N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
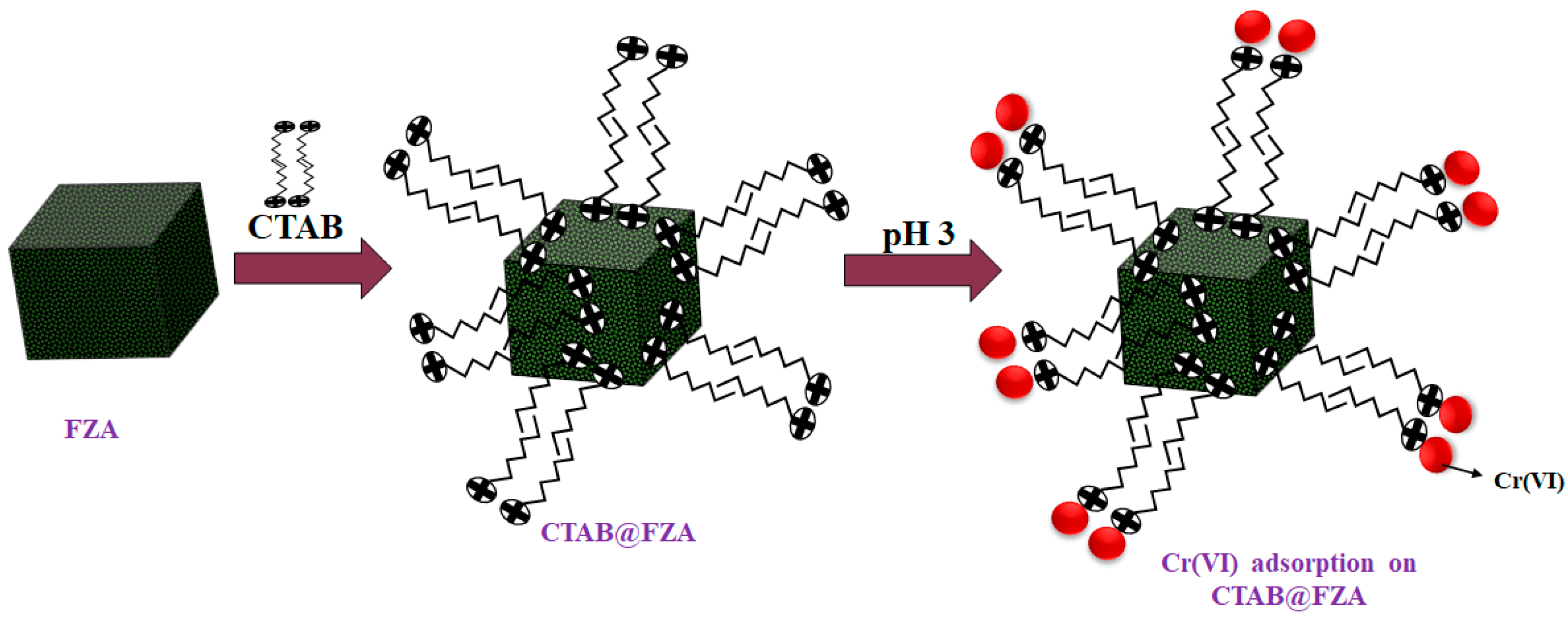
2.2. Synthesis of CTAB@FZA
2.3. Adsorption Experiments
2.4. Sorbent Characterization
3. Results and Discussion
3.1. Characterization
3.1.1. XRD
3.1.2. FTIR and SEM
3.1.3. BET Surface Area Analysis
3.2. Sorption Batch Experiments Results
3.2.1. Effect of pH
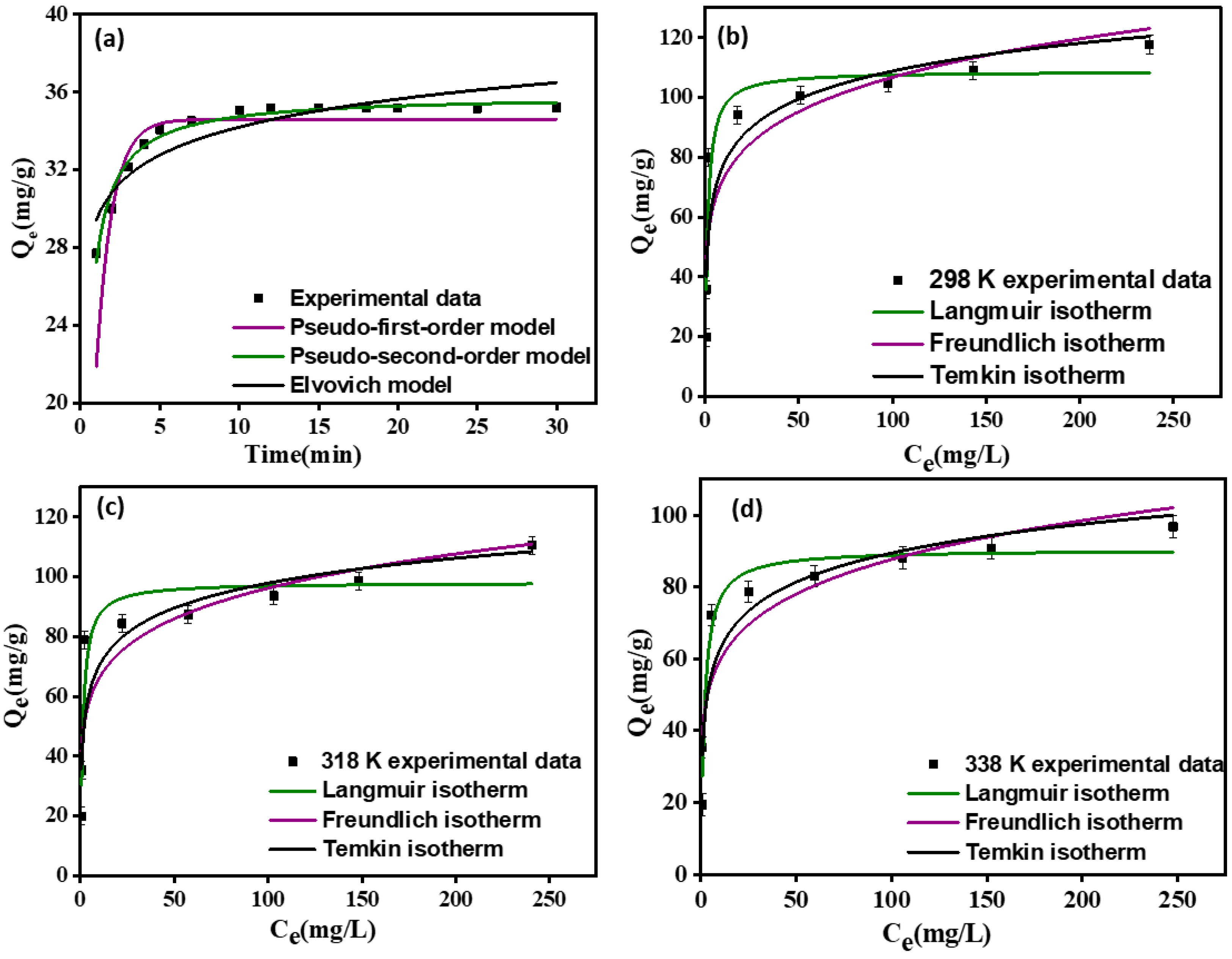
3.2.2. Effect of Contact Time and Adsorption
3.2.3. Adsorbate Concentration and Adsorption Isotherms
3.2.4. Temperature Effect and Adsorption Thermodynamics
3.2.5. Effect of Coexisting Ions
3.3. Application of CTAB@FZA for the Elimination of Chromium from Industrial Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Fu, J.; Lu, Z.; Ding, Z.; Tang, B.; Pang, J. Facile preparation of magnetic mesoporous MnFe2O4@SiO2-CTAB composites for Cr(VI) adsorption and reduction. Environ. Pollut. 2017, 220, 1376–1385. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Zhao, S.; Lei, Y.; Yuan, Y.; He, C.; Gao, C.; Deng, L. Hexavalent chromium removal from aqueous solution by adsorption on modified zeolites coated with Mg-layered double hydroxides. Environ. Sci. Pollut. Res. Int. 2019, 26, 32928–32941. [Google Scholar] [CrossRef]
- Seliem, M.K.; Mobarak, M. Cr(VI) uptake by a new adsorbent of CTAB–modified carbonized coal: Experimental and advanced statistical physics studies. J. Mol. Liq. 2019, 294, 111676. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, K.; Zhang, Y.; Peng, C.; Robledo-Cabrera, A.; Lopez-Valdivieso, A. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling. Environ. Res. 2021, 197, 111151. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Choi, Y.L.; Lingamdinne, L.P.; Koduru, J.R.; Yang, J.K.; Chang, Y.Y.; Karri, R.R. Portable SA/CMC entrapped bimetallic magnetic fly ash zeolite spheres for heavy metals contaminated industrial effluents treatment via batch and column studies. Sci. Rep. 2022, 12, 3430. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Romero-Santana, H.; Monárrez-Cordero, B.E. Removal of Cr(VI) from water by adsorption using low cost clay-perlite-iron membranes. J. Water Proc. Eng. 2020, 38, 101672. [Google Scholar] [CrossRef]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Puteh, M.H.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef]
