Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Powders
2.3. Preparation of Ceramic Samples
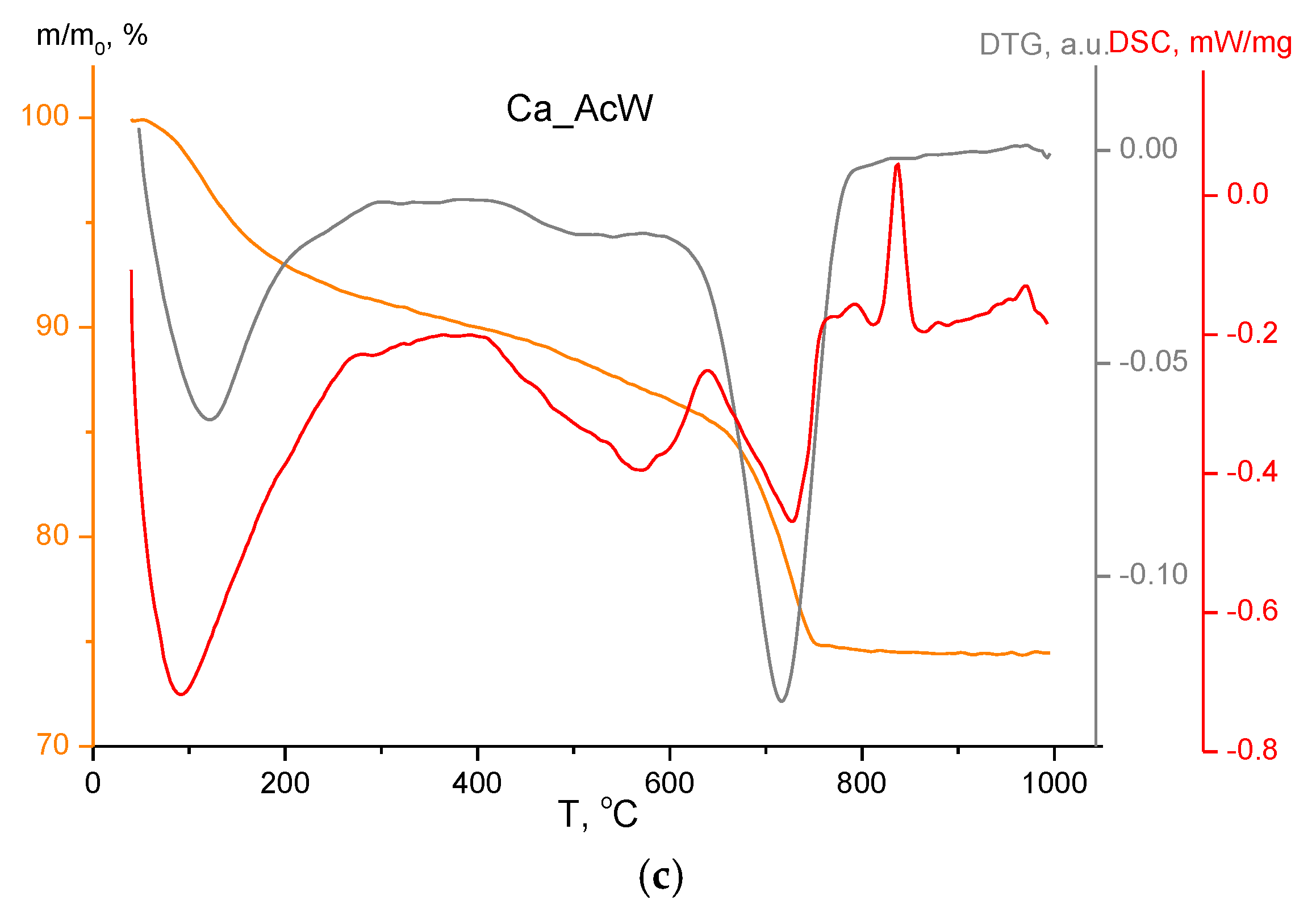
2.4. Methods of Analysis
- D/D0—relative diameter of the sample after the heat treatment, %;
- Dheat treatment—diameter of the sample after the heat treatment, cm;
- Dpress—diameter of the sample after pressing, cm;
- ρ—density of the sample, g/cm3;
- m—weight of the sample, g;
- h—thickness of the sample, cm;
- D—diameter of the sample, cm.
