Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin
Abstract
:1. Introduction
2. Materials Development Using High-Energy Ball-Milled (HEBM) Precursors
2.1. Thermally Induced Chemical Reactions in HEBM Powders and Synthesis of Powder Products
2.1.1. Self-Propagating High-Temperature Synthesis (SHS) from HEBM Powder Precursors
2.1.2. Synthesis in the Thermal Explosion (TE) Mode from HEBM Powder Precursors
2.1.3. Synthesis of Materials by Annealing of HEBM Powder Precursors
2.2. Consolidation of HEBM Powders
2.3. Formation of Coatings from HEBM Powders
2.4. The Role of HEBM in the Microstructure and Phase Formation of the Powder and Bulk Products
3. Emerging Applications of HEBM Powders and Future Research Directions
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Rus. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- Baláž, P. High-energy milling. In Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 103–132. [Google Scholar]
- Pentimalli, M.; Bellusci, M.; Padella, F. High-energy ball milling as a general tool for nanomaterials synthesis and processing. In Handbook of Mechanical Nanostructuring, 1st ed.; Aliofkhazraei, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 663–679. [Google Scholar]
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical milling: A superior nanotechnological tool for fabrication of nanocrystalline and nanocomposite materials. Nanomaterials 2021, 11, 2484. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Smolyakov, V.K. Modeling the synthesis of mechanocomposites in binary systems. Comb. Explos. Shock Waves 2011, 47, 553–562. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Ryabkova, A.I. Mathematical model of the formation of mechanocomposite particles during the mechanical treatment of a powder mixture. J. Phys. Conf. Ser. 2019, 1214, 012013. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013, 58, 383–502. [Google Scholar] [CrossRef]
- Hadef, F. Synthesis and disordering of B2 TM-Al (TM = Fe, Ni, Co) intermetallic alloys by high energy ball milling: A review. Powder Technol. 2017, 311, 556–578. [Google Scholar] [CrossRef]
- Rogachev, A.S. Mechanical activation of heterogeneous exothermic reactions in powder mixtures. Russ. Chem. Rev. 2019, 88, 875–900. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Avvakumov, E.G.; Potkin, A.R.; Samarin, O.I. A planetary mill. USSR Invent. Bull. 1982, 43, 975068. [Google Scholar]
- Kwon, Y.S.; Gerasimov, K.B.; Yoon, S.K. Ball temperatures during mechanical alloying in planetary mills. J. Alloys Compd. 2002, 346, 276–281. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion for Material Synthesis; CRC Press: London, UK, 2014; 424p. [Google Scholar]
- Mukasyan, A.S.; Rogachev, A.S.; ThippaReddy Aruna, S. Combustion synthesis in nanostructured reactive systems. Adv. Powder Technol. 2015, 26, 954–976. [Google Scholar] [CrossRef] [Green Version]
