Cationic Polymerized Epoxy and Radiation Cured Acrylate Blend Nanocomposites Based on WS2 Nanoparticles—Part A: Curing Processes and Kinetics
Abstract
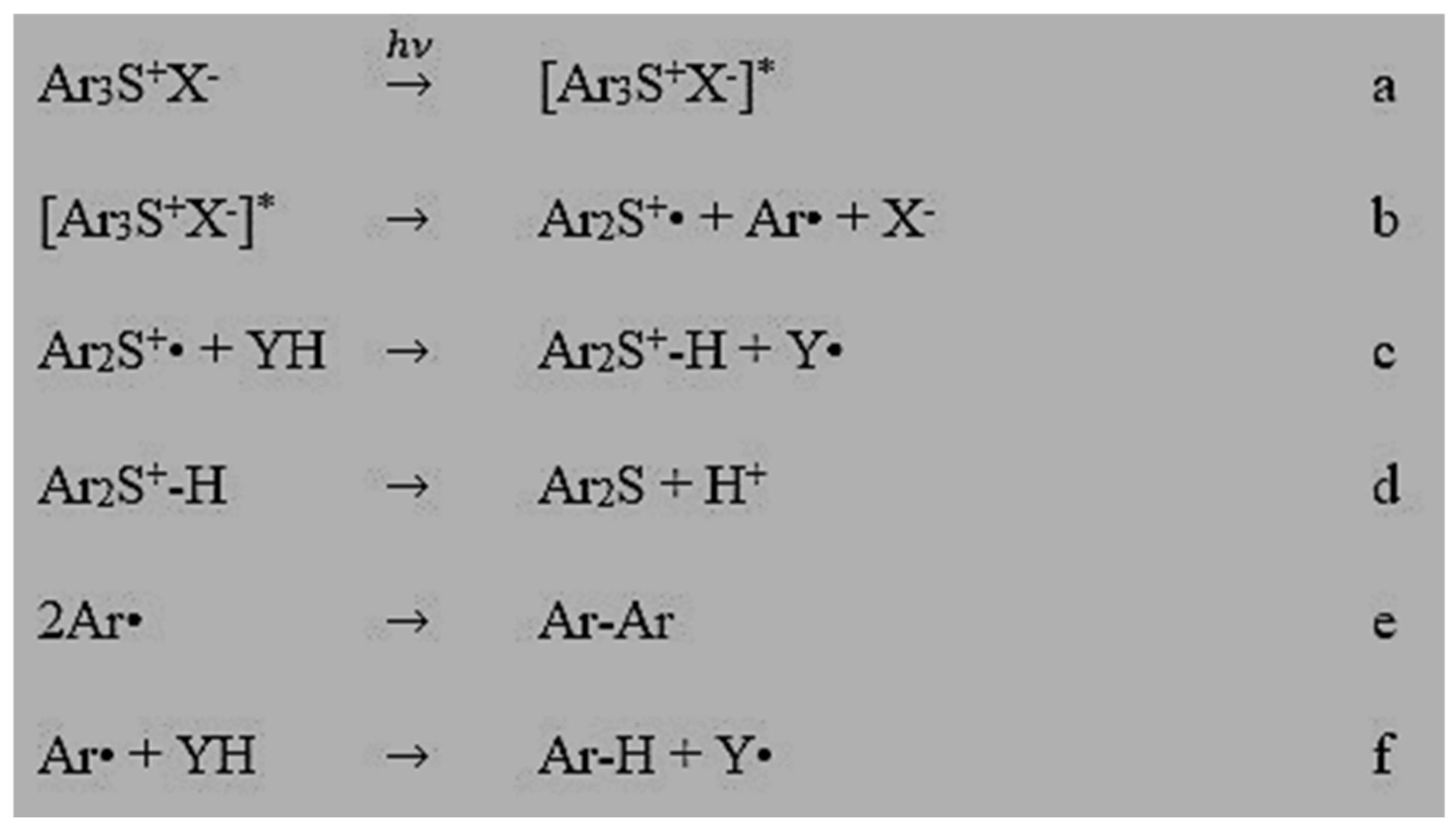
1. Introduction
2. Materials and Samples Preparation
2.1. Materials
2.2. Dispersion and Distribution Techniques
2.3. Sonication/Vortex Multistage Dispersion Technique
2.4. Master-Batch Technique
2.5. Curing System
2.6. Kinetic Analysis of the Curing
2.7. Thermal Curing Procedure
2.8. IF-WS2/Epoxy Interphase Analysis
2.9. Rheometry
2.10. Spectrophotometry
3. Results and Discussions
3.1. Pre-Cured Resin Properties
3.2. Thermal Curing Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crivello, J.V.; Kong, S.; Jang, M. Cationic polymerization: New developments and applications. Macromol. Symp. 2004, 217, 47–61. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F.; Eichhorn, K.J.; Rizza, G. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymer 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Amerio, E.; Bongiovanni, R.; Priola, A.; Gianni, A.D.; Voit, B.; Rizza, G. Preparation and characterization of nanostructured TiO2/epoxy polymeric films. Macromol. Mater. Eng. 2006, 291, 517–523. [Google Scholar] [CrossRef]
- Dabestani, R.; Ivanov, I.N.; Sands, J.M. Cationic Polymerization (Cure Kinetics) of Model Epoxide Systems; Army Research Laboratory: Adelphi, MD, USA, 2002. [Google Scholar]
- Atif, M.; Bongiovanni, R.; Yang, J. Cationically UV-Cured Epoxy Composites. Polym. Rev. 2015, 55, 37–41. [Google Scholar] [CrossRef]
- Crivello, J.V. UV and electron beam-induced cationic polymerization. Nucl. Inst. Methods Phys. Res. B 1999, 151, 8–21. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, X.; Xu, B.; Qian, X.; Song, G.; Nie, J. Cationic photopolymerization of bisphenol A diglycidyl ether epoxy under 385 nm. J. Appl. Polym. Sci. 2013, 130, 3698–3703. [Google Scholar] [CrossRef]
- Crivello, J.V.; Sangermano, M. Visible and Long-Wavelength Photoinitiated Cationic Polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 343–356. [Google Scholar] [CrossRef]
- Crivello, J.V. Synergistic Free Radical Effects in Photoinitiated Cationic Polymerization. Photoinitiated Polym. 2003, 847, 178–186. [Google Scholar]
- Aydogan, B.; Gacal, B.; Yildirim, A.; Yonet, N.; Yuksel, Y. Wavelength tunability in photoinitiated cationic polymerization. In Photochemistry and UV Curing New Trends; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, Indian, 2006; Volume 661, pp. 1–15. ISBN 81-308-0014-4. [Google Scholar]
- Crivello, J.V.; Lam, J.H.W. The Photoinitiated Cationic Polymerization of Epoxy Resins. Am. Chem. Soc. Symp. Ser. 1979, 114, 1–16. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel photoacid generators for cationic photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Chunfu, C.; Li, B.; Wang, C.; Iwasaki, S.; Kanari, M.; Lu, D. UV and Thermal Cure Epoxy Adhesives. In Paint and Coating Indisutry; Yilmaz, F., Ed.; Intech Open: London, UK, 2018; pp. 71–85. [Google Scholar]
