Patent Blue V Dye Adsorption by Fresh and Calcined Zn/Al LDH: Effect of Process Parameters and Experimental Design Optimization
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization
3.1.1. X-ray Diffraction (XRD) Study
3.1.2. Fourier Transform Infrared (FTIR) Analysis
3.1.3. Thermal Analysis (TGA-DTA)
3.2. Dye Removal from Aqueous Solution
3.2.1. Effect of Solution pH
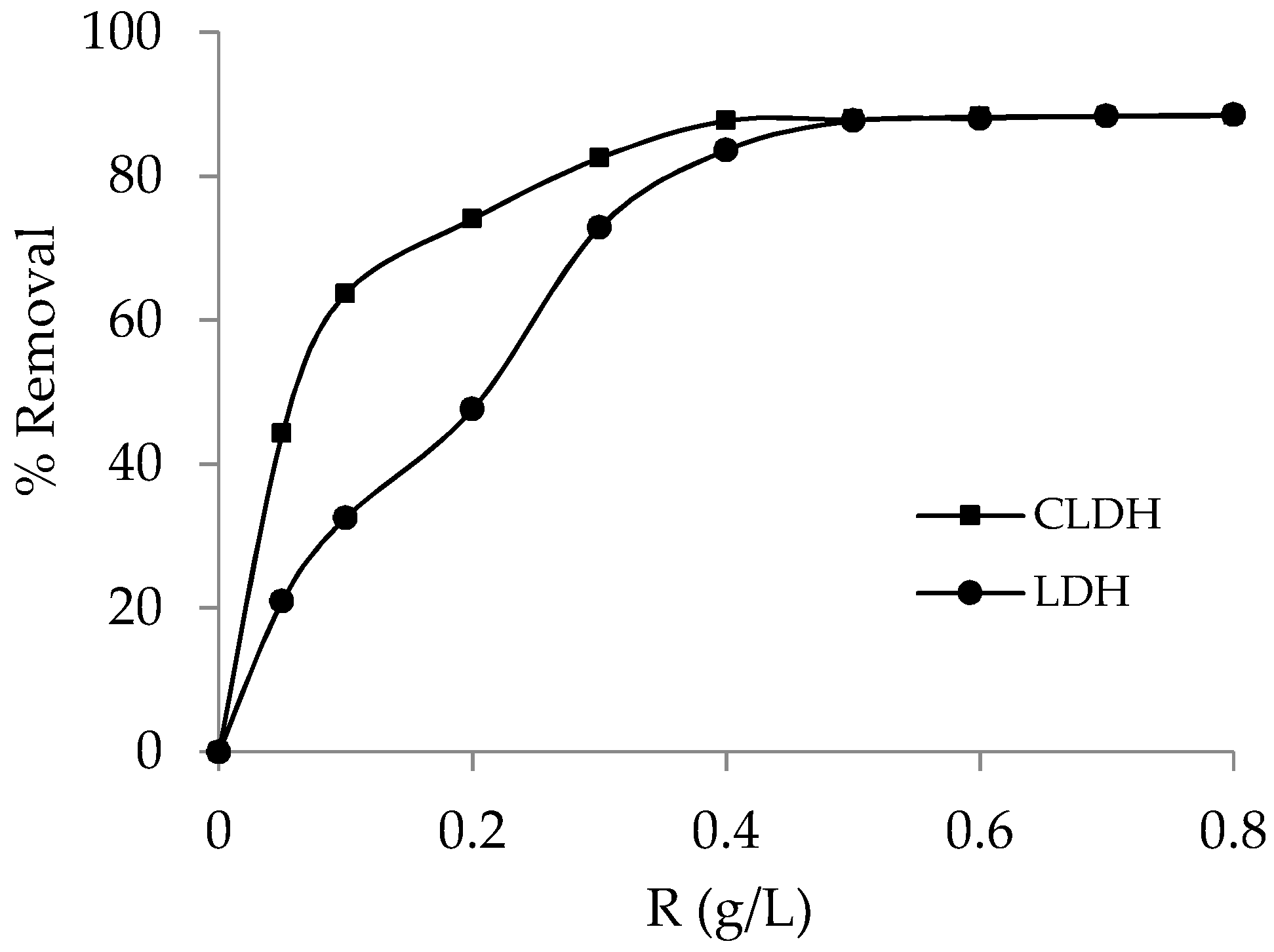
3.2.2. Effect of Adsorbent Dosage
3.2.3. Adsorption Kinetics
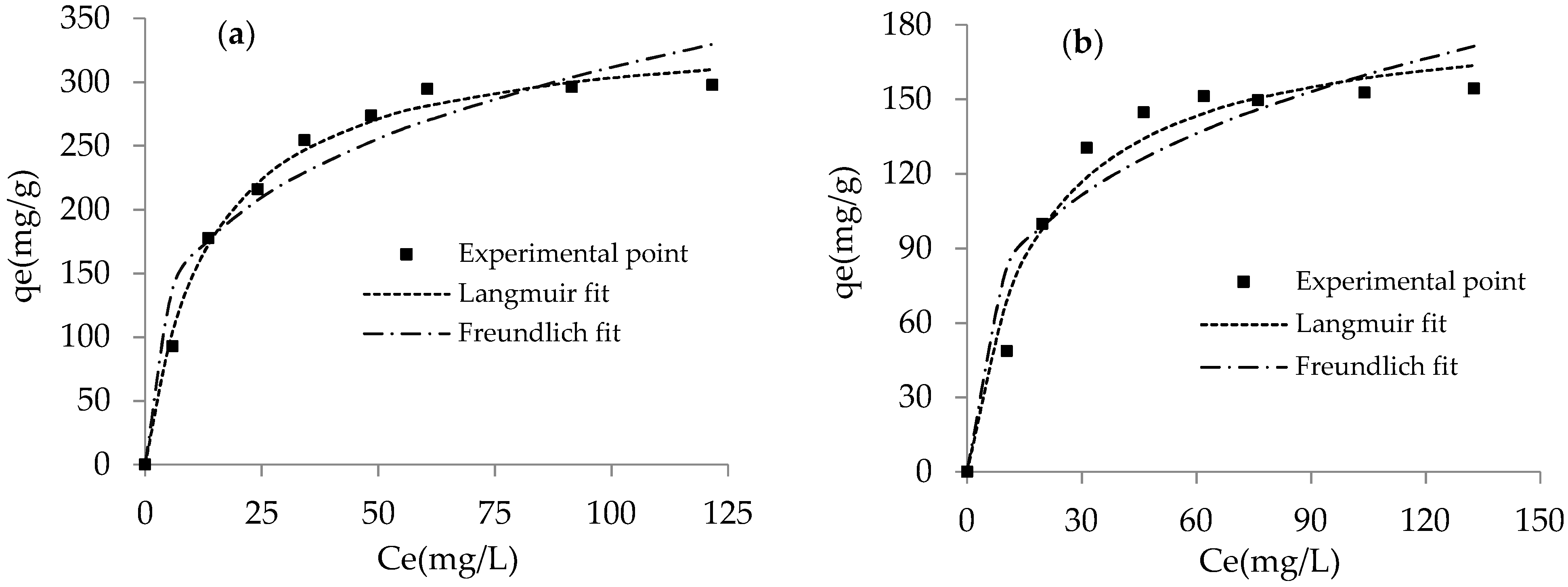
3.2.4. Adsorption Isotherms
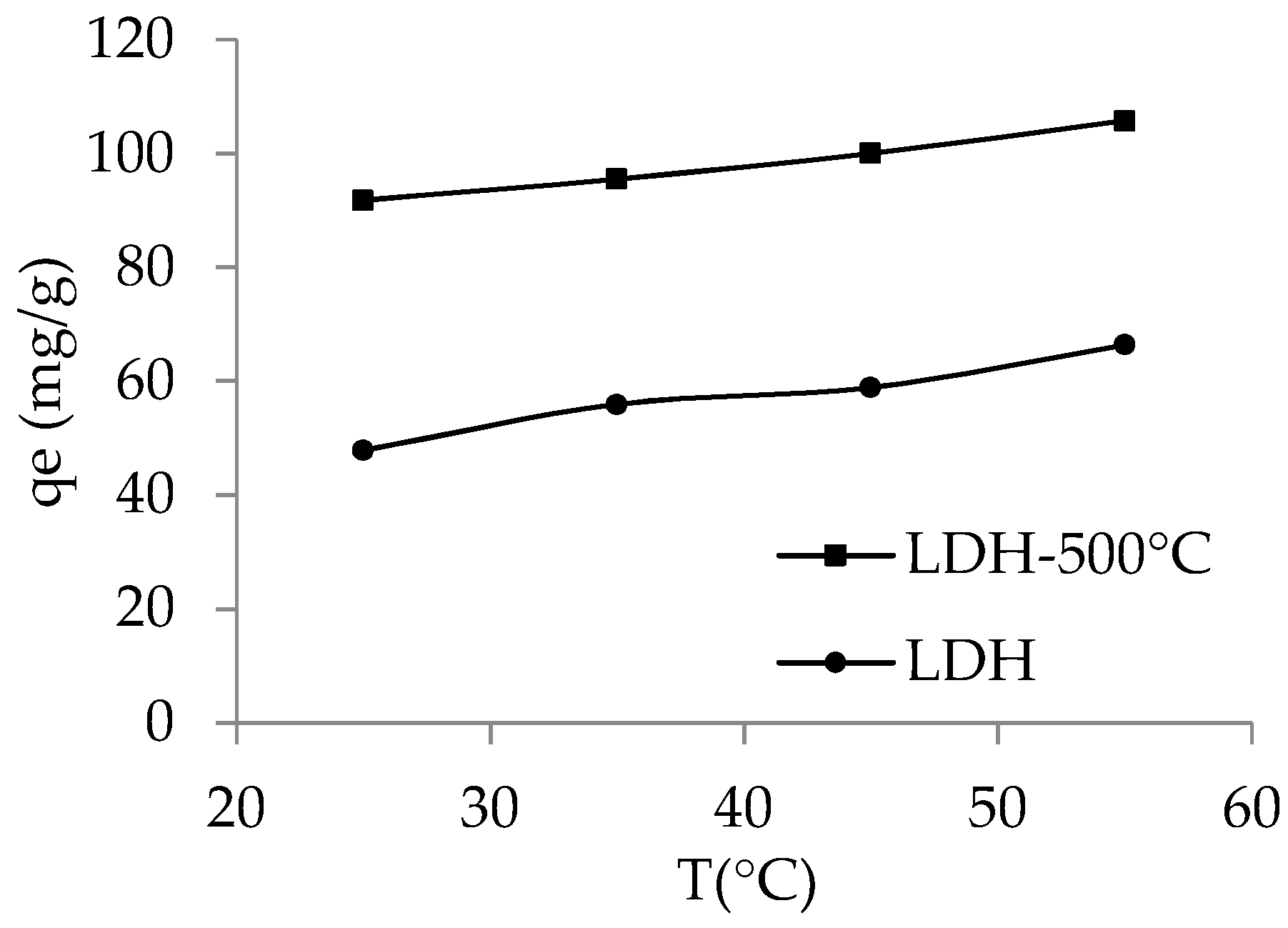
3.2.5. Effect of Temperature
3.3. Process Optimization
3.3.1. Experimental Results
3.3.2. Analysis of Variance (ANOVA)
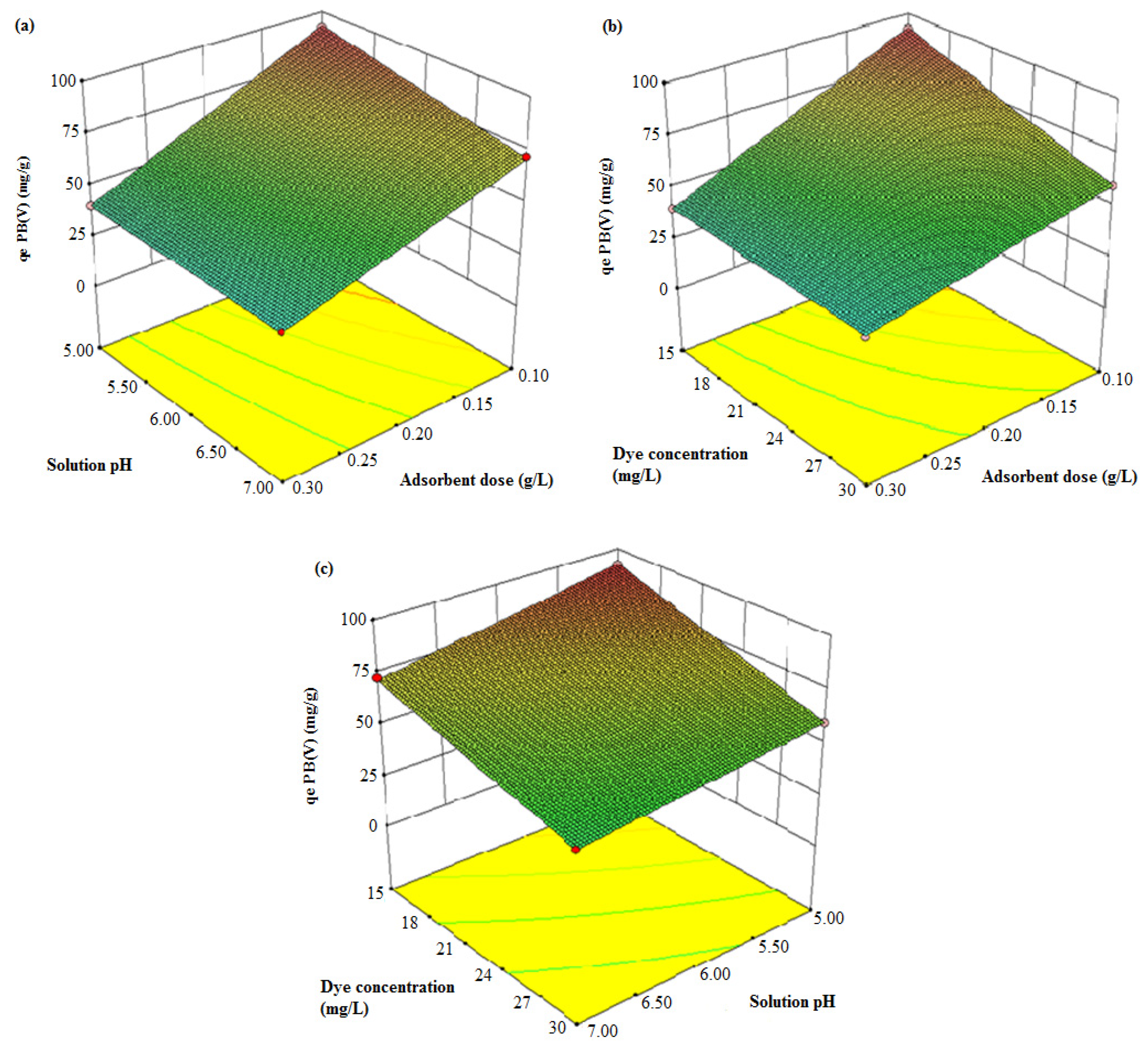
3.3.3. Response Surface Analysis
3.3.4. Optimization Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gowthambabu, V.; Balamurugan, A.; Satheeshkumar, S.; Kanmani, S.S. ZnO nanoparticles as efficient sunlight driven photocatalyst prepared by solution combustion method involved lime juice as biofuel. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119857. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Aihua, W. Potential Patent Blue V Overdose in a Patient Undergoing Free-Flap Breast Reconstruction: A Case Report. A&A Pr. 2021, 15, e01413. [Google Scholar] [CrossRef]