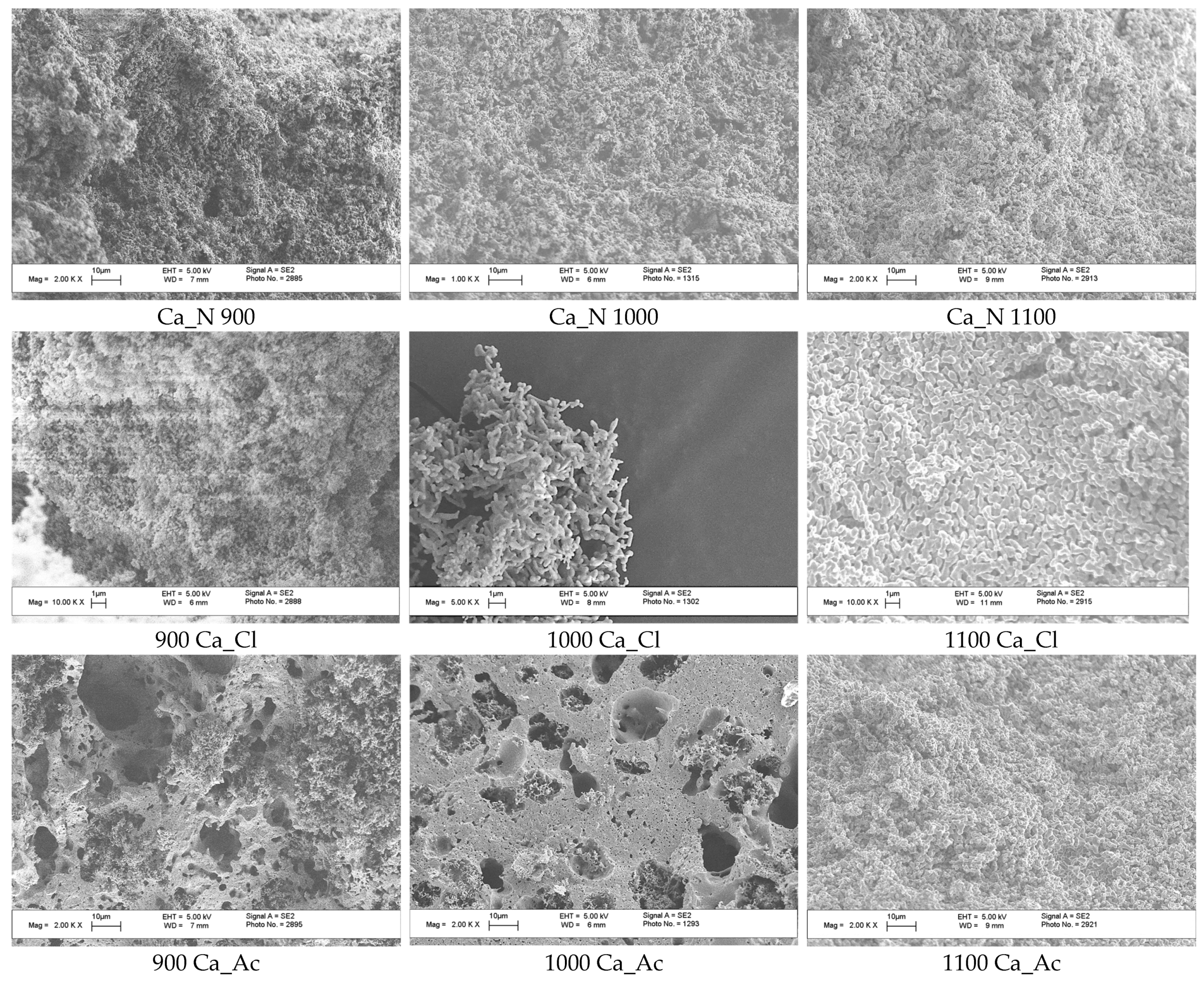
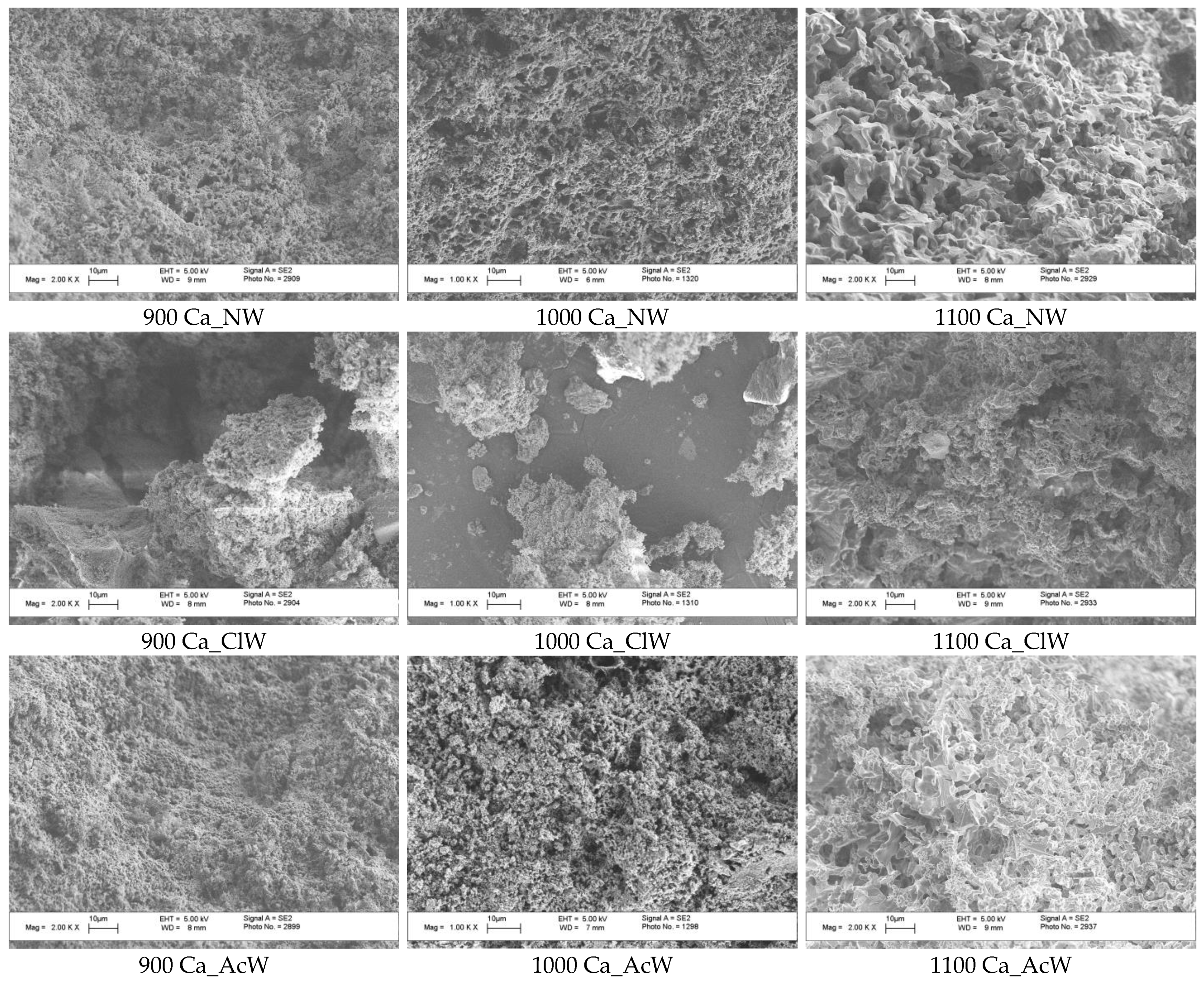
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J.; Stauffer, P.H.; Hendley, J.W., II. Rare Earth Elements: Critical Resources for High Technology; Fact Sheet 087-02; U.S. Geological Survey, US Department of the Interior: Drive Reston, VA, USA, 2022. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Yücel, H.E.; Yıldızhan, F. Evaluation of the Performance of Different Types of Fibrous Concretes Produced by Using Wollastonite. Materials 2022, 15, 6904. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.S.; Carter, J.L.; Boles, W.W.; Allen, C.C.; Allton, J.H. JSC-1: A new lunar soil simulant. Eng. Constr. Oper. Space IV 1994, 2, 857–866. Available online: https://www.lpi.usra.edu/lunar/strategies/jsc_lunar_simulant.pdf (accessed on 30 July 2023).