- Malik, M.S.; Schlogl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Asmussen, S.V.; Sangermano, M.; Vallo, C.I. Visible light polymerization of epoxy monomers using an iodonium salt with camphorquinone/ethyl-4-dimethyl aminobenzoate. Polym. Int. 2013, 62, 1368–1376. [Google Scholar] [CrossRef]
- Golaz, B.; Michaud, V.; Månson, J.A.E. Photo-polymerized epoxy primer for adhesion improvement at thermoplastics/metallic wires interfaces. Compos. Part A Appl. Sci. Manuf. 2013, 48, 171–180. [Google Scholar] [CrossRef]
- Ryu, C.Y.; Spencer, M.J.; Crivello, J.V. Involvement of supramolecular complexes in the capture and release of protonic acids during the cationic ring-opening polymerization of epoxides. Macromolecules 2012, 45, 2233–2241. [Google Scholar] [CrossRef]
- Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Photoinitiated cationic polymerization of epoxy alcohol monomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 389–401. [Google Scholar] [CrossRef]
- Foix, D.; Ramis, X.; Sangermano, M.; Serra, A. Synthesis of a New Hyperbranched-Linear-Hyperbranched Triblock Copolymer and Its Use as a Chemical Modifier for the Cationic Photo and Thermal Curing of Epoxy Resins. J. Polymer Sci. A Polymer Chem. 2012, 50, 1133–1142. [Google Scholar] [CrossRef]
- Belon, C.; Chemtob, A.; Croutxé-Barghorn, C.; Rigolet, S.; Schmitt, M.; Bistac, S.; Le Houérou, V.; Gauthier, C. Nanocomposite coatings via simultaneous organic-inorganic photo-induced polymerization: Synthesis, structural investigation and mechanical characterization. Polym. Int. 2010, 59, 1175–1186. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.P. Recent developments of versatile photoinitiating systems for cationic ring opening polymerization operating at any wavelengths and under low light intensity sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef]
- Park, S.; Kilgallon, L.J.; Yang, Z.; Ryu, D.Y.; Ryu, C.Y. Molecular Origin of the Induction Period in Photoinitiated Cationic Polymerization of Epoxies and Oxetanes. Macromolecules 2019, 52, 1158–1165. [Google Scholar] [CrossRef]
- Lecompère, M.; Allonas, X.; Maréchal, D.; Criqui, A. Dual-cure Photo-thermal Initiating System for Cationic Polymerization of Epoxy under LED Visible Light. Photopolym. Sci. Technol. 2017, 30, 399–404. [Google Scholar] [CrossRef]
- Lecompère, M.; Allonas, X.; Maréchal, D.; Criqui, A. Versatility of Pyrylium Salt/Vinyl Ether Initiating System for Epoxide Dual-Cure Polymerization: Kick-Starting Effect of the Coinitiator. Macromol. Rapid Commun. 2017, 38, 1600660. [Google Scholar] [CrossRef] [PubMed]
- Bomze, D.; Knaack, P.; Liska, R. Successful radical induced cationic frontal polymerization of epoxy-based monomers by C-C labile compounds. R. Soc. Chem. 2015, 6, 8161–8167. [Google Scholar] [CrossRef]
- Mariani, A.; Bidali, S.; Fiori, S.; Sangermano, M.; Malucelli, G.; Bongiovanni, R.; Priola, A. UV-ignited frontal polymerization of an epoxy resin. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2066–2072. [Google Scholar] [CrossRef]