- Costa, D.; Mendonça, M.; Lopes, M.; Fernandes, A.L.; Nunes, S.; Müller, S. Patent blue V dye anaphylaxis: A case report and literature review. Braz. J. Anesthesiol. 2020, 70, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Elhalil, A.; Abdennouri, M.; Barka, N.; Bensitel, M.; Lamonier, C. Effect of aluminium incorporation on physicochemical properties and patent blue V photodegradation of magnesium phosphate materials. Bull. Mater. Sci. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2020, 405, 126674. [Google Scholar] [CrossRef]
- Das, L.; Saha, N.; Ganguli, A.; Das, P.; Bhowal, A.; Bhattacharjee, C. Calcium alginate–bentonite/activated biochar composite beads for removal of dye and Biodegradation of dye-loaded composite after use: Synthesis, removal, mathematical modeling and biodegradation kinetics. Environ. Technol. Innov. 2021, 24, 101955. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Fu, J.; Zelekew, O.A.; Abdeta, A.B.; Kuo, D.-H.; Zhang, J.; Yuan, Z.; Lin, J.; Chen, X. Wool-coiled bimetallic oxysulfide MoSrOS catalyst synthesis for catalytic reduction of toxic organic pollutants and heavy metal ions. J. Sci. Adv. Mater. Devices 2021, 6, 578–586. [Google Scholar] [CrossRef]
- Hussein, T.K.; Jasim, N.A. A comparison study between chemical coagulation and electro-coagulation processes for the treatment of wastewater containing reactive blue dye. Mater. Today Proc. 2021, 42, 1946–1950. [Google Scholar] [CrossRef]