- Papike, J.J.; Simon, S.B.; Laul, J.C. The lunar regolith: Chemistry, mineralogy, and petrology. Rev. Geophys. 1982, 20, 761–826. [Google Scholar] [CrossRef]
- Zhu, C.; Crandall, P.B.; Gillis-Davis, J.J.; Ishii, H.A.; Bradley, J.P.; Corley, L.M.; Kaiser, R.I. Untangling the formation and liberation of water in the lunar regolith. Proc. Natl. Acad. Sci. USA 2019, 116, 11165–11170. [Google Scholar] [CrossRef] [PubMed]
- Ogris, D.M.; Kircher, V.; Gamsjäger, E. Cyclic Solid-Liquid Phase Transformations in the CaO–SiO2 System—Experiments and Modelling. Metall. Mater. Trans. B 2023, 54, 1555–1564. [Google Scholar] [CrossRef]
- Taylor, J.R.; Dinsdale, A.T. Thermodynamic and phase diagram data for the CaO-SiO2 system. Calphad 1990, 14, 71–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Voncken, J.; Yang, Y.; Boom, R.; Wang, N.; Zou, Z. Phase equilibria in the Na2O–CaO–SiO2 system. J. Am. Ceram. Soc. 2011, 94, 3088–3093. [Google Scholar] [CrossRef]
- Roy, D.M. New strong cement materials: Chemically bonded ceramics. Science 1987, 235, 651–658. [Google Scholar] [CrossRef]
- Kozin, A.V.; Fediuk, R.S.; Yarusova, S.B.; Gordienko, P.S.; Lesovik, V.S.; Mosaberpanah, M.A.; Mugahed Amran, Y.A.; Murali, G. Improvement of mechanical characteristics of mortar by using of wollastonite. Mag. Civ. Eng. 2021, 107, 10715. [Google Scholar] [CrossRef]
- Nikonova, N.S.; Tikhomirova, I.N.; Belyakov, A.V.; Zakharov, A.I. Wollastonite in Silicate Matrices. Glass Ceram. 2003, 60, 342–346. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Hassan, A.; Mohamad, Z.; Othman, N. Mechanical properties of wollastonite reinforced thermoplastic composites: A review. Polym. Compos. 2020, 41, 395–429. [Google Scholar] [CrossRef]
- Azarov, G.M.; Maiorova, E.V.; Oborina, M.A.; Belyakov, A.V. Wollastonite raw materials and their applications (a review). Glass Ceram. 1995, 52, 237–240. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, S.; Hu, Y. Enhanced red-emitting phosphor Na2Ca3Si2O8:Eu3+ by charge compensation. J. Mater. Sci. Mater. Electron. 2017, 28, 5262–5269. [Google Scholar] [CrossRef]
- Kolli, N.K.; Kundu, S.; Roy, S. Could Combeite (Na2Ca2Si3O9) Serve as a Potential Biomaterial Platform that could Support the Growth of the Osteoblasts? Silicon 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Singh, P.; Yu, X.; Kumar, A.; Dubey, A.K. Recent advances in silicate-based crystalline bioceramics for orthopedic applications: A review. J. Mater. Sci. 2022, 57, 13109–13151. [Google Scholar] [CrossRef]
- Tikhomirova, I.N.; Makarov, A.V.; Htet, Z.M. Thermal Insulation Materials Based on Expanded Vermiculite and Foamed Liquid Glass. Refract. Ind. Ceram. 2020, 61, 451–455. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Lu, C.; Li, F. Synthesis and characterization of geopolymer from lunar regolith simulant based on natural volcanic scoria. Chin. J. Aeronaut. 2022, 35, 144–159. [Google Scholar] [CrossRef]
- Shukur, M.M.; Al-Majeed, E.A.; Obied, M.M. Characteristic of wollastonite synthesized from local raw materials. Int. J. Eng. Technol. 2014, 4, 426–429. [Google Scholar]
- Obeid, M.M. Crystallization of synthetic wollastonite prepared from local raw materials. Int. J. Mater. Chem. 2014, 4, 79–87. [Google Scholar] [CrossRef]
- Ismail, H.; Mohamad, H. Bioactivity and Biocompatibility Properties of Sustainable Wollastonite Bioceramics from Rice Husk Ash/Rice Straw Ash: A Review. Materials 2021, 14, 5193. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Evolution of Ca2SiO4 and Ca3Si2O7 crystalline phases synthesized from agro-food waste ashes. AIP Conf. Proc. 2019, 2093, 020033. [Google Scholar] [CrossRef]
- Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Sergeev, N. Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics 2022, 5, 550–561. [Google Scholar] [CrossRef]
- Macena, G.S.; Abyzov, A.S.; Fokin, V.M.; Zanotto, E.D.; Ferreira, E.B. Off-stoichiometry effects on crystal nucleation and growth kinetics in soda-lime-silicate glasses. The combeite (Na2O·2CaO·3SiO2)–devitrite (Na2O·3CaO·6SiO2) joint. Acta Mater. 2020, 196, 191–199. [Google Scholar] [CrossRef]
- Baek, J.Y.; Shin, S.H.; Hyun, S.H.; Cho, J.W. Glass structure and crystallization via two distinct thermal histories: Melt crystallization and glass crystallization. J. Eur. Ceram. Soc. 2021, 41, 831–837. [Google Scholar] [CrossRef]
- Ohsato, H.; Sugimura, T. Morphology of synthetic β-wollastonite and para-wollastonite. J. Cryst. Growth 1986, 74, 656–658. [Google Scholar] [CrossRef]
