Mechanical Properties of Dragline Silk Fiber Using a Bottom-Up Approach
Abstract
:1. Introduction
2. Methods and Materials
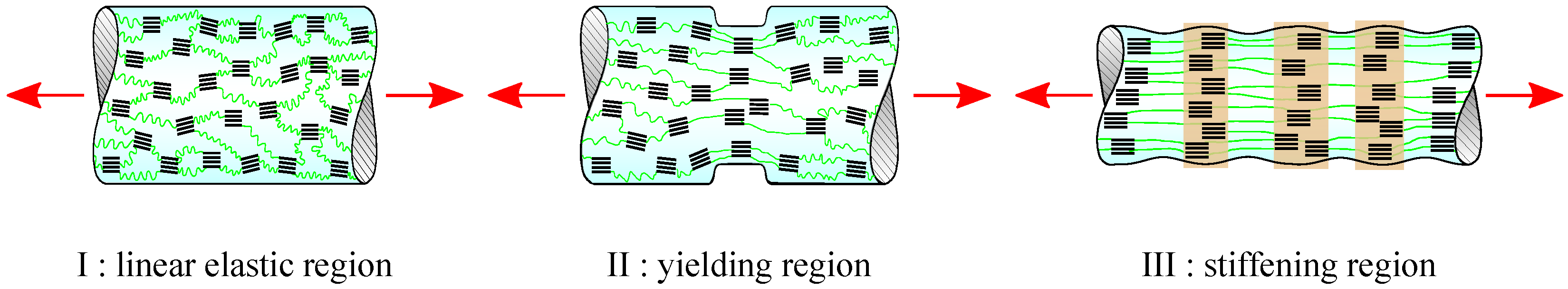
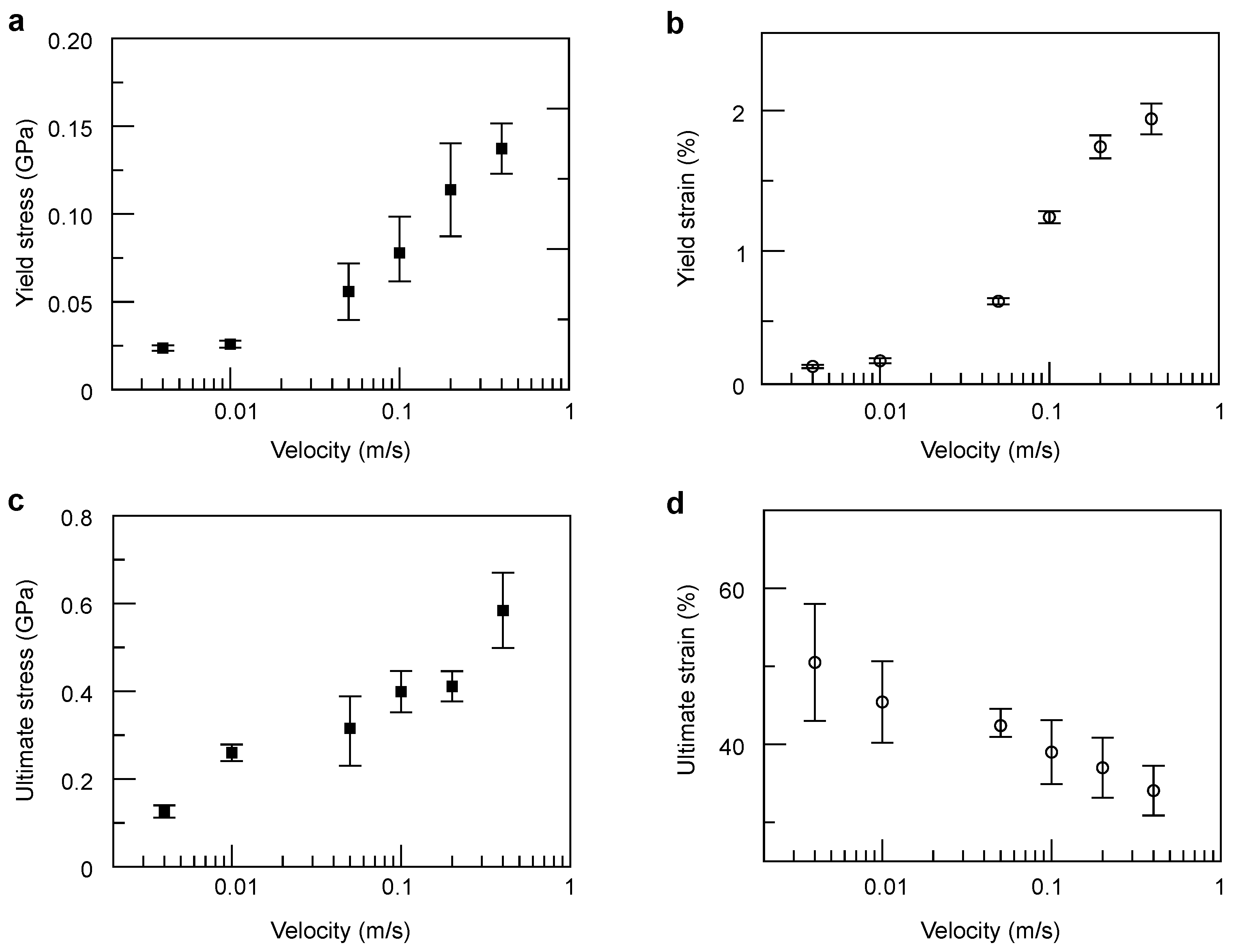
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Candelas, G.C.; Cintron, J. A spider fibroin and its synthesis. J. Exp. Zool. 1981, 216, 1–6. [Google Scholar] [CrossRef]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef]
- Denny, M.W. The physical properties of spider’s silk and their role in the design of orb-webs. J. Exp. Biol. 1976, 65, 483–506. [Google Scholar] [CrossRef]
- Gosline, J.M.; DeMont, M.E.; Denny, M.W. The Structure and Properties ofSpider Silk. Endeavour 1986, 10, 37–43. [Google Scholar] [CrossRef]
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural origin of the strain-hardening of spider silk. Adv. Funct. Mater. 2011, 21, 772–778. [Google Scholar] [CrossRef]
- Kaplan, D.L. Fibrous proteins-silk as a model system. Polym. Degrad. Stab. 1998, 59, 25–32. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Qiao, J.; Wu, Y.; Ren, M.; Wang, Y.; Shen, X.; Wei, X.; Wang, X.; Di, J.; Li, Q. Programmable Contractile Actuations of Twisted Spider Dragline Silk Yarns. ACS Biomater. Sci. Eng. 2021, 7, 482–490. [Google Scholar] [CrossRef]
- Kono, N.; Nakamura, H.; Ohtoshi, R.; Moran, D.A.P.; Shinohara, A.; Yoshida, Y.; Fujiwara, M.; Mori, M.; Tomita, M.; Arakawa, K. Orb-weaving spider Araneus ventricosus genome elucidates the spidroin gene catalogue. Sci. Rep. 2019, 9, 8380. [Google Scholar] [CrossRef]
- Kono, N.; Ohtoshi, R.; Malay, A.D.; Mori, M.; Masunaga, H.; Yoshida, Y.; Nakamura, H.; Numata, K.; Arakawa, K. Darwin’s bark spider shares a spidroin repertoire with Caerostris extrusa but achieves extraordinary silk toughness through gene expression. Open Biol. 2021, 11, 210242. [Google Scholar] [CrossRef]
- Kono, N.; Nakamura, H.; Mori, M.; Yoshida, Y.; Ohtoshi, R.; Malay, A.D.; Moran, D.A.P.; Tomita, M.; Numata, K.; Arakawa, K. Multicomponent nature underlies the extraordinary mechanical properties of spider dragline silk. Proc. Natl. Acad. Sci. USA 2021, 118, e2107065118. [Google Scholar] [CrossRef]