- Luo, H.; Law, W.W.; Wu, Y.; Zhu, W.; Yang, E.-H. Hydrothermal synthesis of needle-like nanocrystalline zeolites from metakaolin and their applications for efficient removal of organic pollutants and heavy metals. Microporous Mesoporous Mater. 2018, 272, 8–15. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Nxumalo, E.N.; Musyoka, N.M.; Mhlanga, S.D. Microwave Irradiation-Assisted Synthesis of Zeolites from Coal Fly Ash: An Optimization Study for a Sustainable and Efficient Production Process. ACS Omega 2020, 5, 25000–25008. [Google Scholar] [CrossRef]
- Behin, J.; Bukhari, S.S.; Dehnavi, V.; Kazemian, H.; Rohani, S. Using Coal Fly Ash and Wastewater for Microwave Synthesis of LTA Zeolite. Chem. Eng. Tech. 2014, 37, 1532–1540. [Google Scholar] [CrossRef]
- Tauanov, Z.; Shah, D.; Inglezakis, V.; Jamwal, P.K. Hydrothermal synthesis of zeolite production from coal fly ash: A heuristic approach and its optimization for system identification of conversion. J. Clean. Prod. 2018, 182, 616–623. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D.N. Fly ash zeolites for water treatment applications. J. Envion. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Song, W.; Shi, T.; Yang, D.; Ye, J.; Zhou, Y.; Feng, Y. Pretreatment effects on the sorption of Cr(VI) onto surfactant-modified zeolite: Mechanism analysis. J. Environ. Manag. 2015, 162, 96–101. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A. Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Jiang, J.-Q.; Ma, X. Adsorption of bisphenol A onto cationic-modified zeolite. Desalin. Water Treat. 2016, 57, 26299–26306. [Google Scholar] [CrossRef]
- Jing, X.; Cao, Y.; Zhang, X.; Wang, D.; Wu, X.; Xu, H. Biosorption of Cr(VI) from simulated wastewater using a cationic surfactant modified spent mushroom. Desalination 2011, 269, 120–127. [Google Scholar] [CrossRef]
- Choi, Y.L.; Angaru, G.K.R.; Kim, D.S.; Ahn, H.Y.; Kim, D.H.; Choi, C.D.; Reddy, K.J.; Yang, J.K.; Chang, Y.Y. Preparation of Na-X and Na-A zeolites from coal fly ash in a thermoelectric power plant and comparison of the adsorption characteristics for Cu (II) with a commercial zeolite. Appl. Chem. Eng. 2019, 30, 749–756. [Google Scholar]