- Maries, A.; Rogers, P.S. Continuous unidirectional crystallization of fibrous metasilicates from melts. J. Mater. Sci. 1978, 13, 2119–2130. [Google Scholar] [CrossRef]
- MacLaren, D.C.; White, M.A. Cement: Its chemistry and properties. J. Chem. Educ. 2003, 80, 623. [Google Scholar] [CrossRef]
- Harris, M.; Simpson, G.; Scrivener, K.; Bowen, P. A method for the reliable and reproducible precipitation of phase pure high Ca/Si ratio (>1.5) synthetic calcium silicate hydrates (CSH). Cem. Concr. Res. 2022, 151, 106623. [Google Scholar] [CrossRef]
- Hunnicutt, W.; Struble, L.; Mondal, P. Effect of synthesis procedure on carbonation of calcium-silicate-hydrate. J. Am. Ceram. Soc. 2017, 100, 3736–3745. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J.; Lu, J. Synthesis of wollastonite nanowires via hydrothermal microemulsion methods. Mater. Lett. 2006, 60, 3007–3010. [Google Scholar] [CrossRef]
- Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings 2023, 13, 374. [Google Scholar] [CrossRef]
- Morsy, R.; Abuelkhair, R.; Elnimr, T. Synthesis and in vitro bioactivity mechanism of synthetic α-wollastonite and β-wollastonite bioceramics. J. Ceram. Sci. Technol. 2016, 7, 65–70. [Google Scholar] [CrossRef]
- Tajuelo Rodriguez, E.; Hunnicutt, W.A.; Mondal, P.; Le Pape, Y. Examination of gamma-irradiated calcium silicate hydrates. Part I: Chemical-structural properties. J. Am. Ceram. Soc. 2020, 103, 558–568. [Google Scholar] [CrossRef]
- Singh, S.P.; Karmakar, B. Mechanochemical synthesis of nano calcium silicate particles at room temperature. New J. Glass Ceram. 2011, 1, 49–52. [Google Scholar] [CrossRef]
- Huang, X.H.; Chang, J. Synthesis of nanocrystalline wollastonite powders by citrate–nitrate gel combustion method. Mater. Chem. Phys. 2009, 115, 1–4. [Google Scholar] [CrossRef]
- Iimori, Y.; Kameshima, Y.; Okada, K.; Hayashi, S. Comparative study of apatite formation on CaSiO3 ceramics in simulated body fluids with different carbonate concentrations. J. Mater. Sci. Mater. Med. 2005, 16, 73–79. [Google Scholar] [CrossRef]
- Martinez, A.; Izquierdo-Barba, I.; Vallet-Regi, M. Bioactivity of a CaO−SiO2 binary glasses system. Chem. Mater. 2000, 12, 3080–3088. [Google Scholar] [CrossRef]
- Blinova, A.A.; Karamirzoev, A.A.; Guseynova, A.R.; Maglakelidze, D.G.; Ilyaeva, T.A.; Gusov, B.A.; Meliksetyants, A.P.; Pirumian, M.M.; Taravanov, M.A.; Pirogov, M.A.; et al. Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids. Micromachines 2023, 14, 245. [Google Scholar] [CrossRef]
- Safronova, T.V. Phase Composition of Ceramic Based on Calcium Hydroxyapatite Powders Containing Byproducts of the Synthesis Reaction. Glass Ceram. 2009, 66, 136–139. [Google Scholar] [CrossRef]
- Gorshkov, V.; Timashev, V.; Saveliev, V. Identification characteristics of compounds containing water. In Methods for Physical and Chemical Analysis of Binders; Gaidzhurov, P., Nekrasov, K., Eds.; Vyshaya Shkola: Moscow, Russia, 1981; pp. 292–294. (In Russian) [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Harman, R.W. Aqueous Solutions of Sodium Silicates. VIII. General Summary and Theory of Constitution. Sodium Silicates as Colloidal Electrolytes. Phys. Chem. 1928, 32, 44–60. [Google Scholar] [CrossRef]
- Singh, N.B. Hydrothermal synthesis of β-dicalcium silicate (β-Ca2SiO4). Prog. Cryst. Growth Charact. Mater. 2006, 52, 77–83. [Google Scholar] [CrossRef]
- Bond, B.D.; Jacobs, P.W.M. The thermal decomposition of sodium nitrate. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 1265–1268. [Google Scholar] [CrossRef]
- Fischer, R.X.; Tillmanns, E. Revised data for combeite, Na2Ca2Si3O9. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1852–1854. [Google Scholar] [CrossRef]
- Ohno, H.; Furukawa, K. X-ray diffraction analysis of molten NaCl near its melting point. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 1981–1985. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl system. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, J.; Si, Y.; Zhu, F.; Li, J. Melting Curve of Potassium Chloride from in situ Ionic Conduction Measurements. Minerals 2020, 10, 250. [Google Scholar] [CrossRef]
- Anwar, J.; Frenkel, D.; Noro, M.G. Calculation of the melting point of NaCl by molecular simulation. J. Chem. Phys. 2003, 118, 728–735. [Google Scholar] [CrossRef]
- Safronova, T.V.; Steklov, M.Y.; Putlyaev, V.I.; Shekhirev, M.A. Na-substituted Ca-deficient carbonate hydroxyapatite for the production of ceramic materials. Compos. Mater. Constr. (Konstr. Iz Kompoz. Mater.) 2006, N4, 34–39. Available online: https://www.elibrary.ru/item.asp?id=11846526 (accessed on 30 July 2023). (In Russian).