- Park, H.J.; Ryu, C.Y.; Crivello, J.V. Photoinitiated cationic polymerization of limonene 1,2-oxide and α-pinene oxide. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 109–117. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Moszner, N.; Yagci, Y. Visible light initiated free radical promoted cationic polymerization using acylgermane based photoinitiator in the presence of onium salts. Macromolecules 2008, 41, 6714–6718. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, A.M.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Visible-Light-Sensitive Photoredox Catalysis by Iron Complexes: Applications in Cationic and Radical Polymerization Reactions. J. Polym. Sci. A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Horcajada, P.; Livage, C.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Iron-Based Metal-Organic Frameworks (MOF) as Photocatalysts for Radical and Cationic Polymerizations under Near UV and Visible LEDs (385–405 nm). Macromol. Chem. Phys. 2016, 217, 2534–2540. [Google Scholar] [CrossRef]
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762. [Google Scholar] [CrossRef]
- Wang, D.; Szillat, F.; Fouassier, J.P.; Lalevée, J. Remarkable Versatility of Silane/Iodonium Salt as Redox Free Radical, Cationic, and Photopolymerization Initiators. Macromolecules 2019, 52, 5638–5645. [Google Scholar] [CrossRef]
- Haifaa, M.; Dumur, F.; Telitel, S.; Vidal, L.; Xiao, P.; Versace, D.L.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; et al. PhotoInitiating Systems of Polymerization and In-Situ Incorporation of Metal Nanoparticles in Polymer Matrixes Upon Visible Lights: Push-Pull Malonate and Malonitrile Based Dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar]
- Hoppe, C.C.; Ficek, B.A.; Eom, H.S.; Scranton, A.B. Cationic photopolymerization of epoxides containing carbon black nanoparticles. Polymer 2010, 51, 6151–6160. [Google Scholar] [CrossRef]
- Dickey, M.D.; Gupta, S.; Leach, K.A.; Collister, E.; Willson, C.G.; Russell, T.P. Novel 3-D structures in polymer films by coupling external and internal fields. Langmuir 2006, 22, 4315–4318. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New horizons in cationic photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Yagci, Y. Initiation of Cationic Polymerization by Photoinduced Electron Transfer. Macromol. Symp. 1998, 134, 177–188. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. Photosensitized Cationic Polymerizations Using Dialkylphenacylsulfonium and Dialkyl(4-hydroxyphenyl)sulfonium Salt Photoinitiators. Macromolecules 1981, 14, 1141–1147. [Google Scholar] [CrossRef]
- Nelson, E.W.; Carter, T.P.; Scranton, A.B. The role of the triplet state in the photosensitization of cationic polymerizations by anthracene. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 247–256. [Google Scholar] [CrossRef]
- Crivello, J.V.; Ortiz, R.A. Benzyl alcohols as accelerators in the photoinitiated cationic polymerization of epoxide monomers. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2298–2309. [Google Scholar] [CrossRef]
- Nakajima, A.; Tomotake, A.; Kida, S.; Konica Minolta IJ Technologies. Development of New Cationic UV Curable Inkjet Ink. 2006. Available online: https://www.konicaminolta.com/inkjet/technology/report/pdf/200809_ppic08.pdf (accessed on 20 October 2022).