- Boumya, W.; Khnifira, M.; Machrouhi, A.; Abdennouri, M.; Achak, M.; Qourzal, S.; Tounsadi, H.; Barka, N. Box–Behnken design for the understand of adsorption behaviors of cationic and anionic dyes by activated carbon. Desalin. Water Treat. 2021, 212, 204–211. [Google Scholar] [CrossRef]
- El-Abboubi, M.; Taoufik, N.; Mahjoubi, F.; Oussama, A.; Kzaiber, F.; Barka, N. Sorption of methyl orange dye by dodecyl-sulfate intercalated Mg-Al layered double hydroxides. Mater. Today Proc. 2020, 37, 3894–3897. [Google Scholar] [CrossRef]
- Elhalil, A.; Abdennouri, M.; Sadiq, M.; Kadmi, Y.; Favier, L.; Barka, N. Synthesis of Ba doped ZnO-Al2O3 nanocomposite from layered double hydroxide structure and their photocatalytic activity for the degradation of caffeine. J. Appl. Surf. Interfaces 2018, 4, 9–16. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Sadiq, M.; Abdennouri, M.; Kadmi, Y.; Favier, L.; Qourzal, S.; Barka, N. Enhanced photocatalytic degradation of caffeine as a model pharmaceutical pollutant by Ag-ZnO-Al2O3 nanocomposite. Desalination Water Treat. 2017, 94, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.S.; Schwarz, J.A. Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci. 1989, 130, 157–164. [Google Scholar] [CrossRef]
- Sahoo, G.P.; Samanta, S.; Bhui, D.K.; Pyne, S.; Maity, A.; Misra, A. Hydrothermal synthesis of hexagonal ZnO microstructures in HPMC polymer matrix and their catalytic activities. J. Mol. Liq. 2015, 212, 665–670. [Google Scholar] [CrossRef]