- Hillert, M.; Sundman, B.; Wang, X. An assessment of the CaO-SiO2 system. Metall. Trans. B 1990, 21, 303–312. [Google Scholar] [CrossRef]
- Zadov, A.E.; Gazeev, V.M.; Pertsev, N.N.; Gurbanov, A.G.; Gobechiya, E.R.; Yamnova, N.A.; Chukanov, N.V. Calcioolivine, γ-Ca2SiO4, an Old and New Mineral species. Geol. Ore Depos. 2009, 51, 741–749. [Google Scholar] [CrossRef]
- Soboleva, E.N.; Yuritsyn, N.S.; Ugolkov, V.L. Kinetics of crystal nucleation of Na2O·2CaO·3SiO2-based solid solutions in glasses of the Na2SiO3—CaSiO3 pseudobinary join. Glass Phys. Chem. 2004, 30, 481–486. [Google Scholar] [CrossRef]
- Gusev, S.A.; Shekhirev, M.A.; Safronova, T.V.; Putlayev, V.I.; Skvortsova, Z.N.; Protsenko, P.V. Wetting and spreading of molten NaCl and CaCl2 over polycrystalline hydroxyapatite. Mendeleev Commun. 2014, 24, 12–14. [Google Scholar] [CrossRef]


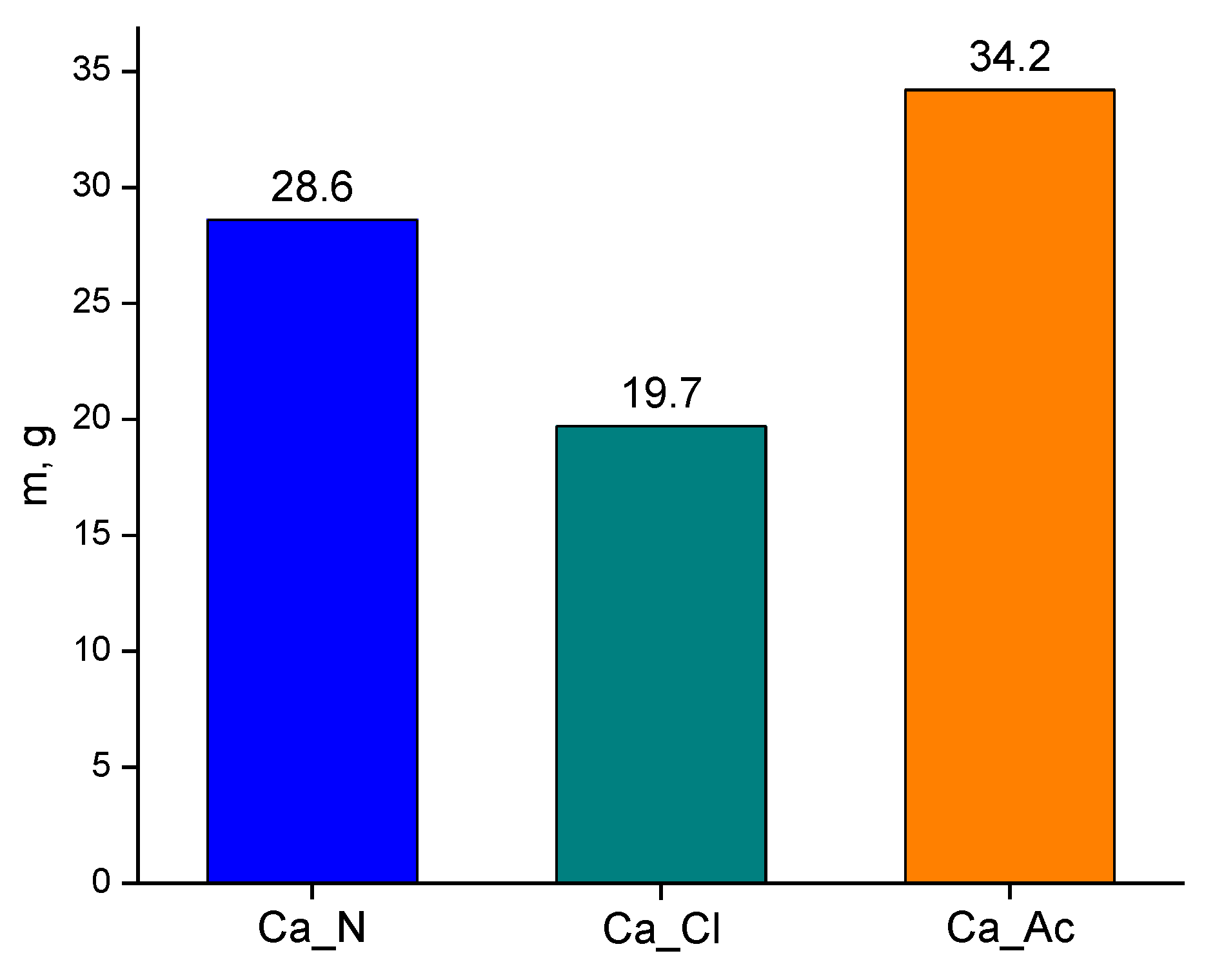












| Labeling | Salts Used for Solutions Preparation 1 | Stage of Washing | |
|---|---|---|---|
| Resource of SiO32− | Resource of Ca2+ | ||
| Ca_N | Na2SiO3·5H2O | Ca(NO3)2·4H2O | not used |
| Ca_NW | used | ||
| Ca_Cl | CaCl2·6H2O | not used | |
| Ca_ClW | used | ||
| Ca_Ac | Ca(CH3COO)2·H2O | not used | |
| Ca_AcW | used | ||
| Powder | Phase Composition According XRD |
|---|---|