- Baikerikar, K.K.; Scranton, A.B. Photopolymerizable liquid encapsulants for microelectronic devices: Thermal and mechanical properties of systems with reduced in-mold cure times. J. Appl. Polym. Sci. 2001, 81, 3449–3461. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. Tungsten dichalcogenides (WS2, WSe2, and WTe2): Materials chemistry and applications. J. Mater. Chem. A 2017, 5, 18299–18325. [Google Scholar] [CrossRef]
- Sharif, M.; Pourabbas, B.; Sangermano, M.; Moghadam, F.S.; Mohammadi, M.; Roppolo, I.; Fazli, A. The effect of graphene oxide on UV curing kinetics and properties of SU8 nanocomposites. Polym. Int. 2017, 66, 405–417. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.K.; Kim, K.H.; Kwon, T.Y. Degree of conversion of dual-cured resin cement light-cured through three fibre posts within human root canals: An ex vivo study. Int. Endod. J. 2009, 42, 667–674. [Google Scholar] [CrossRef]
- Pandey, R. Photopolymers in 3D Printing Applications. 2014. Available online: https://www.theseus.fi/bitstream/handle/10024/80083/pandey_ramji.pdf?sequence=1&isAllowed=y (accessed on 20 October 2022).
- Verschueren, K.; Kaurb, B. Cycloaliphatic Epoxide Resins for Cationic UV-Cure. 1996. Available online: https://www.osti.gov/etdeweb/servlets/purl/20051955 (accessed on 20 October 2022).
- Otorgust, G.; Sedova, A.; Dodiuk, H.; Kenig, S.; Tenne, R. Carbon and tungsten disulfide nanotubes and fullerene-like nanostructures in thermoset adhesives: A critical review. Rev. Adhes. Adhes. 2015, 3, 311–363. [Google Scholar] [CrossRef]
- Haba, D.; Brunner, A.J.; Pinter, G. Dispersion of fullerene-like WS2 nanoparticles within epoxy and the resulting fracture mechanics. Compos. Sci. Technol. 2015, 119, 55–61. [Google Scholar] [CrossRef]
- Shneider, M.; Dodiuk, H.; Kenig, S.; Tenne, R. The effect of tungsten sulfide fullerene-like nanoparticles on the toughness of epoxy adhesives. J. Adhes. Sci. Technol. 2010, 24, 1083–1095. [Google Scholar] [CrossRef]
- Haba, D.; Barbezat, M.; Ayalur-Karunakaran, S.; Schlögl, S.; Brunner, A.J.; Pinter, G. Significance of epoxy network properties for the toughening effect of flaky and fullerene-like WS2 nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1738–1747. [Google Scholar] [CrossRef]
- Haba, D. Toughening of Epoxy with WS2 Nanoparticles. Ph.D. Thesis, Montanunitversitaet Leoben, Leoben, Austria, 2016. Available online: https://pure.unileoben.ac.at/portal/files/1835750/AC13362385n01vt.pdf (accessed on 20 October 2022).