- Sheffer, M.M.; Hoppe, A.; Krehenwinkel, H.; Uhl, G.; Kuss, A.W.; Jensen, L.; Jensen, C.; Gillespie, R.G.; Hoff, K.J.; Prost, S. Chromosome-level reference genome of the European wasp spider Argiope bruennichi: A resource for studies on range expansion and evolutionary adaptation. Gigascience 2021, 10, giaa148. [Google Scholar] [CrossRef]
- Miller, J.; Vienneau-Hathaway, J.; Dendev, E.; Lan, M.; Ayoub, N.A. The common house spider, Parasteatoda tepidariorum, maintains silk gene expression on sub-optimal diet. PLoS ONE 2020, 15, e0237286. [Google Scholar] [CrossRef]
- Glišovic, A.; Vehoff, T.; Davies, R.J.; Salditt, T. Strain dependent structural changes of spider dragline silk. Macromolecules 2008, 41, 390–398. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 271–275. [Google Scholar] [CrossRef]
- van Beek, J.D.; Hess, S.; Vollrath, F.; Meier, B.H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. USA 2002, 99, 10266–10271. [Google Scholar] [CrossRef] [Green Version]
- Lefèvre, T.; Rousseau, M.; Pézolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef] [Green Version]
- Cunniff, P.M.; Fossey, S.A.; Auerbach, M.A.; Song, J.W.; Kaplan, D.L.; Adams, W.W.; Eby, R.K.; Mahoney, D.; Vezie, D.L. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994, 5, 401–410. [Google Scholar] [CrossRef]
- Simmons, A.; Ray, E.; Jelinski, L.W. Solid-state 13C NMR of Nephila clavipes dragline silk establishes structure and identity of crystalline regions. Macromolecules 1994, 27, 5235–5237. [Google Scholar] [CrossRef]
- Oroudjev, E.; Soares, J.; Arcidiacono, S.; Thompson, J.B.; Fossey, S.A.; Hansma, H.G. Segmented nanofibers of spider dragline silk: Atomic force microscopy and single-molecule force spectroscopy. Proc. Natl. Acad. Sci. USA 2002, 99, 6460–6465. [Google Scholar] [CrossRef] [Green Version]
- Hinman, M.B.; Lewis, R.V. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J. Biol. Chem. 1992, 267, 19320–19324. [Google Scholar] [CrossRef]
- Porter, D.; Vollrath, F.; Shao, Z. Predicting the mechanical properties of spider silk as a model nanostructured polymer. Eur. Phys. J. E 2005, 16, 199–206. [Google Scholar] [CrossRef]