- Elhalil, A.; Farnane, M.; Machrouhi, A.; Mahjoubi, F.Z.; Elmoubarki, R.; Tounsadi, H.; Abdennouri, M.; Barka, N. Effect of molar ratio and calcination temperature on the adsorption performance of Zn/Al layered double hydroxide nanoparticles in the removal of pharmaceutical pollutants. J. Sci. Adv. Mater. Devices 2018, 3, 188–195. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Boumya, W.; Mahjoubi, F.; Elhalil, A.; Sadiq, M.; Barka, N. Ni-Fe-SDS and Ni-Fe-SO4 layered double hydroxides: Preparation, characterization and application in dyes removal. Mater. Today Proc. 2020, 37, 3871–3875. [Google Scholar] [CrossRef]
- Li, S.; Bai, Z.; Zhao, D. Characterization and friction performance of Zn/Mg/Al-CO3 layered double hydroxides. Appl. Surf. Sci. 2013, 284, 7–12. [Google Scholar] [CrossRef]
- Rives, V. Characterisation of Layered Double Hydroxides and Their Decomposition Products. Mater. Mater. Chem. Phys. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Lagergren, S. About the theorie of so-called adsorption of soluble substance. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids, Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]






| Name | Molecular Structure | MW (g/mol) | λmax (nm) |
|---|---|---|---|
| Patent blue V (Acid blue 3) |  | 582.66 | 637 |
| Factors | Levels | |
|---|---|---|
| Low (−) | High (+) | |
| A. Adsorbent dosage (mg/g) | 0.1 | 0.3 |
| B. Solution pH | 5 | 7 |
| C. Dye concentration (mg/L) | 15 | 30 |
| D. Nature of adsorbent | LDH | CLDH |