- Hosseini Hashemi, M.S.; Eslami, F.; Karimzadeh, R. Organic contaminants removal from industrial wastewater by CTAB treated synthetic zeolite Y. J. Environ. Manag. 2019, 233, 785–792. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Choi, Y.L.; Lingamdinne, L.P.; Choi, J.S.; Kim, D.S.; Koduru, J.R.; Yang, J.K.; Chang, Y.Y. Facile synthesis of economical feasible fly ash-based zeolite-supported nano zerovalent iron and nickel bimetallic composite for the potential removal of heavy metals from industrial effluents. Chemosphere 2021, 267, 128889. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Ramos, R.; Jacobo-Azuara, A.; Diaz-Flores, P.E.; Guerrero-Coronado, R.M.; Mendoza-Barron, J.; Berber-Mendoza, M.S. Adsorption of chromium(VI) from an aqueous solution on a surfactant-modified zeolite, Colloids and Surfaces A. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 35–41. [Google Scholar] [CrossRef]
- Majumder, R.; Sheikh, L.; Naskar, A.; Vineeta; Mukherjee, M.; Tripathy, S. Depletion of Cr(VI) from aqueous solution by heat dried biomass of a newly isolated fungus Arthrinium malaysianum: A mechanistic approach. Sci. Rep. 2017, 7, 11254. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Abhilash; Pandey, B.D. Mechanism elucidation and adsorbent characterization for removal of Cr(VI) by native fungal adsorbent. Sustain. Envion. Res. 2018, 28, 289–297. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Cheng, J.; Pan, Y.; Yang, G.; Liu, Y.; Wang, L.; Leng, Y.; Tuo, X. Synthesis and characterization of CTAB-modified bentonite composites for the removal of Cs+. J. Radioanal. Nucl. Chem. 2021, 329, 451–461. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, Q.; Chen, J.; Chen, Z.; Gu, Y. Preparation of magnetically recoverable bentonite-Fe3O4-MnO2 composite particles for Cd(II) removal from aqueous solutions. J. Colloid Interface Sci. 2018, 513, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, H.; Hou, S.; Wang, S. Preparation of Fe3O4@SiO2@MnO2 microspheres as an adsorbent for Th(IV) removal from aqueous solution. J. Radioanal. Nucl. Chem. 2021, 329, 253–263. [Google Scholar] [CrossRef]
- de Barros, H.R.; Piovan, L.; Sassaki, G.L.; de Araujo Sabry, D.; Mattoso, N.; Nunes, A.M.; Meneghetti, M.R.; Riegel-Vidotti, I.C. Surface interactions of gold nanorods and polysaccharides: From clusters to individual nanoparticles. Carbohydr. Polym. 2016, 152, 479–486. [Google Scholar] [CrossRef]
- Sathvika, T.; Kumar Saraswathi, A.R.; Rajesh, V.; Rajesh, N. Confluence of montmorillonite and Rhizobium towards the adsorption of chromium(vi) from aqueous medium. RSC Adv. 2019, 9, 28478–28489. [Google Scholar] [CrossRef]