- Brookes, V.L.; Young, R.J.; Vollrath, F. Deformation micromechanics of spider silk. J. Mater. Sci. 2008, 43, 3728–3732. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Ishii, T.; Masunaga, H.; Numata, K. Spider dragline silk composite films doped with linear and telechelic polyalanine: Effect of polyalanine on the structure and mechanical properties. Sci. Rep. 2018, 8, 3564. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, K.; Malay, A.D.; Masunaga, H.; Norma-Rashid, Y.; Numata, K. Simultaneous effect of strain rate and humidity on the structure and mechanical behavior of spider silk. Commun. Mater. 2020, 1, 10. [Google Scholar]
- Termonia, Y. Molecular Modeling of Spider Silk Elasticity. Macromolecules 1994, 27, 7378–7381. [Google Scholar] [CrossRef]
- Becker, N.; Oroudjev, E.; Mutz, S.; Cleveland, J.P.; Hansma, P.K.; Hayashi, C.Y.; Makarov, D.E.; Hansma, H.G. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003, 2, 278–283. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Hierarchical chain model of spider capture silk elasticity. Phys. Rev. Lett. 2005, 94, 028104. [Google Scholar] [CrossRef] [Green Version]
- Nova, A.; Keten, S.; Pugno, N.M.; Redaelli, A.; Buehler, M.J. Molecular and Nanostructural Mechanisms of Deformation, Strength and Toughness of Spider Silk Fibrils. Nano Lett. 2010, 10, 2626–2634. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, M.; Xiao, S.; Markert, B.; Stacklies, W.; Gräter, F. Silk fiber mechanics from multiscale force distribution analysis. Biophys. J. 2011, 100, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.A.; Patil, S.P.; Greving, I.; Lämmel, M.; Gkagkas, K.; Seydel, T.; Müller, M.; Markert, B.; Gräter, F. Stress-induced long-range ordering in spider silk. Sci. Rep. 2017, 7, 15273. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.P.; Markert, B.; Gräter, F. Refining a Bottom-up Computational Approach for Spider Silk Fibre Mechanics. In Proceedings of the 3rd GAMM Seminar on Continuums Biomechanics, Essen, Germany, 20–21 January 2012; Volume II-21, pp. 75–87. [Google Scholar]