| Adsorbent | qexp (mg/g) | Pseudo First-Order | Pseudo Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qcal (mg/g) | K1 (1/min) | r2 | qcal (mg/g) | K2 (g/mg min) | r2 | ||
| CLDH | 99.36 | 94.52 | 0.347 | 0.991 | 101.47 | 0.005 | 0.998 |
| LDH | 49.48 | 47.47 | 0.368 | 0.959 | 50.83 | 0.012 | 0.990 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | r2 | KF (mg1–1/n/g·L−1/n) | n | r2 | |
| CLDH | 344.37 | 0.074 | 0.993 | 83.231 | 3.487 | 0.943 |
| LDH | 185.40 | 0.057 | 0.967 | 41.608 | 3.453 | 0.906 |
| Adsorbent | T (K) | KD | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/K·mol) |
|---|---|---|---|---|---|
| CLDH | 298 | 16.48 | −6.94 | 11.67 | 62.41 |
| 308 | 18.36 | −7.46 | |||
| 318 | 21.06 | −8.09 | |||
| 328 | 25.31 | −8.82 | |||
| LDH | 298 | 4.79 | −3.89 | 13.71 | 59.01 |
| 308 | 6.10 | −4.63 | |||
| 318 | 6.64 | −5.01 | |||
| 328 | 8.18 | −5.73 |
| Run | Coded Values | Actual Values | qe (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | ||
| 1 | −1 | −1 | −1 | −1 | 5 | 0.1 | 15 | LDH | 48.62 |
| 2 | −1 | 1 | −1 | −1 | 5 | 0.3 | 15 | LDH | 32.36 |
| 3 | 1 | −1 | −1 | −1 | 7 | 0.1 | 15 | LDH | 26.67 |
| 4 | 1 | 1 | −1 | −1 | 7 | 0.3 | 15 | LDH | 20.45 |
| 5 | −1 | −1 | 1 | −1 | 5 | 0.1 | 30 | LDH | 15.90 |
| 6 | −1 | 1 | 1 | −1 | 5 | 0.3 | 30 | LDH | 22.00 |
| 7 | 1 | −1 | 1 | −1 | 7 | 0.1 | 30 | LDH | 11.81 |
| 8 | 1 | 1 | 1 | −1 | 7 | 0.3 | 30 | LDH | 14.20 |
| 9 | −1 | −1 | −1 | 1 | 5 | 0.1 | 15 | CLDH | 92.24 |
| 10 | −1 | 1 | −1 | 1 | 5 | 0.3 | 15 | CLDH | 40.47 |