- Vidyuk, T.M.; Korchagin, M.A.; Dudina, D.V.; Bokhonov, B.B. Synthesis of ceramic and composite materials using a combination of self-propagating high-temperature synthesis and spark plasma sintering (Review). Comb. Explos. Shock Waves 2021, 57, 385–397. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigor'eva, T.F.; Bokhonov, B.B.; Sharafutdinov, M.R.; Barinova, A.P.; Lyakhov, N. Solid-state combustion in mechanically activated SHS systems. I. Effect of activation time on process parameters and combustion product composition. Comb. Explos. Shock Waves 2003, 39, 43–50. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Grigor'eva, T.F.; Bokhonov, B.B.; Sharafutdinov, M.R.; Barinova, A.P.; Lyakhov, N. Solid-state combustion in mechanically activated SHS systems. II. Effect of mechanical activation conditions on process parameters and combustion product composition. Comb. Explos. Shock Waves 2003, 39, 51–58. [Google Scholar] [CrossRef]
- Ditenberg, I.A.; Osipov, D.A.; Korchagin, M.A.; Smirnov, I.V.; Grinyaev, K.V.; Gavrilov, A.I. Influence of ball milling duration on the morphology, features of the structural-phase state and microhardness of 3Ni-Al powder mixture. Adv. Powder Technol. 2021, 32, 3447–3455. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Bokhonov, B.B. Self-propagating high-temperature synthesis of quasicrystals. Comb. Explos. Shock Waves 2004, 40, 438–444. [Google Scholar] [CrossRef]
- Bokhonov, B.B. Mechanical alloying and self-propagating high-temperature synthesis of stable icosahedral quasicrystals. J. Alloys Compd. 2008, 461, 150–153. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Gavrilov, A.I.; Grishina, I.V.; Dudina, D.V.; Ukhina, A.V.; Bokhonov, B.B.; Lyakhov, N.Z. Self-propagating high-temperature synthesis of Ti3SiC2 and Ti3AlC2 single-phase MAX phases in mechanically activated mixtures of initial reactants. Comb. Explos. Shock Waves 2022, 58, 46–53. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Gavrilov, A.I.; Dudina, D.V.; Bokhonov, B.B.; Bulina, N.V. Hedvall effect in self-propagating high-temperature synthesis in mechanically activated compositions. Comb. Explos. Shock Waves 2021, 57, 640–650. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Bokhonov, B.B. Combustion of mechanically activated 3Ti+2BN mixtures. Comb. Explos. Shock Waves 2010, 46, 170–177. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Gavrilov, A.I.; Zarko, V.E.; Kiskin, A.B.; Iordan, Y.V.; Trushlyakov, V.I. Self-propagating high-temperature synthesis in mechanically activated mixtures of boron carbide and titanium. Comb. Explos. Shock Waves 2017, 53, 669–677. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Dudina, D.V.; Korchagin, M.A.; Lomovsky, O.I. Microstructure changes in TiB2-Cu nanocomposite under sintering. J. Mater. Sci. 2004, 39, 5325–5331. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.-S.; Lomovsky, O.I.; Korchagin, M.; Mali, V.; Dudina, D. A synthetic route for metal-ceramic interpenetrating phase composites. Mater. Lett. 2006, 60, 3723–3726. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Dudina, D.V. Application of self-propagating high-temperature synthesis and mechanical activation for obtaining nanocomposites. Comb. Explos. Shock Waves 2007, 43, 176–187. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Dudina, D.V.; Lomovsky, O.I.; Korchagin, M.A.; Mali, V.I. Nanocomposites TiB2-Cu: Consolidation and erosion behavior. J. Mater. Sci. 2005, 40, 3491–3495. [Google Scholar] [CrossRef]