- Dovi, E.; Kani, A.N.; Aryee, A.A.; Jie, M.; Li, J.; Li, Z.; Qu, L.; Han, R. Decontamination of bisphenol A and Congo red dye from solution by using CTAB functionalised walnut shell. Environ. Sci. Pollut. Res. Int. 2021, 28, 28732–28749. [Google Scholar] [CrossRef]
- Guan, Q.; Gao, K.; Ning, P.; Miao, R.; He, L. Efficient removal of low-concentration Cr(vi) from aqueous solution by 4A/HACC particles. New J. Chem. 2019, 43, 17220–17230. [Google Scholar] [CrossRef]
- Aloulou, H.; Ghorbel, A.; Aloulou, W.; Ben Amar, R.; Khemakhem, S. Removal of fluoride ions (F(-)) from aqueous solutions using modified Turkish zeolite with quaternary ammonium. Environ. Technol. 2021, 42, 1353–1365. [Google Scholar] [CrossRef]
- Hailu, S.L.; Nair, B.U.; Redi-Abshiro, M.; Diaz, I.; Tessema, M. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng. 2017, 5, 3319–3329. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Su, Y.; Du, C. A novel and green strategy for efficient removing Cr(VI) by modified kaolinite-rich coal gangue. Appl. Clay Sci. 2021, 211, 106208. [Google Scholar] [CrossRef]
- Liang, M.; Ding, Y.; Zhang, Q.; Wang, D.; Li, H.; Lu, L. Removal of aqueous Cr(VI) by magnetic biochar derived from bagasse. Sci. Rep. 2020, 10, 21473. [Google Scholar] [CrossRef]
- Belachew, N.; Hinsene, H. Preparation of cationic surfactant-modified kaolin for enhanced adsorption of hexavalent chromium from aqueous solution. Appl. Water Sci. 2019, 10, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, J.; Yang, Y.; Liu, X. Surface modified leaves with high efficiency for the removal of aqueous Cr(VI). Appl. Surf. Sci. 2019, 484, 189–196. [Google Scholar] [CrossRef]
- Cai, W.; Gu, M.; Jin, W.; Zhou, J. CTAB-functionalized C@SiO2 double-shelled hollow microspheres with enhanced and selective adsorption performance for Cr(VI). J. Alloy. Compd. 2019, 777, 1304–1312. [Google Scholar] [CrossRef]
- Cai, W.; Dionysiou, D.D.; Fu, F.; Tang, B. CTAB-intercalated molybdenum disulfide nanosheets for enhanced simultaneous removal of Cr(VI) and Ni(II) from aqueous solutions. J. Hazard. Mater. 2020, 396, 122728. [Google Scholar] [CrossRef]
