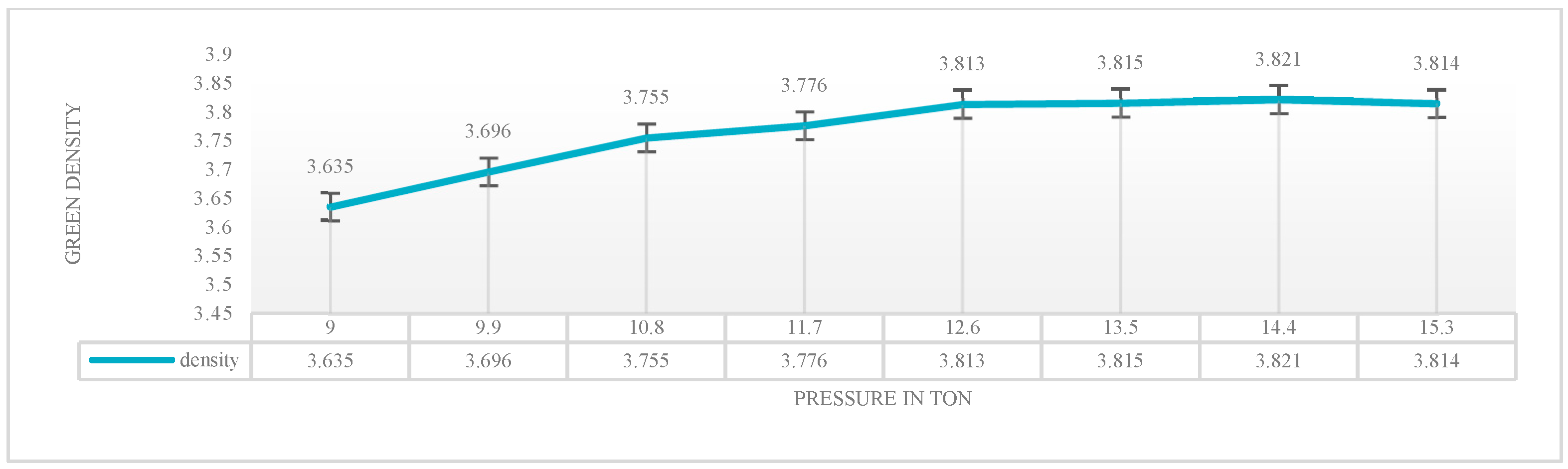
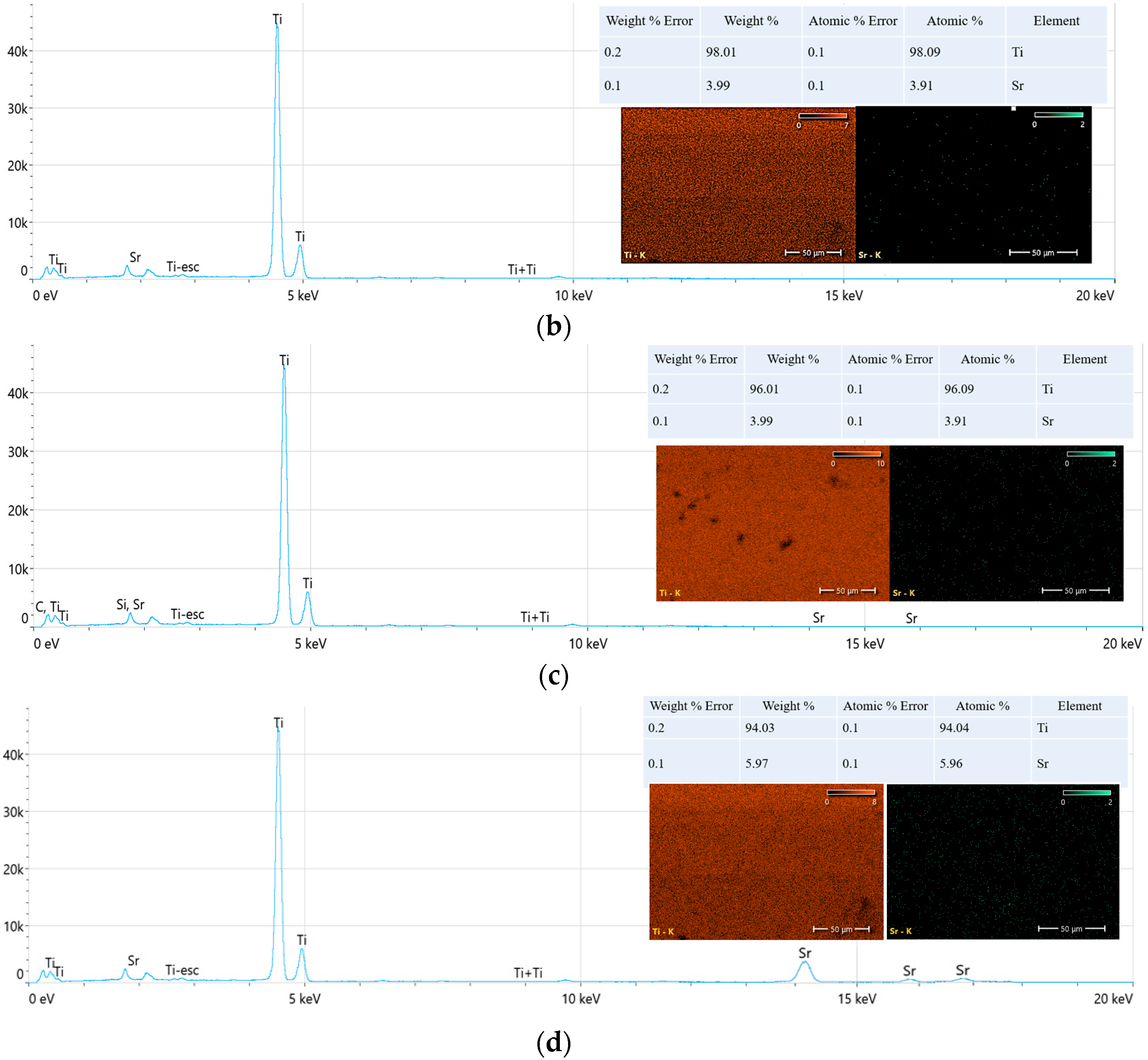
1. Introduction
Titanium is an indispensable element for high-performance components because of its remarkable combination of physical and mechanical properties and distinctive properties. Among structural metals, titanium has the best ratio of mechanical properties with respect to its density, excellent corrosion resistance, and good biocompatibility [
1]. However, the embedded materials must be solid and sufficiently resilient to survive the physiological loads applied and must operate for a much longer duration [
2]. Titanium is relatively unchanged when it is inserted into the human body as an implant because of its high corrosion resistance, and this property makes titanium biologically inert. Thus, titanium cannot osseointegrate with human bone cells [
3]. Positive modulation of biological processes is limited, given that osteoinduction (the induction of bone apposition) cannot be accomplished [
4]. Although titanium has a high content in the Earth’s crust, it is more expensive and is a rare material compared with other technological materials in the medical field. The unfortunate and unavoidable high cost of Ti is exacerbated in nearly every step of manufacturing from mineral to products [
5]. The usual metallurgical procedures are inefficient when dealing with Ti, which is extremely reactive at high temperatures [
6], and difficult and poor machining causes significant material loss. For such reasons, powder metallurgy (PM), as a processing route, is considered one of the most suitable fabrication methods and a practical means of producing complex Ti parts directly at a low cost. The PM method has enhanced chemical and microstructural homogeneity in addition to the ability to create different composite structures [
7]. Implant-related parameters, including the chemical composition and implant design, implant surface topography, material, form, diameter, length, implant surface treatments, and coatings, all contribute to osseointegration [
8]. According to the literature, utilizing inorganic antibacterial compounds outperforms using organic antibacterial materials in terms of toxicity, specificity of action, and durability [
9].