- Kim, J.S.; Dudina, D.V.; Kim, J.C.; Kwon, Y.S.; Park, J.J.; Rhee, C.K. Properties of Cu-based nanocomposites produced by mechanically activated self-propagating high-temperature synthesis and spark-plasma sintering. J. Nanosci. Nanotechnol. 2010, 10, 252–257. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Gabdrashova, S.E.; Dudina, D.V.; Bokhonov, B.B.; Bulina, N.V.; Kuznetsov, V.L.; Ishchenko, A.V. Combustion characteristics and structure of carbon nanotube/titanium composites. J. Thermal Anal. Calorim. 2019, 137, 1903–1910. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Dudina, D.V.; Bokhonov, B.B.; Bulina, N.V.; Ukhina, A.V.; Batraev, I.S. Synthesis of nickel boride by thermal explosion in ball-milled powder mixtures. J. Mater. Sci. 2018, 53, 13592–13599. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Bulina, N.V. Superadiabatic regime of the thermal explosion in a mechanically activated mixture of tungsten with carbon black. Comb. Explos. Shock Waves 2016, 52, 225–233. [Google Scholar] [CrossRef]
- Filimonov, V.Y.; Korchagin, M.A.; Dietenberg, I.A.; Tyumentsev, A.N.; Lyakhov, N.Z. High temperature synthesis of single-phase Ti3Al intermetallic compound in mechanically activated powder mixture. Powder Technol. 2013, 235, 606–613. [Google Scholar] [CrossRef]
- Korchagin, M.A. Thermal explosion in mechanically activated low-calorific-value compositions. Comb. Explos. Shock Waves 2015, 51, 578–586. [Google Scholar] [CrossRef]
- Korchagin, M.A.; Avvakumov, E.G.; Lepezin, G.G.; Vinokurova, O.B. Thermal explosion and self-propagating high-temperature synthesis in mechanically activated SiO2-Al mixtures. Comb. Explos. Shock Waves 2014, 50, 641–646. [Google Scholar] [CrossRef]
- Filimonov, V.Y.; Korchagin, M.A.; Smirnov, E.V.; Lyakhov, N.Z. Macrokinetics of solid-phase synthesis of an activated 3Ni + Al mixture in the thermal explosion mode. Comb. Explos. Shock Waves 2010, 46, 449–456. [Google Scholar] [CrossRef]
- Dudina, D.V.; Vidyuk, T.M.; Gavrilov, A.I.; Ukhina, A.V.; Bokhonov, B.B.; Legan, M.A.; Matvienko, A.A.; Korchagin, M.A. Separating the reaction and spark plasma sintering effects during the formation of TiC–Cu composites from mechanically milled Ti–C–3Cu mixtures. Ceram. Int. 2021, 47, 12494–12504. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Ukhina, A.V.; Bulanova, U.E.; Legan, M.A.; Novoselov, A.N.; Esikov, M.A.; Anisimov, A.G. Manufacturing of TiC-Cu composites by mechanical milling and spark plasma sintering using different carbon sources. Surf. Interf. 2021, 27, 101445. [Google Scholar] [CrossRef]
- Bokhonov, B.; Korchagin, M. The formation of graphite encapsulated metal nanoparticles during mechanical activation and annealing of soot with iron and nickel. J. Alloys Compd. 2002, 333, 308–320. [Google Scholar] [CrossRef]