| Ca_N | Ca1,5SiO3,5·xH2O, CaCO3 (calcite), CaCO3 (aragonite), NaNO3 |
| Ca_Cl | Ca1,5SiO3,5·xH2O, CaCO3 (calcite), CaCO3 (aragonite), NaCl |
| Ca_Ac | Ca1,5SiO3,5·xH2O, CaCO3(calcite) |
| Ca_NW | Ca1,5SiO3,5·xH2O, CaCO3 (calcite), CaCO3 (aragonite) |
| Ca_ClW | Ca1,5SiO3,5·xH2O, CaCO3 (calcite), CaCO3 (aragonite) |
| Ca_AcW | Ca1,5SiO3,5·xH2O, CaCO3 (calcite) |
| Powder | By-Product | Molar Mass of By-Product, c.u. | Theoretical Weight of By-Product According to Synthesis Conditions, mexp., g | Weight of By-Product Extracted from Mother Liquor, mextract., g | Part of By-Product Extracted, mextract./mexp., % | Part of By-Product Remaining in Powder, % |
|---|---|---|---|---|---|---|
| Ca_N | NaNO3 | 85.0 | 42.5 | 28.6 | 67.3 | 32.7 |
| Ca_Cl | NaCl | 58.4 | 26.2 | 19.7 | 75.1 | 24.9 |
| Ca_Ac | NaCH3COO | 82.0 | 41.0 | 34.2 | 83.4 | 16.6 |
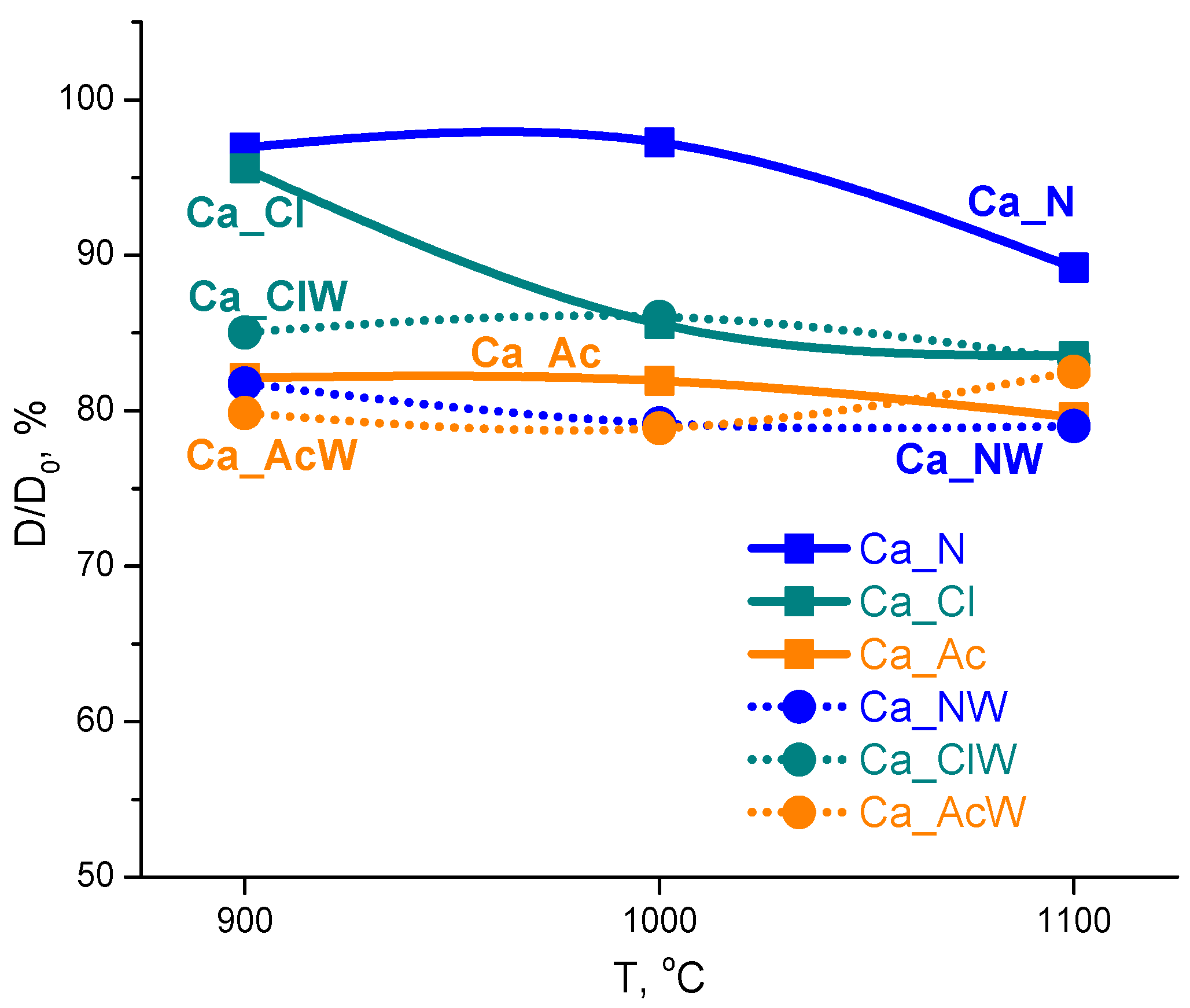
| Ca_N | Ca_Cl | Ca_Ac | Ca_NW | Ca_ClW | Ca_AcW | |
|---|---|---|---|---|---|---|
| Powders | 0.46 | 0.54 | 0.44 | 0.34 | 0.33 | 0.37 |
| Preceramic powder compacts | 1.09 | 1.41 | 1.08 | 0.85 | 0.89 | 0.87 |
| Ceramics after 900 | 0.81 | 0.68 * | 1.34 | 1.25 | 1.10 * | 1.23 |
| Ceramics after 1000 | 0.78 | 0.91 * | 1.33 | 1.20 | 1.03 * | 1.18 |