| 11 | 1 | −1 | −1 | 1 | 7 | 0.1 | 15 | CLDH | 72.48 |
| 12 | 1 | 1 | −1 | 1 | 7 | 0.3 | 15 | CLDH | 37.88 |
| 13 | −1 | −1 | 1 | 1 | 5 | 0.1 | 30 | CLDH | 59.80 |
| 14 | −1 | 1 | 1 | 1 | 5 | 0.3 | 30 | CLDH | 36.56 |
| 15 | 1 | −1 | 1 | 1 | 7 | 0.1 | 30 | CLDH | 47.78 |
| 16 | 1 | 1 | 1 | 1 | 7 | 0.3 | 30 | CLDH | 32.59 |
| Main Coefficient | Value |
|---|---|
| b0 | 38.24 |
| b1 | −8.67 |
| b2 | −5.26 |
| b3 | −8.16 |
| b4 | 14.24 |
| b12 | 1.97 |
| b13 | 4.93 |
| b14 | −6.93 |
| b23 | 1.77 |
| b24 | 0.13 |
| b34 | 0.05 |
| b123 | −1.43 |
| b124 | 1.18 |
| b134 | 1.06 |
| b234 | −0.08 |
| b1234 | 0.04 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 7310.92 | 7 | 609.24 | 361.05 | 0.0002 | significant |
| A | 1203.9 | 1 | 1203.9 | 713.46 | 0.0001 | |
| B | 441.96 | 1 | 441.96 | 261.91 | 0.0005 | |
| C | 1064.86 | 1 | 1064.86 | 631.06 | 0.0001 | |
| D | 3242.99 | 1 | 3242.99 | 1921.87 | <0.0001 | |
| AB | 62.21 | 1 | 62.21 | 36.87 | 0.0090 | |
| AC | 389.18 | 1 | 389.18 | 230.64 | 0.0006 | |
| BC | 50.16 | 1 | 50.16 | 29.72 | 0.0121 | |
| Residual | 5.06 | 3 | 1.69 | |||
| Cor Total | 7315.99 | 10 |
| Run | Actual | Predicted | Residual |
|---|---|---|---|
| 1 | 20.45 | 20.76 | −0.31 |
| 2 | 14.20 | 14.82 | −0.62 |
| 3 | 72.48 | 71.59 | 0.89 |
| 4 | 59.80 | 60.11 | −0.31 |
| 5 | 48.62 | 48.00 | 0.62 |
| 6 | 92.24 | 92.86 | −0.62 |
| 7 | 32.59 | 31.97 | 0.62 |
| 8 | 15.90 | 15.59 | 0.31 |
| 9 | 47.78 | 47.74 | 0.04 |