- Bokhonov, B.; Borisova, Y.; Korchagin, M. Formation of encapsulated molybdenum carbide particles by annealing mechanically activated mixtures of amorphous carbon with molybdenum. Carbon 2004, 42, 2067–2071. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V. Synthesis of ZrC and HfC nanoparticles encapsulated in graphitic shells from mechanically milled Zr-C and Hf-C powder mixtures. Ceram. Int. 2017, 43, 14529–14532. [Google Scholar] [CrossRef]
- Bokhonov, B.; Korchagin, M.; Borisova, Y. Formation of nanosized particles encapsulated in boron nitride during low-temperature annealing of mechanochemically treated Fe–BN mixtures. J. Alloys Compd. 2004, 372, 141–147. [Google Scholar] [CrossRef]
- Dudina, D.V.; Mukherjee, A.K. Reactive Spark Plasma Sintering: Successes and challenges of nanomaterial synthesis. J. Nanomater. 2013, 2013, 625218. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cao, G. Mechanochemically activated powders as precursors for spark plasma sintering (SPS) processes. In High-Energy Ball Milling: Mechanochemical Processing of Nanopowders; Sopicka-Lizer, M., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 275–303. [Google Scholar]
- Olevsky, E.A.; Dudina, D.V. Field-Assisted Sintering: Science and Applications; Springer International Publishing: Cham, Switzerland, 2018; 425p. [Google Scholar]
- Mukasyan, A.S.; Rogachev, A.S.; Moskovskikh, D.O.; Yermekova, Z.S. Reactive spark plasma sintering of exothermic systems: A critical review. Ceram. Int. 2022, 48, 2988–2998. [Google Scholar] [CrossRef]
- Dudina, D.V.; Vidyuk, T.M.; Korchagin, M.A. Synthesis of ceramic reinforcements in metallic matrices during spark plasma sintering: Consideration of reactant/matrix mutual chemistry. Ceramics 2021, 4, 592–599. [Google Scholar] [CrossRef]
- Cabouro, G.; Chevalier, S.; Gaffet, E.; Grin, Y.; Bernard, F. Reactive sintering of molybdenum disilicide by spark plasma sintering from mechanically activated powder mixtures: Processing parameters and properties. J. Alloys Compd. 2008, 465, 344–355. [Google Scholar] [CrossRef]
- Paris, S.; Gaffet, E.; Bernard, F.; Munir, Z.A. Spark plasma synthesis from mechanically activated powders: A versatile route for producing dense nanostructured iron aluminides. Scr. Mater. 2004, 50, 691–696. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Skripkina, T.S.; Ukhina, A.V.; Anisimov, A.G.; Bokhonov, B.B. Melting at the inter-particle contacts during Spark Plasma Sintering: Direct microstructural evidence and relation to particle morphology. Vacuum 2020, 181, 109566. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V. Recrystallisation-accompanied phase separation in Ag–Fe and Ag–Ni nanocomposites: A route to structure tailoring of nanoporous silver. RSC Adv. 2013, 3, 12655. [Google Scholar] [CrossRef]
- Petrov, S.A.; Bokhonov, B.B.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Ukhina, A.V.; Bakina, O.V.; Lerner, M.I. Fe-Ag pseudo-alloys obtained by wire electric explosion, ball milling and spark plasma sintering. Mater. Lett. 2022, 323, 132536. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Dudina, D.V.; Ukhina, A.V.; Korchagin, M.A.; Bulina, N.V.; Mali, V.I.; Anisimov, A.G. Formation of self-supporting porous graphite structures by Spark Plasma Sintering of nickel-amorphous carbon mixtures. J. Phys. Chem. Solids 2015, 76, 192–202. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Korchagin, M.A.; Ukhina, A.V.; Dudina, D.V. Structural and morphological transformations in cobalt-carbon mixtures during ball milling, annealing and Spark Plasma Sintering. Vacuum 2018, 157, 210–215. [Google Scholar] [CrossRef]