- Patil, S.P.; Markert, B.; Gräter, F. Rate-Dependent Behavior of the Amorphous Phase of Spider Dragline Silk. Biophys. J. 2014, 106, 2511–2518. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.P.; Xiao, S.; Gkagkas, K.; Markert, B.; Gräter, F. Viscous friction between crystalline and amorphous phase of dragline silk. PLoS ONE 2014, 9, e104832. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.P. Multiscale Modeling of Spider Dragline Silk. Ph.D. Thesis, Universitäetsbibliothek der RWTH Aachen University, Aachen, Germany, 2015. [Google Scholar]
- Du, N.; Liu, X.Y.; Narayanan, J.; Li, L.; Lim, M.L.M.; Li, D. Design of superior spider silk: From nanostructure to mechanical properties. Biophys. J. 2006, 91, 4528–4535. [Google Scholar] [CrossRef] [Green Version]
- Sintya, E.; Alam, P. Localised semicrystalline phases of MaSp1 proteins show high sensitivity to overshearing in β-sheet nanocrystals. Int. J. Biol. Macromol. 2016, 92, 1006–1011. [Google Scholar] [CrossRef]
- LS-DYNA Keyword User’s Manual; Livermore Software Technology Corporation: Livermore, CA, USA, 2010.
- Madsen, B.; Shao, Z.Z.; Vollrath, F. Variability in the mechanical properties of spider silks on three levels: Interspecific, intraspecific and intraindividual. Int. J. Biol. Macromol. 1999, 24, 301–306. [Google Scholar] [CrossRef]
- Madsen, B.; Vollrath, F. Mechanics and morphology of silk drawn from anesthetized spiders. Naturwissenschaften 2000, 87, 148–153. [Google Scholar] [CrossRef]
- Garrido, M.A.; Elices, M.; Viney, C.; Pérez-Rigueiro, J. The variability and interdependence of spider drag line tensile properties. Polymer 2002, 43, 4495–4502. [Google Scholar] [CrossRef]
- Vollrath, F.; Madsen, B.; Shao, Z.Z. The effect of spinning conditions on the mechanics of a spider’s dragline silk. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 2339–2346. [Google Scholar] [CrossRef] [Green Version]
- Vehoff, T.; Glišović, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical properties of spider dragline silk: Humidity, hysteresis, and relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef] [Green Version]
- Denny, M.W. Silks–their properties and functions. Symp. Soc. Exp. Biol. 1980, 34, 247–272. [Google Scholar] [PubMed]