| 10 | 40.47 | 40.50 | −0.03 |
| 11 | 32.36 | 32.32 | 0.04 |
| 12 | 37.88 | 37.57 | 0.31 |
| 13 | 11.81 | 11.85 | −0.04 |
| 14 | 36.56 | 37.45 | −0.87 |
| 15 | 22.01 | 21.11 | 0.89 |
| 16 | 26.67 | 27.56 | −0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machrouhi, A.; Taoufik, N.; Elhalil, A.; Tounsadi, H.; Rais, Z.; Barka, N. Patent Blue V Dye Adsorption by Fresh and Calcined Zn/Al LDH: Effect of Process Parameters and Experimental Design Optimization. J. Compos. Sci. 2022, 6, 115. https://doi.org/10.3390/jcs6040115
Machrouhi A, Taoufik N, Elhalil A, Tounsadi H, Rais Z, Barka N. Patent Blue V Dye Adsorption by Fresh and Calcined Zn/Al LDH: Effect of Process Parameters and Experimental Design Optimization. Journal of Composites Science. 2022; 6(4):115. https://doi.org/10.3390/jcs6040115
Chicago/Turabian StyleMachrouhi, Aicha, Nawal Taoufik, Alaâeddine Elhalil, Hanane Tounsadi, Zakia Rais, and Noureddine Barka. 2022. "Patent Blue V Dye Adsorption by Fresh and Calcined Zn/Al LDH: Effect of Process Parameters and Experimental Design Optimization" Journal of Composites Science 6, no. 4: 115. https://doi.org/10.3390/jcs6040115
APA StyleMachrouhi, A., Taoufik, N., Elhalil, A., Tounsadi, H., Rais, Z., & Barka, N. (2022). Patent Blue V Dye Adsorption by Fresh and Calcined Zn/Al LDH: Effect of Process Parameters and Experimental Design Optimization. Journal of Composites Science, 6(4), 115. https://doi.org/10.3390/jcs6040115