- Dudina, D.V.; Ukhina, A.V.; Bokhonov, B.B.; Korchagin, M.A.; Bulina, N.V.; Kato, H. The influence of the formation of Fe3C on graphitization in a carbon-rich iron-amorphous carbon mixture processed by Spark Plasma Sintering and annealing. Ceram. Int. 2017, 43, 11902–11906. [Google Scholar] [CrossRef]
- Lomovsky, O.I.; Dudina, D.V.; Ulianitsky, V.Y.; Zlobin, S.; Kosarev, V.; Klinkov, S.; Korchagin, M.; Kwon, D.H.; Kim, J.S.; Kwon, Y.S. Cold and detonation spraying of TiB2-Cu nanocomposites. Mater. Sci. Forum 2007, 534, 1373–1376. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Lomovsky, O.I.; Dudina, D.; Kosarev, V.; Klinkov, S.; Kwon, D.; Smurov, I. Cold spraying of in situ produced TiB2-Cu nanocomposite powders. Comp. Sci. Technol. 2007, 67, 2292–2296. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Bokhonov, B.B.; Ukhina, A.V.; Esikov, M.A.; Shikalov, S.V.; Kosarev, V.F. Spark plasma sintering treatment of cold sprayed materials for synthesis and structural modification: A case study using TiC-Cu composites. Mater. Lett. X 2022, 14, 100140. [Google Scholar] [CrossRef]
- Dudina, D.V.; Batraev, I.S.; Ulianitsky, V.Y.; Korchagin, M.A. Possibilities of the computer-controlled detonation spraying method: A chemistry viewpoint. Ceram. Int. 2014, 40, 3253–3260. [Google Scholar] [CrossRef]
- Dudina, D.V.; Korchagin, M.A.; Zlobin, S.B.; Ulianitsky, V.Y.; Lomovsky, O.I.; Bulina, N.V.; Bataev, I.A.; Bataev, V.A. Compositional variations in the coatings formed by detonation spraying of Ti3Al at different O2/C2H2 ratios. Intermetallics 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Dudina, D.V.; Pribytkov, G.A.; Krinitcyn, M.G.; Korchagin, M.A.; Bulina, N.V.; Bokhonov, B.B.; Batraev, I.S.; Rybin, D.K.; Ulianitsky, V.Y. Detonation spraying behavior of TiCx-Ti powders and the role of reactive processes in the coating formation. Ceram. Int. 2016, 42, 690–696. [Google Scholar] [CrossRef]
- Dudina, D.V.; Batraev, I.S.; Ulianitsky, V.Y.; Bulina, N.V.; Korchagin, M.A.; Lomovsky, O. Detonation spraying of Ti–Al intermetallics: Phase and microstructure development of the coatings. Mater. Manuf. Proc. 2015, 30, 724–729. [Google Scholar] [CrossRef]
- Dudina, D.V.; Batraev, I.S.; Ulianitsky, V.Y.; Bulina, N.V.; Korchagin, M.A.; Bataev, I.A.; Jorge, A.M. Formation routes of nanocomposite coatings in detonation spraying of Ti3SiC2-Cu powders. J. Thermal Spray Technol. 2014, 23, 1116–1123. [Google Scholar] [CrossRef]
- Dudina, D.V.; Batraev, I.S.; Ulianitsky, V.Y.; Korchagin, M.A.; Golubkova, G.V.; Abramov, S.Y.; Lomovsky, O. Control of interfacial interaction during detonation spraying of Ti3SiC2–Cu composites. Inorg. Mater. 2014, 50, 35–39. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Dudina, D.V.; Shtertser, A.A.; Smurov, I. Computer-controlled detonation spraying: Flexible control of the coating chemistry and microstructure. Metals 2019, 9, 1244. [Google Scholar] [CrossRef] [Green Version]
