0.65 | 30.5 | 5.8 | 113.2 | 2360 | −24.8 |
| 3% SBA-15-epoxy | 0.86 | 33.1 | 6.4 | 155.8 | 3949 | −22.3 |
| 3% SBA-15-amino | 1.04 | 52.9 | 5.7 | 323.0 | 4274 | −25.0 |
| Mesoporous Silica Content | Tensile Strength | Izod Impact Test | DMA | ||||
|---|---|---|---|---|---|---|---|
| Stress at Break | Elongation at Break | Modulus | Toughness | Impact Strength | Storage Modulus at Glassy State 1 | Tg 2 | |
| (MPa) | (%) | (MPa) | (kJ/m3) | (kJ/m2) | (MPa) | (°C) | |
| ±5 | ±1 | ±0.5 | ±10 | ±0.3 | ±200 | ±1 | |
| - | 45.1 | 5.5 | 2349 | 1427 | 0.720 | 1203 | 151.3 |
| 1% SBA-15 (10) | 54.4 | 6.2 | 2541 | 1964 | 1.109 | 1351 | 151.3 |
| 3% SBA-15 (10) | 60.4 | 6.6 | 2463 | 2286 | 1.597 | 1448 | 147.5 |
| 6% SBA-15 (10) | 39.2 | 3.3 | 2653 | 671 | 2.138 | 2064 | 143.6 |
| 9% SBA-15 (10) | 33.2 | 2.6 | 2827 | 456 | 0.941 | 2054 | 139.7 |
| 3% SBA-15 (5) | 22.3 | 1.9 | 2599 | 228 | 1.370 | 1211 | 143.9 |
| 3% SBA-15 (sc) | 37.3 | 5.4 | 2548 | 1285 | 1.940 | 1514 | 148.0 |
| 3% SBA-15-propyl | 29.5 | 2.7 | 2594 | 431 | 0.708 | 1320 | 137.0 |
| 3% SBA-15-epoxy | 35.3 | 2.5 | 3106 | 471 | 2.164 | 1465 | 136.1 |
| 3% SBA-15-amino | 44.6 | 4.0 | 2785 | 976 | 1.354 | 1607 | 150.0 |
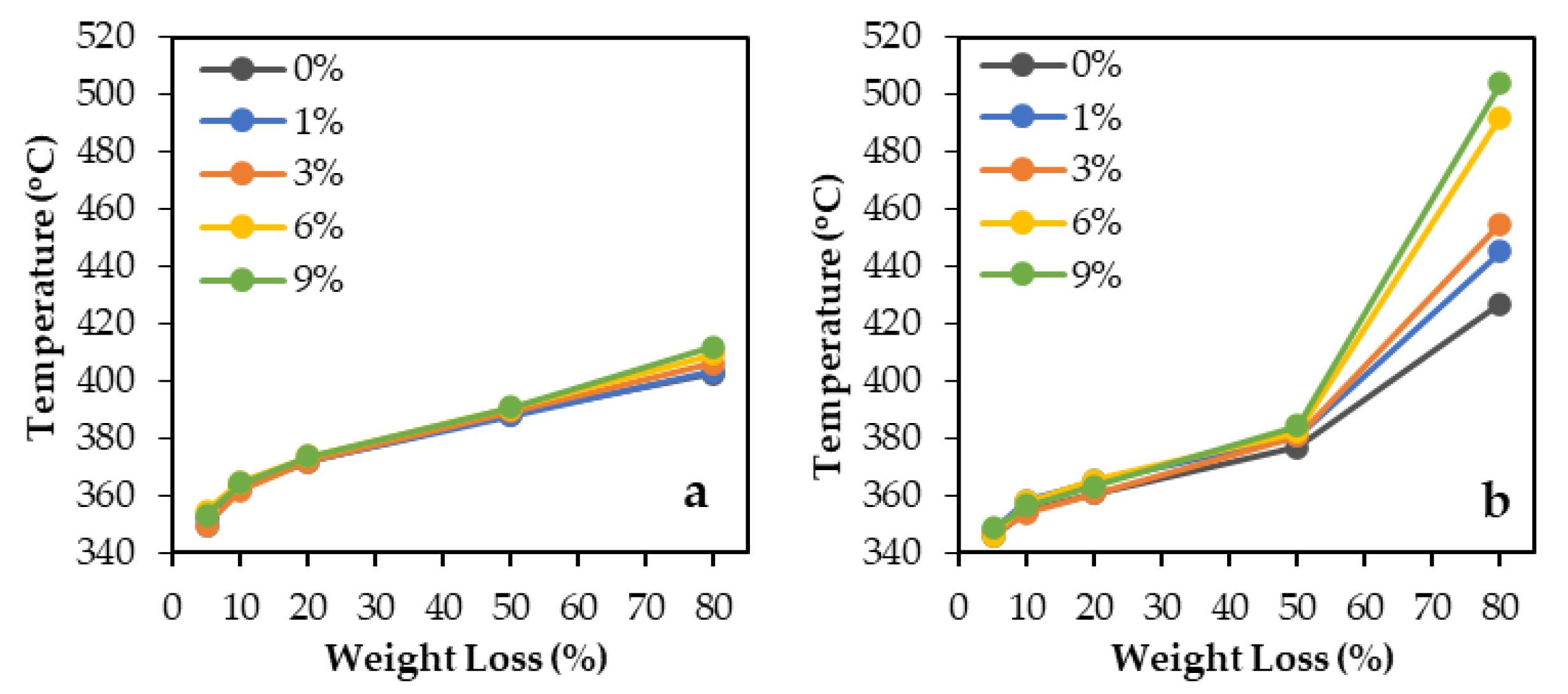
| Epoxy System | Silica Filler | Temperature Values of Specific Weight Losses (%) | ||||
|---|---|---|---|---|---|---|
| T5 | T10 | T20 | T50 | T80 | ||
| (°C) | (°C) | (°C) | (°C) | (°C) | ||
| Rubbery (DEGBA/ D2000) | - | 352.5 | 363.4 | 372.0 | 388.1 | 402.6 |
| 1% SBA-15 (10) | 349.4 | 362.3 | 371.9 | 388.0 | 403.2 | |
| 3% SBA-15 (10) | 349.4 | 361.6 | 371.7 | 389.1 | 406.2 | |
| 6% SBA-15 (10) | 354.5 | 364.5 | 373.5 | 390.3 | 409.2 | |
| 9% SBA-15 (10) | 353.0 | 364.2 | 373.7 | 390.6 | 411.8 | |
| 3% SBA-15 (5) | 348.1 | 360.9 | 370.8 | 386.6 | 402.1 | |