- Thostenson, E.T.; Chou, T.W. Processing-structure-multi-functional property relationship in carbon nanotube/epoxy composites. Carbon N. Y. 2006, 44, 3022–3029. [Google Scholar] [CrossRef]
- Shneider, M.; Dodiuk, H.; Tenne, R.; Kenig, S. Nanoinduced Morphology and Enhanced Properties of Epoxy Containing Tungsten Disulfide Nanoparticles. Polym. Eng. Sci. 2013, 53, 2624–2632. [Google Scholar] [CrossRef]
- Haba, D.; Griesser, T.; Müller, U.; Brunner, A.J. Comparative investigation of different silane surface functionalizations of fullerene-like WS2. J. Mater. Sci. 2015, 50, 5125–5135. [Google Scholar]
- Sangermano, M.; Priola, A.; Kortaberria, G.; Jimeno, A.; Garcia, I.; Mondragon, I.; Rizza, G. Photopolymerization of epoxy coatings containing iron-oxide nanoparticles. Macromol. Mater. Eng. 2007, 292, 956–961. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Turcato, E.A.; di Gianni, A.; Ronchetti, S. Epoxy coatings containing clays and organoclays: Effect of the filler and its water content on the UV-curing process. Prog. Org. Coat. 2008, 62, 336–343. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Verdejo, R.; Lopez-Manchado, M.A.; Sangermano, M. Epoxy-Graphene UV-cured nanocomposites. Polymer 2011, 52, 4664–4669. [Google Scholar] [CrossRef]
- Chemtob, A.; Versace, D.L.; Belon, C.; Croutxé-Barghorn, C.; Rigolet, S. Concomitant organic-inorganic UV-curing catalyzed by photoacids. Macromolecules 2008, 41, 7390–7398. [Google Scholar] [CrossRef]
- Däbritz, F.; Voit, B.; Naguib, M.; Sangermano, M. Hyperstar poly (ester-methacrylate) s as additives in thermally and photocured epoxy resins. Polymer 2011, 52, 5723–5731. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Pisoni, A.; Jacimovic, J.; Gaál, R.; Náfrádi, B.; Berger, H.; Révay, Z.; Forró, L. Anisotropic transport properties of tungsten disulfide. Scr. Mater. 2016, 114, 48–50. [Google Scholar] [CrossRef]
- Garrett, K.W.; Rosenberg, H.M. The thermal conductivity of epoxy-resin/powder composite materials. J. Phys. D Appl. Phys. 1974, 7, 1247–1258. [Google Scholar] [CrossRef]
- Raichman, D.; Strawser, D.A.; Lellouche, J.P. Covalent functionalization/polycarboxylation of tungsten disulfide inorganic nanotubes (INTs-WS2). Nano Res. 2015, 8, 1454–1463. [Google Scholar] [CrossRef]












| Comparison Criteria | WS2-TO | WS2-C |
|---|---|---|
| Geometry (d-space- interlayer layer spacing according to XRD) | Spherical (2θ = 14.1°; 6.26 Å) | Oval (2θ = 14.2°; 6.22 Å) |
| Diameter | 80 nm | 90 nm |
| Moisture Content | 6.7% weight loss | 1.5% weight loss |
| pH value | 4.9 | 7.2 |
| Oxygen/Tungsten ratio by XPS | 0.55 | 0.83 |
| Sample Name | PGE | PGEnTA Neat | PGEnTA 1.0 wt.% WS2-C | PGEnTA 1.0 wt.%WS2-TO |
|---|---|---|---|---|
| DC [%] | 64 | 40 | 45 | 55 |
| Exo. Peak [°C] | 121 | 166 | 160 | 145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gershoni, G.; Dodiuk, H.; Tenne, R.; Kenig, S. Cationic Polymerized Epoxy and Radiation Cured Acrylate Blend Nanocomposites Based on WS2 Nanoparticles—Part A: Curing Processes and Kinetics. J. Compos. Sci. 2023, 7, 41. https://doi.org/10.3390/jcs7010041
Gershoni G, Dodiuk H, Tenne R, Kenig S. Cationic Polymerized Epoxy and Radiation Cured Acrylate Blend Nanocomposites Based on WS2 Nanoparticles—Part A: Curing Processes and Kinetics. Journal of Composites Science. 2023; 7(1):41. https://doi.org/10.3390/jcs7010041
Chicago/Turabian StyleGershoni, Gilad, Hanna Dodiuk, Reshef Tenne, and Samuel Kenig. 2023. "Cationic Polymerized Epoxy and Radiation Cured Acrylate Blend Nanocomposites Based on WS2 Nanoparticles—Part A: Curing Processes and Kinetics" Journal of Composites Science 7, no. 1: 41. https://doi.org/10.3390/jcs7010041
APA StyleGershoni, G., Dodiuk, H., Tenne, R., & Kenig, S. (2023). Cationic Polymerized Epoxy and Radiation Cured Acrylate Blend Nanocomposites Based on WS2 Nanoparticles—Part A: Curing Processes and Kinetics. Journal of Composites Science, 7(1), 41. https://doi.org/10.3390/jcs7010041