- Ulianitsky, V.Y.; Rybin, D.K.; Ukhina, A.V.; Bokhonov, B.B.; Dudina, D.V.; Samodurova, M.N.; Trofimov, E.A. Structure and composition of Fe-Co-Ni and Fe-Co-Ni-Cu coatings obtained by detonation spraying of powder mixtures. Mater. Lett. 2021, 290, 129498. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Korchagin, M.A.; Gavrilov, A.I.; Batraev, I.S.; Rybin, D.K.; Ukhina, A.V.; Dudina, D.V.; Samodurova, M.N.; Trofimov, E.A. FeCoNiCu alloys obtained by detonation spraying and spark plasma sintering of high-energy ball-milled powders. J. Therm. Spray Technol. 2022, 31, 1067–1075. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Rybin, D.K.; Sova, A.; Moghaddam, A.O.; Samodurova, M.; Doubenskaia, M.; Trofimov, E. Formation of metal composites by detonation spray of powder mixtures. Int. J. Adv. Manuf. Technol. 2021, 117, 81–95. [Google Scholar] [CrossRef]
- Kuchumova, I.D.; Cherkasova, N.Y.; Batraev, I.S.; Shikalov, V.S.; Ukhina, A.V.; Koga, G.Y.; Jorge, A.M. Wear-resistant Fe-based metallic glass-Al2O3 composite coatings produced by detonation spraying. J. Therm. Spray Technol. 2022, 31, 1355–1365. [Google Scholar] [CrossRef]
- Shikalov, V.S.; Vidyuk, T.M.; Filippov, A.A.; Kuchumova, I.D. Microstructure, mechanical and tribological properties of cold sprayed Cu–W coatings. Int. J. Refract. Metals Hard Mater. 2022, 106, 105866. [Google Scholar] [CrossRef]
- Dudina, D.V.; Grigoreva, T.F.; Kvashnin, V.I.; Devyatkina, E.T.; Vosmerikov, S.V.; Ukhina, A.V.; Novoselov, A.N.; Legan, M.A.; Esikov, M.A.; Lukyanov, Y.L.; et al. Microstructure and properties of Cu-10 wt% Al bronze obtained by high-energy mechanical milling and spark plasma sintering. Mater. Lett. 2022, 312, 131671. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Ukhina, A.V.; Dudina, D.V.; Anisimov, A.G.; Mali, V.I.; Batraev, I.S. Carbon uptake during Spark Plasma Sintering: Investigation through the analysis of the carbide “footprint” in a Ni–W alloy. RSC Adv. 2015, 5, 80228–80237. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Ukhina, A.V.; Anisimov, A.G.; Mali, V.I.; Esikov, M.A.; Batraev, I.S.; Kuznechik, O.O.; Pilinevich, L.P. Reactivity of materials towards carbon of graphite foil during Spark Plasma Sintering: A case study using Ni–W powders. Mater. Lett. 2016, 168, 62–67. [Google Scholar] [CrossRef]
- Shevtsova, L.I.; Korchagin, M.A.; Esikov, M.A.; Lozhkina, E.; Lozhkin, V.; Samoylenko, V.; Nemolochnov, D.; Malicov, V. Ni3Al+B material obtained by mechanical activation followed by spark plasma sintering. Mater. Today Proc. 2019, 12, 120–123. [Google Scholar] [CrossRef]