- Forghani, M.; Azizi, A.; Livani, M.J.; Kafshgari, L.A. Adsorption of lead(II) and chromium(VI) from aqueous environment onto metal-organic framework MIL-100(Fe): Synthesis, kinetics, equilibrium and thermodynamics. J. Solid State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Lingamdinne, L.P.; Choi, Y.L.; Koduru, J.R.; Yang, J.K.; Chang, Y.Y. Encapsulated zerovalent iron/nickel-fly ash zeolite foam for treating industrial wastewater contaminated by heavy metals. Mater. Today Chem. 2021, 22, 100577. [Google Scholar] [CrossRef]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, B.T.; Tran, H.N.; Chao, H.-P. Efficient Removal of Cr(VI) from Water by Biochar and Activated Carbon Prepared through Hydrothermal Carbonization and Pyrolysis: Adsorption-Coupled Reduction Mechanism. Water 2019, 11, 1164. [Google Scholar] [CrossRef]
- Lv, G.; Li, Z.; Jiang, W.T.; Ackley, C.; Fenske, N.; Demarco, N. Removal of Cr(VI) from water using Fe(II)-modified natural zeolite. Chem. Eng. Res. Des. 2014, 92, 384–390. [Google Scholar] [CrossRef]
- Chanda, R.; Hosain, M.; Sumi, S.A.; Sultana, M.; Islam, S.; Biswas, B.K. Removal of Chromium (VI) and Lead (II) from Aqueous Solu-tion Using Domestic Rice Husk Ash-(RHA-) Based Zeolite Faujasite. Adsorpt. Sci. Technol. 2022, 2022, 4544611. [Google Scholar] [CrossRef]
- Nasanjargal, S.; Munkhpurev, B.A.; Kano, N.; Kim, H.J.; Ganchimeg, Y. The removal of chromium (VI) from aqueous solution by amine-functionalized zeolite: Kinetics, thermodynamics, and equilibrium Study. J. Environ. Prot. 2021, 12, 654–675. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, H.; Wang, Z.; Zhang, T.; Yang, D.; Qiu, F. Efficient removal of As (capital SHA, Cyrillic) via the synergistic effect of oxidation and absorption by FeOOH@MnO2@CAM nano-hybrid adsorption membrane. Chemosphere 2020, 258, 127329. [Google Scholar] [CrossRef]
- Alley, E.R. Water Quality Control Handbook; McGraw-Hill: New York, NY, USA, 2007; Volume 2. [Google Scholar]
- Duranoğlu, D.; Buyruklardan Kaya, İ.G.; Beker, U.; Şenkal, B.F. Synthesis and adsorption properties of polymeric and polymer-based hybrid adsorbent for hexavalent chromium removal. Chem. Eng. J. 2012, 181–182, 103–112. [Google Scholar] [CrossRef]






| Adsorbent | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| FZA | 28.46 | 0.04 | 5.34 |
| CTAB@FZA | 0.78 | 0.01 | 48.09 |
| Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | Elvovich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg/g) | K1 (min−1) | R2 | Qe (mg/g) | K2 (g/mg·min) | R2 | α (mg/g·min) | β (g/mg) | R2 | |