| Ceramics after 1100 | 0.94 | 0.90 | 1.35 | 1.21 | 1.08 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronova, T.V.; Sterlikov, G.S.; Kaimonov, M.R.; Shatalova, T.B.; Filippov, Y.Y.; Toshev, O.U.; Roslyakov, I.V.; Kozlov, D.A.; Tikhomirova, I.N.; Akhmedov, M.R. Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate). J. Compos. Sci. 2023, 7, 408. https://doi.org/10.3390/jcs7100408
Safronova TV, Sterlikov GS, Kaimonov MR, Shatalova TB, Filippov YY, Toshev OU, Roslyakov IV, Kozlov DA, Tikhomirova IN, Akhmedov MR. Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate). Journal of Composites Science. 2023; 7(10):408. https://doi.org/10.3390/jcs7100408
Chicago/Turabian StyleSafronova, Tatiana V., Gleb S. Sterlikov, Maksim R. Kaimonov, Tatiana B. Shatalova, Yaroslav Y. Filippov, Otabek U. Toshev, Ilya V. Roslyakov, Daniil A. Kozlov, Irina N. Tikhomirova, and Muslim R. Akhmedov. 2023. "Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate)" Journal of Composites Science 7, no. 10: 408. https://doi.org/10.3390/jcs7100408
APA StyleSafronova, T. V., Sterlikov, G. S., Kaimonov, M. R., Shatalova, T. B., Filippov, Y. Y., Toshev, O. U., Roslyakov, I. V., Kozlov, D. A., Tikhomirova, I. N., & Akhmedov, M. R. (2023). Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate). Journal of Composites Science, 7(10), 408. https://doi.org/10.3390/jcs7100408