- Dudina, D.V.; Vidyuk, T.M.; Korchagin, M.A.; Gavrilov, A.I.; Bulina, N.V.; Esikov, M.A.; Datekyu, M.; Kato, H. Interaction of a Ti–Cu alloy with carbon: Synthesis of composites and model experiments. Materials 2019, 12, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudina, D.V.; Korchagin, M.A.; Gavrilov, A.I.; Bulina, N.V.; Batraev, I.S.; Esikov, M.A.; Batraev, I.S.; Esikov, M.A.; Georgarakis, K.; Kato, H. Formation of TiC-Cu nanocomposites by a reaction between Ti25Cu75 melt-spun alloy and carbon. Mater. Lett. 2019, 235, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Dudina, D.V.; Mali, V.I.; Anisimov, A.G.; Bulina, N.V.; Korchagin, M.A.; Lomovsky, O.I.; Bataev, I.A.; Bataev, V.A. Ti3SiC2-Cu composites by mechanical milling and Spark Plasma Sintering: Possible microstructure formation scenarios. Metals Mater. Int. 2013, 19, 1235–1241. [Google Scholar] [CrossRef]
- Dudina, D.V.; Mali, V.I.; Anisimov, A.G.; Lomovsky, O.I. Shock compression of Ti–B–Cu powder mixtures: Microstructural aspects. Mater. Sci. Eng. A 2009, 503, 41–44. [Google Scholar] [CrossRef]
- Khimich, M.A.; Prosolov, K.A.; Mishurova, T.; Evsevleev, S.; Monforte, X.; Teuschl, A.; Slezak, P.; Ibragimov, E.; Saprykin, A.; Kovalevskaya, Z.; et al. Advances in laser additive manufacturing of Ti-Nb alloys: From nanostructured powders to bulk objects. Nanomaterials 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Nepapushev, A.A.; Moskovskikh, D.O.; Vorotilo, K.V.; Rogachev, A.S. TiAl-based materials by in situ selective laser melting of Ti/Al reactive composites. Metals 2020, 10, 1505. [Google Scholar] [CrossRef]
- Shekhawat, D.; Vauth, M.; Pezoldt, J. Size dependent properties of reactive materials. Inorganics 2022, 10, 56. [Google Scholar] [CrossRef]
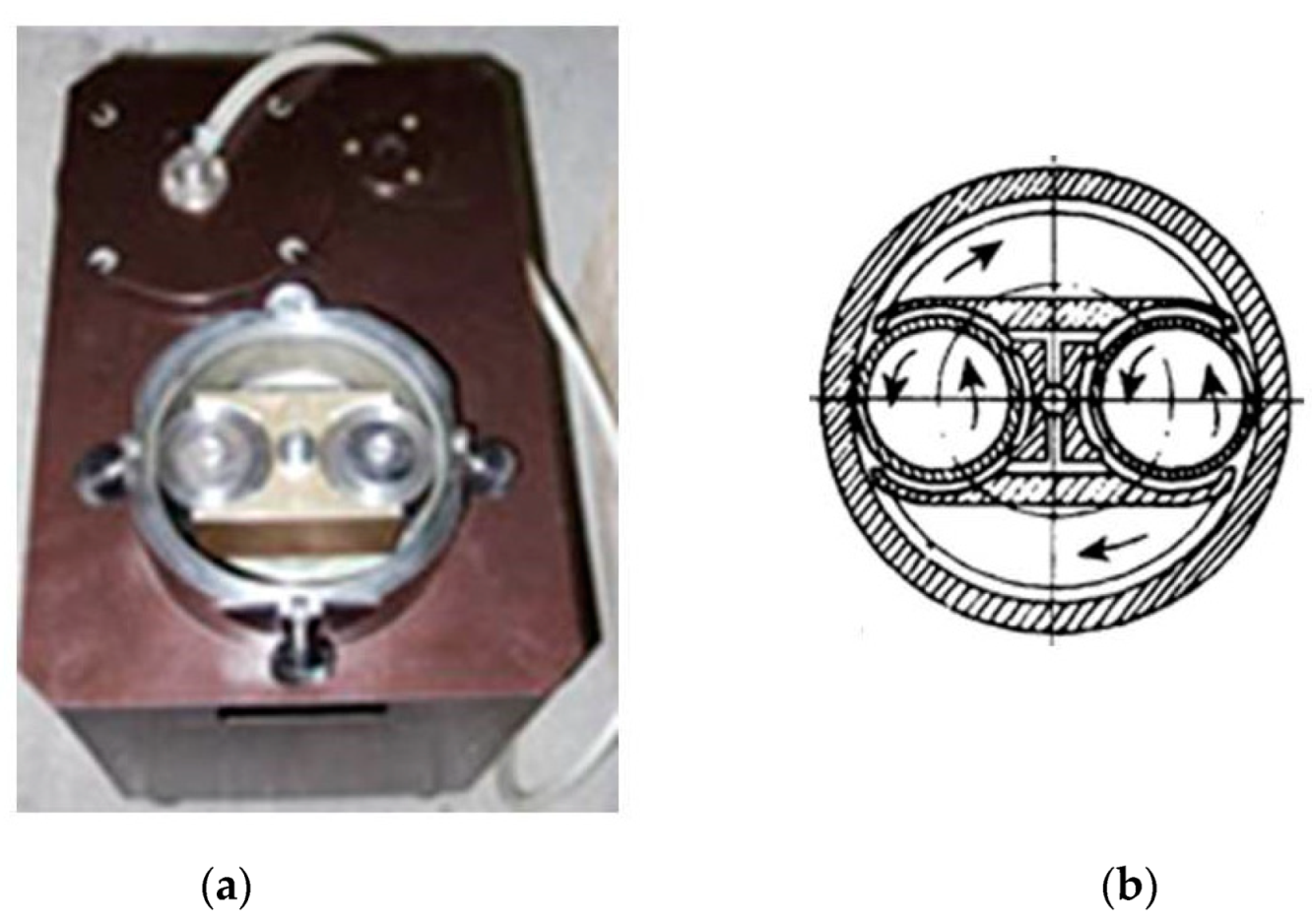
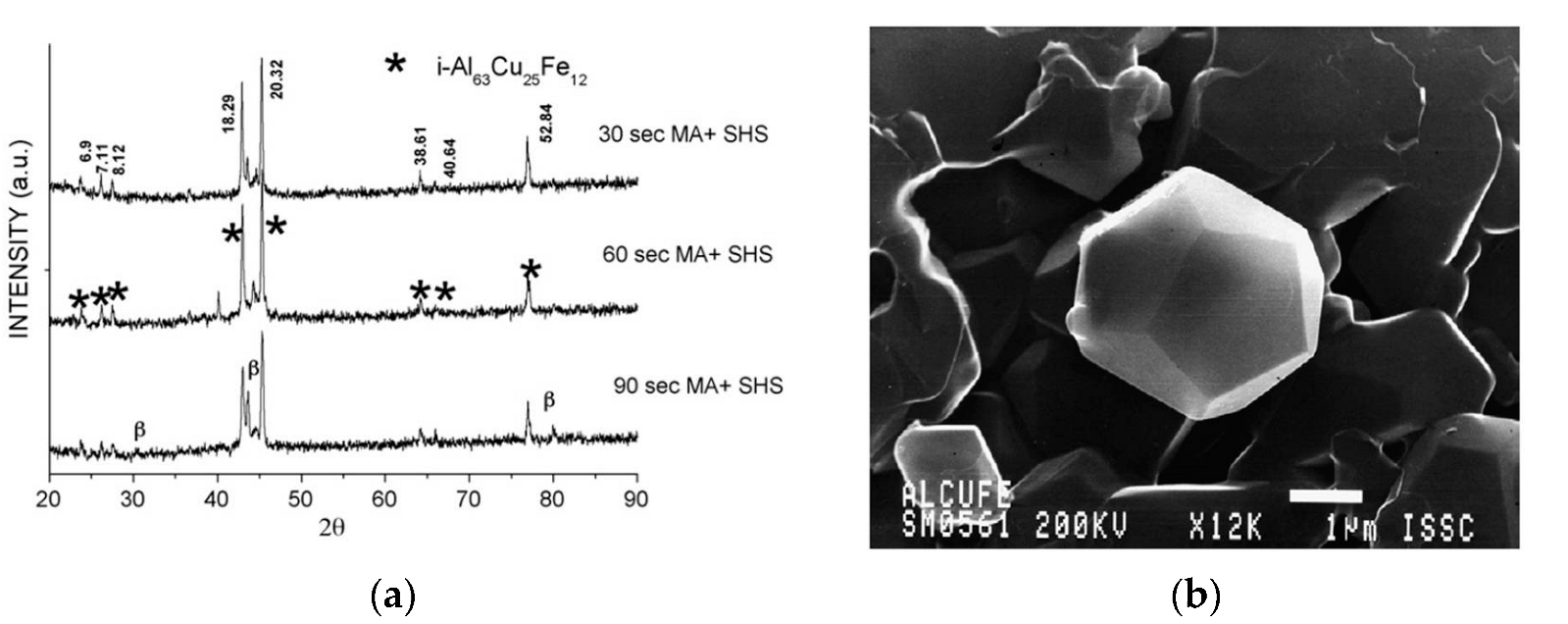
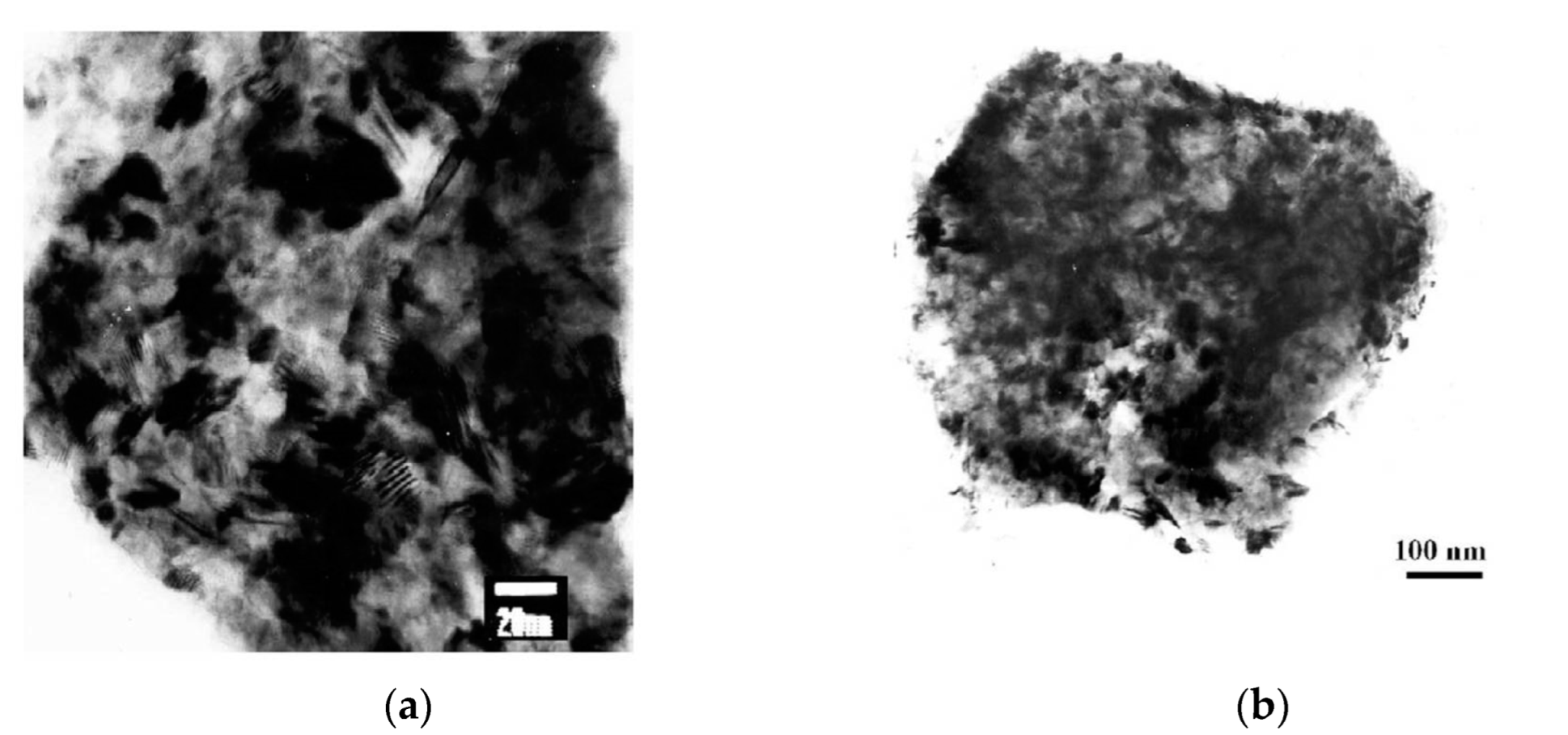

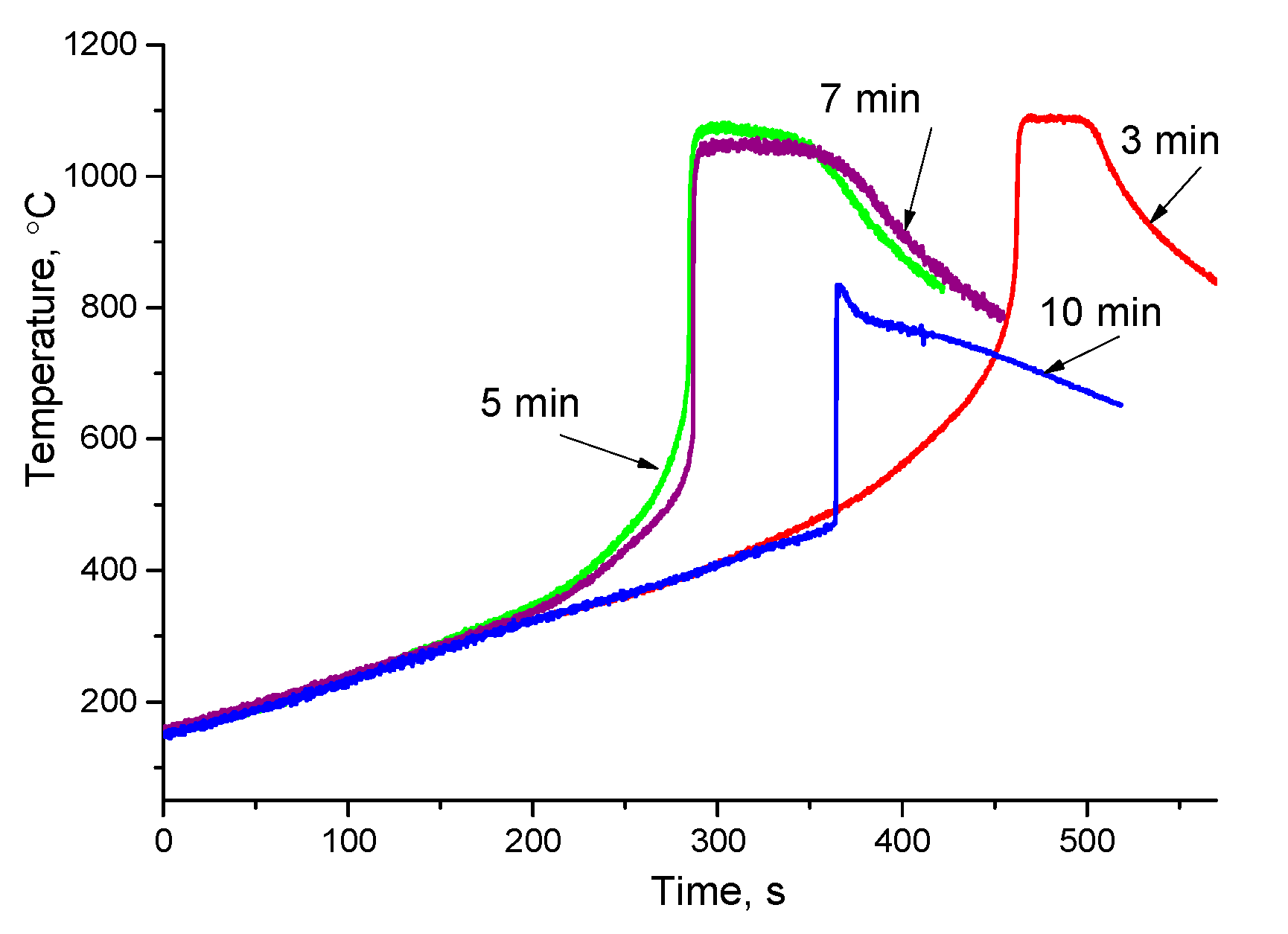

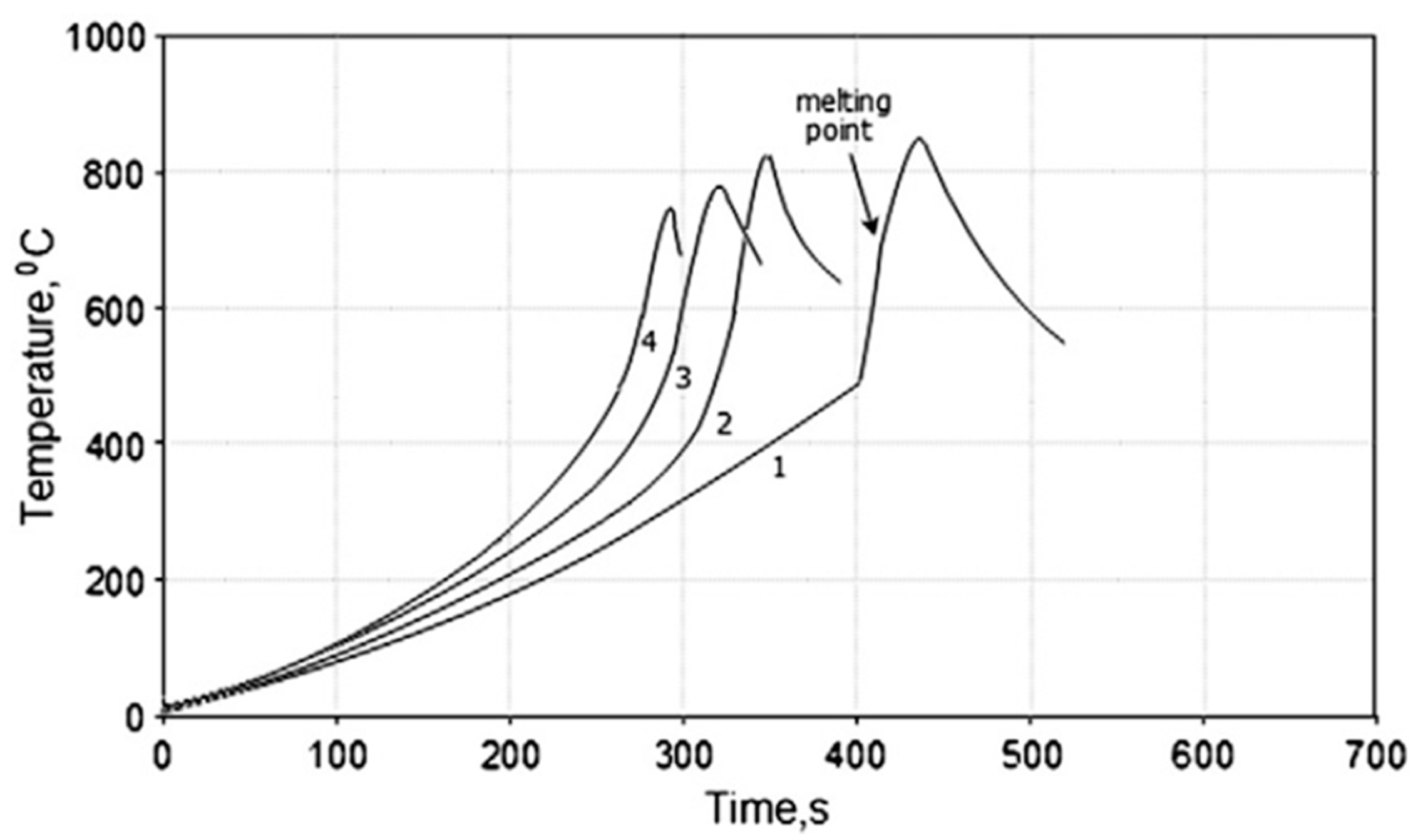
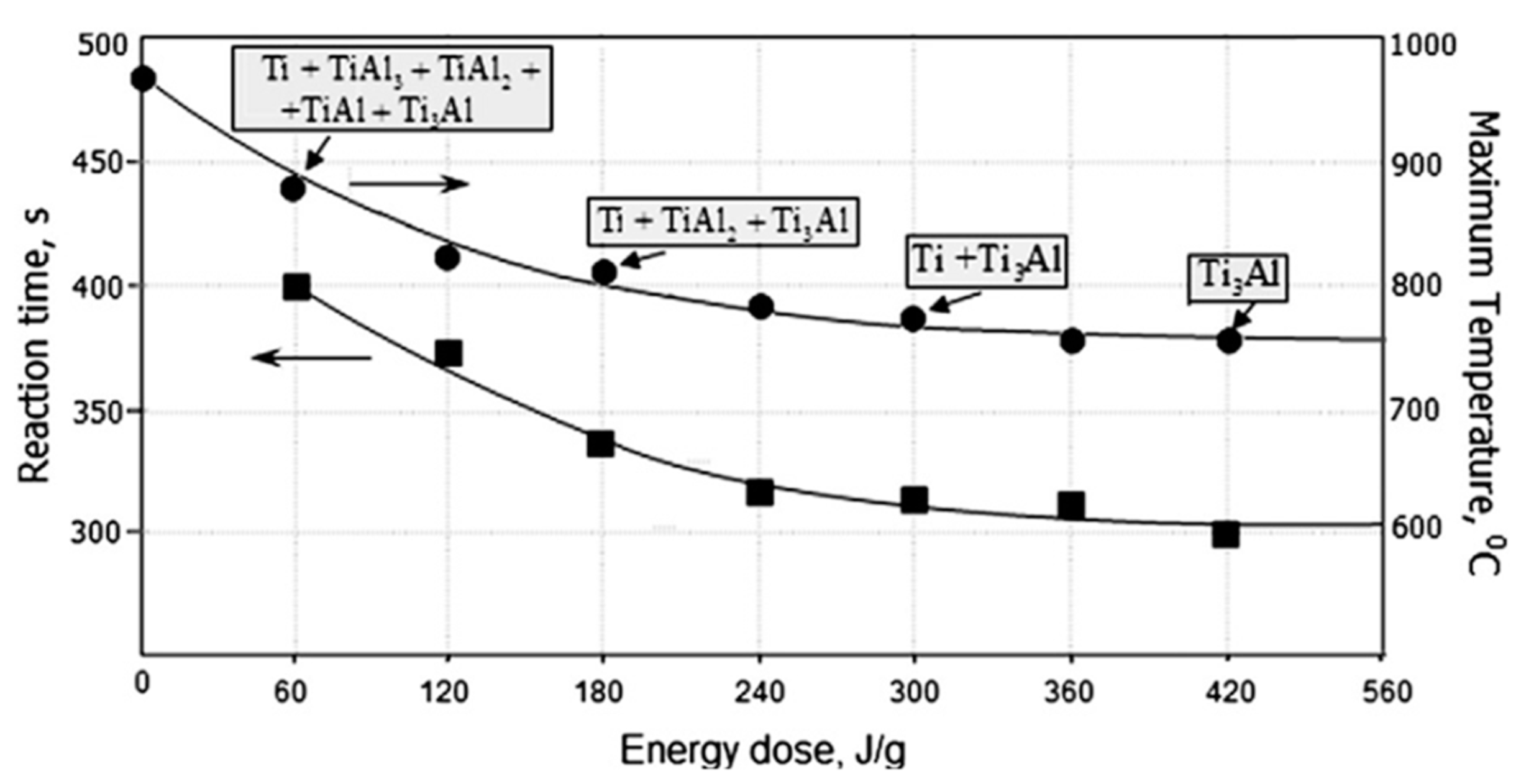


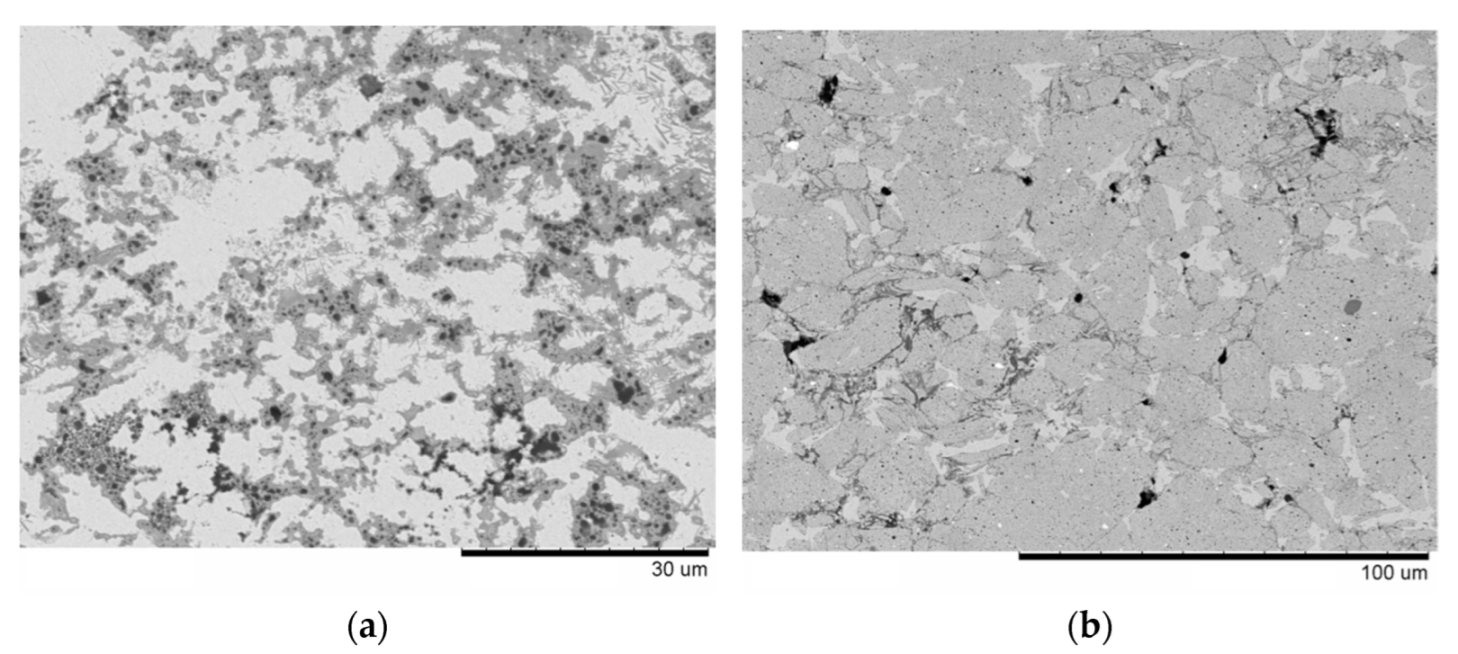

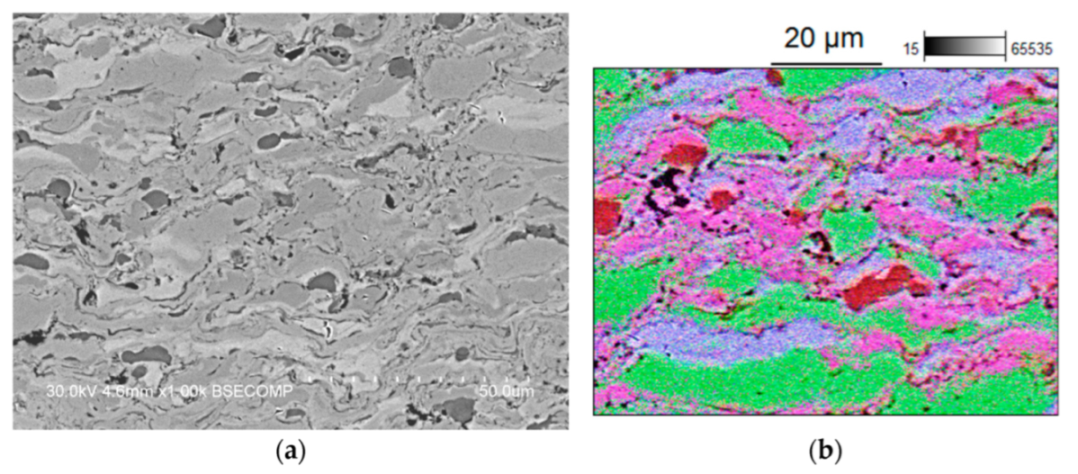
| Powder Mixture Subjected to HEBM | Processing Method of HEBM Powders | Phase Composition of the Product | Product State/Features | Role of HEBM | Reference |
|---|---|---|---|---|---|
| Powder products | |||||
| Ni + Al | SHS | Ni3Al | powder | implementation of solid state combustion, formation of a single-phase nanocrystalline product | [17] |
| Ti + Al | TE | Ti3Al | powder | formation of a single-phase nanocrystalline product | [34] |
| Ni + B | TE | Ni3B | powder | formation of a single-phase product | [32] |
| Ti + C + Si | SHS | Ti3SiC2 | powder | formation of a single-phase product | [22] |
| Ti + Al + C | SHS | Ti3AlC2 | powder | formation of a single-phase product | [22] |
| Fe−C | annealing | Fe(Fe3C)@C | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a graphitic shell | [40] |
| Ni−C | annealing | Ni@C | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a graphitic shell | [40] |
| Mo + C | annealing | Mo2C@C | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a graphitic shell | [41] |
| Zr + C | annealing | ZrC@C | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a graphitic shell | [42] |
| Hf + C | annealing | HfC@C | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a graphitic shell | [42] |
| Fe + BN | annealing | Fe2B@BN | powder: core-shell particles | formation of non-equilibrium structures decomposing to form a BN shell | [43] |