- Köhler, T.; Vollrath, F. Thread biomechanics in the two orb-weaving spiders Araneus diadematus (Araneae, Araneidae) and Uloborus walckenaerius (Araneae, Uloboridae). J. Exp. Zool. 1995, 271, 1–17. [Google Scholar] [CrossRef]
- Work, R.W. The force-elongation behavior of web fibers and silks forcibly obtained from orb-web-spinning spiders. Text. Res. J. 1976, 46, 485–492. [Google Scholar] [CrossRef]

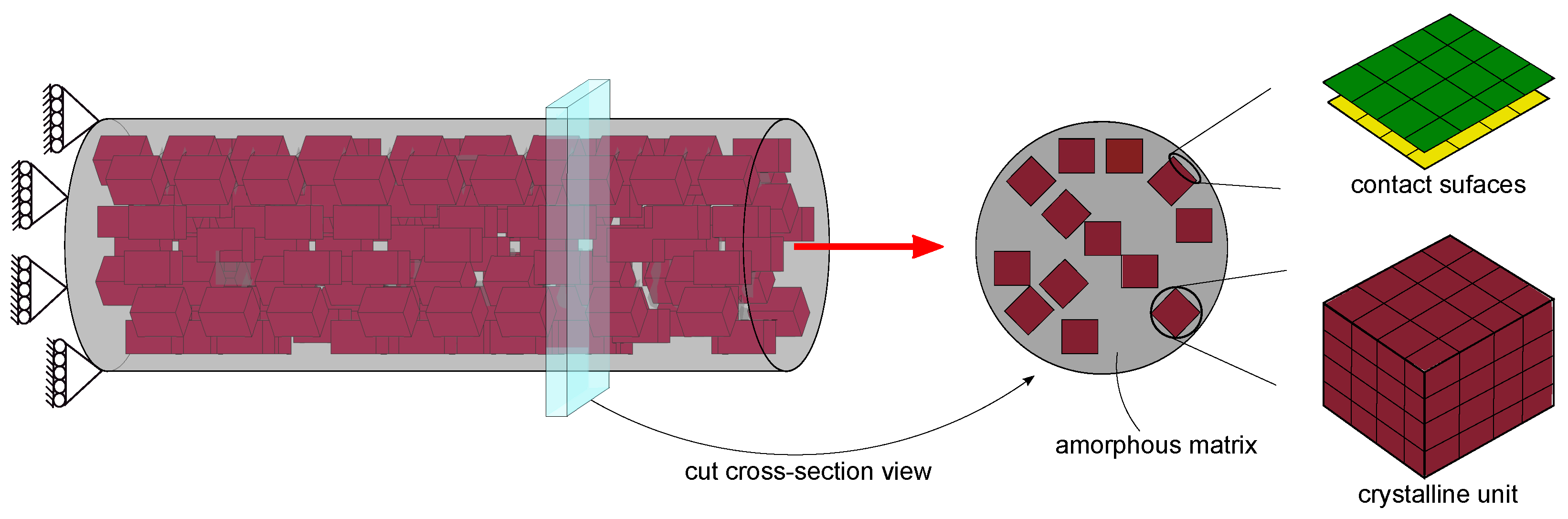



| Structural Component | Mechanical Behavior a | Description b |
|---|---|---|
| crystalline unit | irreversible deformation | crystal cubes are randomly |
| (plastic kinematic material) | distributed with 25% vol. | |
| amorphous phase | rate-dependent deformation | filled the remaining space |
| (viscoelastic material) | in the model | |
| crystalline-amorphous | viscous friction | lubricated film of 2D shell |
| interface | (surface to surface contact) | elements of the null material |
| Mechanical Properties | 3D Fiber Model | Experimental Data a |
|---|---|---|
| ultimate Strength, (GPa) | 0.50–0.67 | 0.65–1.61 |
| extensibility, | 0.32–0.36 | 0.23–0.45 |
| initial stiffness, E (GPa) | 6.5–8.1 | 3.8–10.0 |
| post-yield stiffness, E (GPa) | 1.1–1.7 | 1.5–5.1 |
| toughness (MJ/m) | 101–135 | 120–225 |
| hysteresis (%) | 70–74 | 65–70.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.P.; Kulkarni, A.; Markert, B. Mechanical Properties of Dragline Silk Fiber Using a Bottom-Up Approach. J. Compos. Sci. 2022, 6, 95. https://doi.org/10.3390/jcs6030095
Patil SP, Kulkarni A, Markert B. Mechanical Properties of Dragline Silk Fiber Using a Bottom-Up Approach. Journal of Composites Science. 2022; 6(3):95. https://doi.org/10.3390/jcs6030095
Chicago/Turabian StylePatil, Sandeep P., Ambarish Kulkarni, and Bernd Markert. 2022. "Mechanical Properties of Dragline Silk Fiber Using a Bottom-Up Approach" Journal of Composites Science 6, no. 3: 95. https://doi.org/10.3390/jcs6030095
APA StylePatil, S. P., Kulkarni, A., & Markert, B. (2022). Mechanical Properties of Dragline Silk Fiber Using a Bottom-Up Approach. Journal of Composites Science, 6(3), 95. https://doi.org/10.3390/jcs6030095