| 3% SBA-15 (sc) | 351.4 | 363.0 | 372.4 | 388.9 | 405.4 | |
| 3% SBA-15-propyl | 353.6 | 364.0 | 373.2 | 389.1 | 405.5 | |
| 3% SBA-15-epoxy | 352.7 | 364.0 | 373.6 | 390.2 | 407.6 | |
| 3% SBA-15-amino | 347.5 | 361.1 | 370.6 | 387.7 | 403.4 | |
| Glassy (DEGBA/IPD) | - | 345.5 | 354.4 | 360.6 | 376.8 | 426.9 |
| 1% SBA-15 (10) | 347.9 | 358.0 | 364.9 | 380.7 | 445.2 | |
| 3% SBA-15 (10) | 346.4 | 353.8 | 360.6 | 380.6 | 454.8 | |
| 6% SBA-15 (10) | 345.9 | 357.7 | 365.4 | 382.2 | 491.8 | |
| 9% SBA-15 (10) | 348.4 | 356.2 | 363.3 | 384.2 | 503.7 | |
| 3% SBA-15 (5) | 346.8 | 355.0 | 362.2 | 378.7 | 435.4 | |
| 3% SBA-15 (sc) | 348.4 | 356.4 | 363.2 | 379.9 | 440.2 | |
| 3% SBA-15-propyl | 349.9 | 357.2 | 363.8 | 380.0 | 439.2 | |
| 3% SBA-15-epoxy | 346.4 | 355.6 | 363.0 | 379.6 | 436.4 | |
| 3% SBA-15-amino | 342.8 | 354.4 | 363.7 | 382.5 | 454.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkiliopoulos, D.; Bikiaris, D.; Efstathiadis, D.; Triantafyllidis, K. Glassy and Rubbery Epoxy Composites with Mesoporous Silica. J. Compos. Sci. 2023, 7, 243. https://doi.org/10.3390/jcs7060243
Gkiliopoulos D, Bikiaris D, Efstathiadis D, Triantafyllidis K. Glassy and Rubbery Epoxy Composites with Mesoporous Silica. Journal of Composites Science. 2023; 7(6):243. https://doi.org/10.3390/jcs7060243
Chicago/Turabian StyleGkiliopoulos, Dimitrios, Dimitrios Bikiaris, Doukas Efstathiadis, and Konstantinos Triantafyllidis. 2023. "Glassy and Rubbery Epoxy Composites with Mesoporous Silica" Journal of Composites Science 7, no. 6: 243. https://doi.org/10.3390/jcs7060243
APA StyleGkiliopoulos, D., Bikiaris, D., Efstathiadis, D., & Triantafyllidis, K. (2023). Glassy and Rubbery Epoxy Composites with Mesoporous Silica. Journal of Composites Science, 7(6), 243. https://doi.org/10.3390/jcs7060243