| Al + Cu + Fe | SHS | quasicrystalline phase | powder | formation of a single-phase product | [21] |
| Al + Ni + Co | SHS | quasicrystalline phase | powder | enabled SHS, formation of a single-phase product | [20] |
| Ti + BN | SHS | TiB2-TiN | powder | formation of a nanocrystalline composite ceramic powder | [24] |
| Ti + B4C | SHS | TiC-TiB2 | powder | formation of a submicron composite ceramic powder | [25] |
| Bulk alloys, ceramics, and composites | |||||
| Ti + C + Cu | reactive SPS | TiC−Cu | bulk composite | formation of a fine-grained composite, particle morphology control | [38,39,51] |
| Ti + B + Cu | SHS, SPS | TiB2−Cu | bulk composite | formation of a fine-grained composite | [26,27,30] |
| Cu + Al | SPS | Cu(Al) | bulk alloy | alloy formation | [72] |
| Ni + C | SPS | Ni-graphite | bulk composite | mixing, structural refinement | [54] |
| Co + C | SPS | Co-graphite | bulk composite | mixing, structural refinement | [55] |
| Ni + W | SPS | Ni(W)-Ni2W4C-WC | bulk composite | alloy formation | [73,74] |
| Fe + Ag | SPS | Fe−Ag | bulk pseudo-alloy | structural refinement | [52,53] |
| Ni + Al + B | SPS | Ni3Al−Ni(Al) alloyed with B | bulk composite | alloying with a minor additive | [75] |
| Ti25Cu75 + C | SPS | TiC−Cu | bulk composite | grinding of the alloy particles and mixing | [76,77] |
| Ti3SiC2 + Cu | SPS | Ti3SiC2−Cu. TiCx−Cu(Si) | bulk composite | mixing, particle morphology variation | [78] |
| Ti + B + Cu | shock consolidation | TiB2-Cu | bulk composite | reaction completeness during consolidation | [79] |
| Ti + B + Cu | SHS, shock consolidation | TiB2−Cu | bulk composite | formation of a fine-grained composite | [29] |
| Coatings | |||||
| Fe + Co + Ni + Cu | DS | FeCoNiCu | coating, fcc alloy structure | alloy formation | [67] |
| Ti3SiC2 + Cu | DS | Ti3SC2−Cu, TiCx-Cu(Si) | coating | formation of composite particles | [64,65] |
| Ti + C | TE, DS | Ti-titanium carbide (carbonitride) | coating | mixing, formation of composite particles | [62] |
| Ti + Al | TE, DS | titanium aluminides-titanium oxynitrides | coating | formation of a single-phase feedstock powder | [61,63] |
| Ti + B + Cu | SHS, DS | TiB2−Cu | coating | formation of composite particles and a fine-grained composite | [57] |
| Ti + B + Cu | SHS, CS | TiB2−Cu | coating | formation of composite particles and a fine-grained composite | [57,58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudina, D.V.; Bokhonov, B.B. Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin. J. Compos. Sci. 2022, 6, 188. https://doi.org/10.3390/jcs6070188
Dudina DV, Bokhonov BB. Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin. Journal of Composites Science. 2022; 6(7):188. https://doi.org/10.3390/jcs6070188
Chicago/Turabian StyleDudina, Dina V., and Boris B. Bokhonov. 2022. "Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin" Journal of Composites Science 6, no. 7: 188. https://doi.org/10.3390/jcs6070188
APA StyleDudina, D. V., & Bokhonov, B. B. (2022). Materials Development Using High-Energy Ball Milling: A Review Dedicated to the Memory of M.A. Korchagin. Journal of Composites Science, 6(7), 188. https://doi.org/10.3390/jcs6070188