Numerous studies have shown that modifying surfaces with inorganic metal elements such as zinc (Zn), magnesium (Mg), and strontium (Sr) can hasten osseointegration and encourage the formation of new bones [
10]. As a result, ceramic oxides or metallic dispersions are used as reinforcing agents [
11]. Sr is thought to have multiple action mechanisms. One strontium compound, Sr ranelate, is a combination of sodium ranelate and strontium chloride (SrCl
2·6H
2O). Numerous works have demonstrated that Sr can stimulate the calcium-sensing receptor (Ca SR) found in the membrane of osteoclasts and osteoblasts, which proposes that it plays an important role in the anabolic effects of Sr on osteoblast cells. Sr is an intriguing candidate for a surface coating because of its mentioned features, given that it can combine the mechanical characteristics of a Ti-based implant with a relatively rough surface [
12]. Sr oxide influences bone production and resorption by promoting osteoblast survival and differentiation. Preosteoclast differentiation was also observed to be decreased concurrently by SrO [
13]. SrO is an intriguing choice for a surface coating because of its mentioned features, considering that it can combine the mechanical capabilities of Ti-based implants with a surface that is only moderately rough with the benefits of Sr for the repair of bones. Sr can inhibit the osteoclast differentiation of preosteoclast cells and promote the expression of outcome cells and protein secretion to stimulate bone repair [
14]. Also, these compounds have a high hardness and are highly coherent to the base, improving the physical and mechanical properties of base alloys [
15].
Porous materials have been used to additionally lower the elastic modulus of Ti-based alloys. The elastic modulus is a quality that is difficult to change. In this study, focused elements could be added for the purpose of changing the elastic modulus; otherwise, the porosity in alloys that are utilized to make the final product determines the value of the elastic modulus [
16]. Modern Ti alloys have an elasticity modulus that is three to four times higher than that of human bones, which may be uncomfortable for patients and increase the risk of implant failure. The reason for this is that the implant can withstand much mechanical stress, which weakens the bones and causes wear and reduced bone density. This condition is referred to as stress shielding [
17]. Powder metallurgy (PM) is one method for achieving porosity at the surface of a component. This method can create implants with interconnected pores that encourage osseointegration by allowing the development of bones. A collection of methods known as PM is used to create, characterize, and consolidate metallic or ceramic powders through sintering and compacting [
6]. Increasing the value of the micro surface roughness is an advantage of PM composite materials. Depending on completed wettability and mechanical tests, bioactive composite alloys could be utilized as materials to create biomedical implants with enhanced osseointegration potential and mechanical compatibility for demanding load-bearing applications [
18]. The biocompatibility, implant characteristics, and corrosion resistance of dental implants have all been demonstrated to be improved by the addition of SrO to Ti [
19].
A novel type of biomedical ceramic–metal composite, which is known as a bioactive composite metal, is the subject of this study, and it is made possible by such factors. It is made using an efficient PM method. The hybrid structure of the composite alloy consists of a biodegradable strontium oxide component, ceramic elements in Ti, and a bioinert Ti matrix, which is permanent following implantation and offers implant mechanical performance. In terms of tensile property application, dental implants, which are frequently subjected to demanding tensile loading, should be directly compared with cp Ti alloy. This study offers the idea of a Sr oxide–cp titanium composite alloy in which testing is conducted to evaluate the microstructure and mechanical properties in a fabricated condition. This paper focuses on the recent developments of bioactive composite Ti-based alloys for future biomedical applications. The present aims of this work are to develop a Ti-SrO composite and investigate its properties, such as the microstructure and physio-mechanical properties.