| 35.22 | 34.60 | 1.00 | 0.4010 | 35.64 | 0.08 | 0.9723 | 2.88 | 0.48 | 0.8135 |
| Temperature (K) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q max (mg/g) | KL (L/mg) | R2 | KF (mg/g)(L/mg)1/n | n (L/g) | R2 | KT (L/mg) | Bt | R2 | |
| 298 | 108.76 | 0.81 | 0.9071 | 50.15 | 6.09 | 0.7789 | 32.84 | 13.43 | 0.842 |
| 318 | 98.1 | 0.79 | 0.8805 | 45.53 | 6.15 | 0.7699 | 34.7 | 12.00 | 0.8224 |
| 338 | 90.45 | 0.54 | 0.9419 | 40.51 | 5.96 | 0.8141 | 21.92 | 11.62 | 0.8852 |
| Adsorbent | Q Max (mg/g) | Conditions (pH; Temperature) | Ref |
|---|---|---|---|
| Zeolite/MgAL-LDHs | 15.69 | 6–7; 298 K | [2] |
| MIL−100(Fe) | 30.45 | 2; 298 K | [40] |
| Alginate supported Fe/Ni-Zeolite | 7.36 | 3; 298 K | [41] |
| Ball milled activated carbon | 28.90 | 7; 295 K | [4] |
| Tectona grandis sawdust biochar | 83.50 | 3; 303 K | [42] |
| Fe(II)-Natural Zeolite | 0.7 | 4; 298 K | [43] |
| Surface modified zeolite-Y | 1.95 | 3–4; 298 K | [16] |
| Rice Husk Ash-Based Zeolite FAU | 3.56 | 5; 298 K | [44] |
| Amine-Functionalized Zeolite | 13.5 | 3; 298 K | [45] |
| CTAB@FZA | 108.76 | 3; 298 K | Present study |
| Temperature (K) | Qmax (mg/g) | ln Kc | ∆G° (KJ/mol) | ∆H° (KJ/mol) | ∆S° (J/K/mol) | R2 |
|---|---|---|---|---|---|---|
| 298 | 108.76 | 4.48 | −11.52 | −12.06 | −2.69 | 0.8825 |
| 318 | 98.1 | 4.35 | −11.11 | |||
| 338 | 90.45 | 3.90 | −10.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angaru, G.K.R.; Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.-Y. N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent. J. Compos. Sci. 2022, 6, 256. https://doi.org/10.3390/jcs6090256
Angaru GKR, Lingamdinne LP, Koduru JR, Chang Y-Y. N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent. Journal of Composites Science. 2022; 6(9):256. https://doi.org/10.3390/jcs6090256
Chicago/Turabian StyleAngaru, Ganesh Kumar Reddy, Lakshmi Prasanna Lingamdinne, Janardhan Reddy Koduru, and Yoon-Young Chang. 2022. "N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent" Journal of Composites Science 6, no. 9: 256. https://doi.org/10.3390/jcs6090256
APA StyleAngaru, G. K. R., Lingamdinne, L. P., Koduru, J. R., & Chang, Y.-Y. (2022). N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent. Journal of Composites Science, 6(9), 256. https://doi.org/10.3390/jcs6090256