5. Conclusions
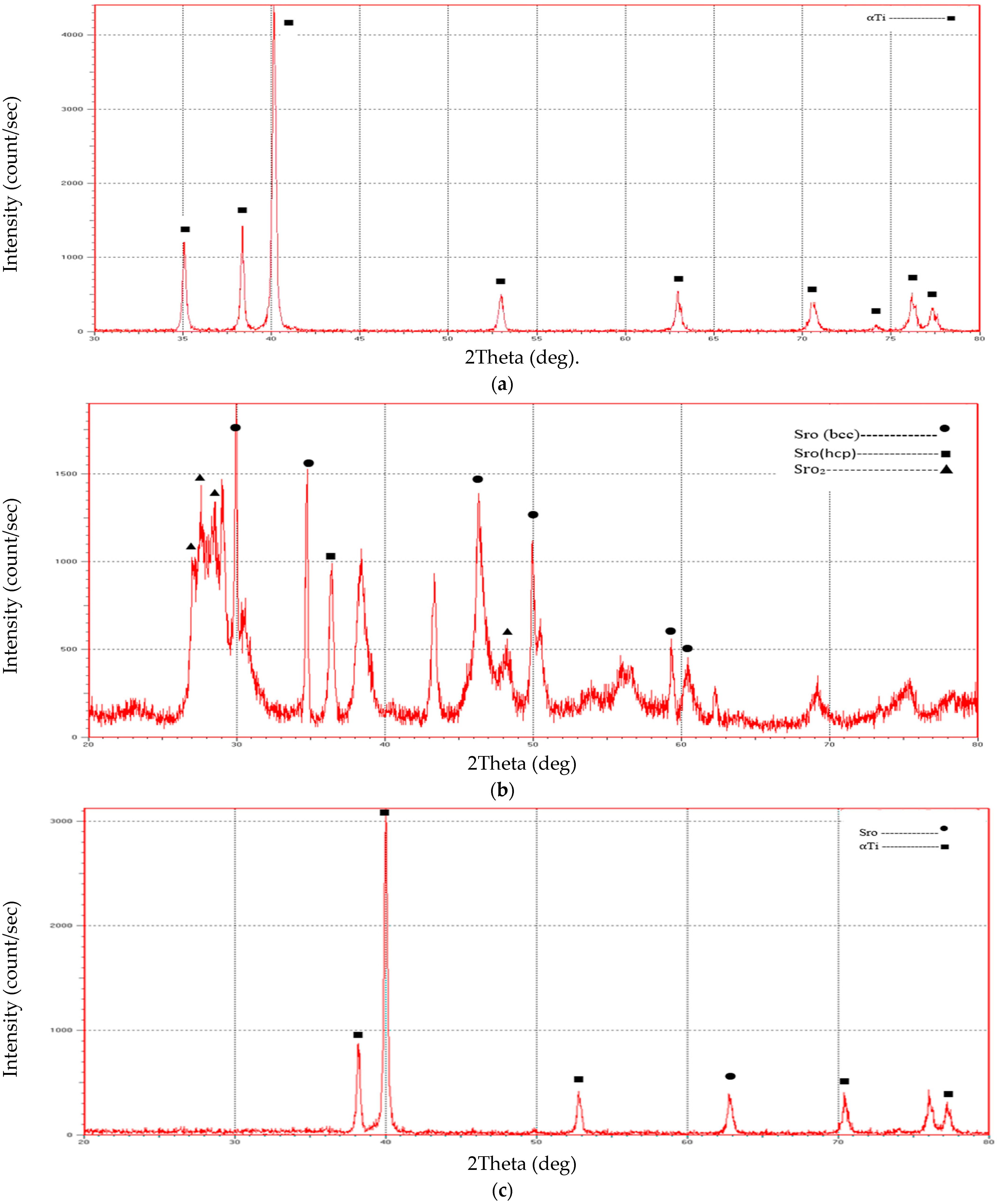
A novel and highly promising Ti composite alloy (Ti-SrO alloy) was prepared in this study using the PM approach. Prepared samples of Ti-SrO composite had additions of 2%, 4%, and 6% by wt%. Chemical studies, measurements of the porosity and density, and measurements of the microhardness, XRD, roughness, dimensional tensile strength, and contact angle were used to describe them. According to the XRD measurements, increasing the SrO percentages caused the fraction of SrO in the composite to climb to its maximum. Only the ⍺-Ti phases were formed in the structure of the Ti alloy and Ti-SrO alloys at different percentages (2%, 4%, and 6% by wt%), and phase transformation was not observed in the base alloy. Increases in the porosity, Brinell hardness, and diametral tensile strength were observed compared with those of the cp Ti alloys.
Only the contact angle measurements decreased with the increase in SrO additive materials from 2%, 4%, and 6% by wt%. The Ti-SrO composite was found to have the greatest potential for biomedical applications. By using surgical implants made of Ti-SrO alloy, the stress shielding phenomenon will essentially be eliminated, which makes such alloys more comfortable for patients. A model will be created and applied to the body of the organism, namely the leg region, to identify the concentration of stresses and the weaknesses of alloys. Such new biomedical alloys will make excellent conducting biocomposite replacements for orthopedic and dental implants because of their intriguing properties. However, the body of research to date has still focused on the characterization of the Ti-SrO alloys and only some of their physio-mechanical properties. Therefore, further biological studies are necessary to evaluate the potential of Ti-SrO alloys to optimize their use for biomedical applications.