The Variance of the Polypropylene α Relaxation Temperature in iPP/a-PP-pPBMA/Mica Composites
Abstract
:1. Introduction
2. Materials and Methods
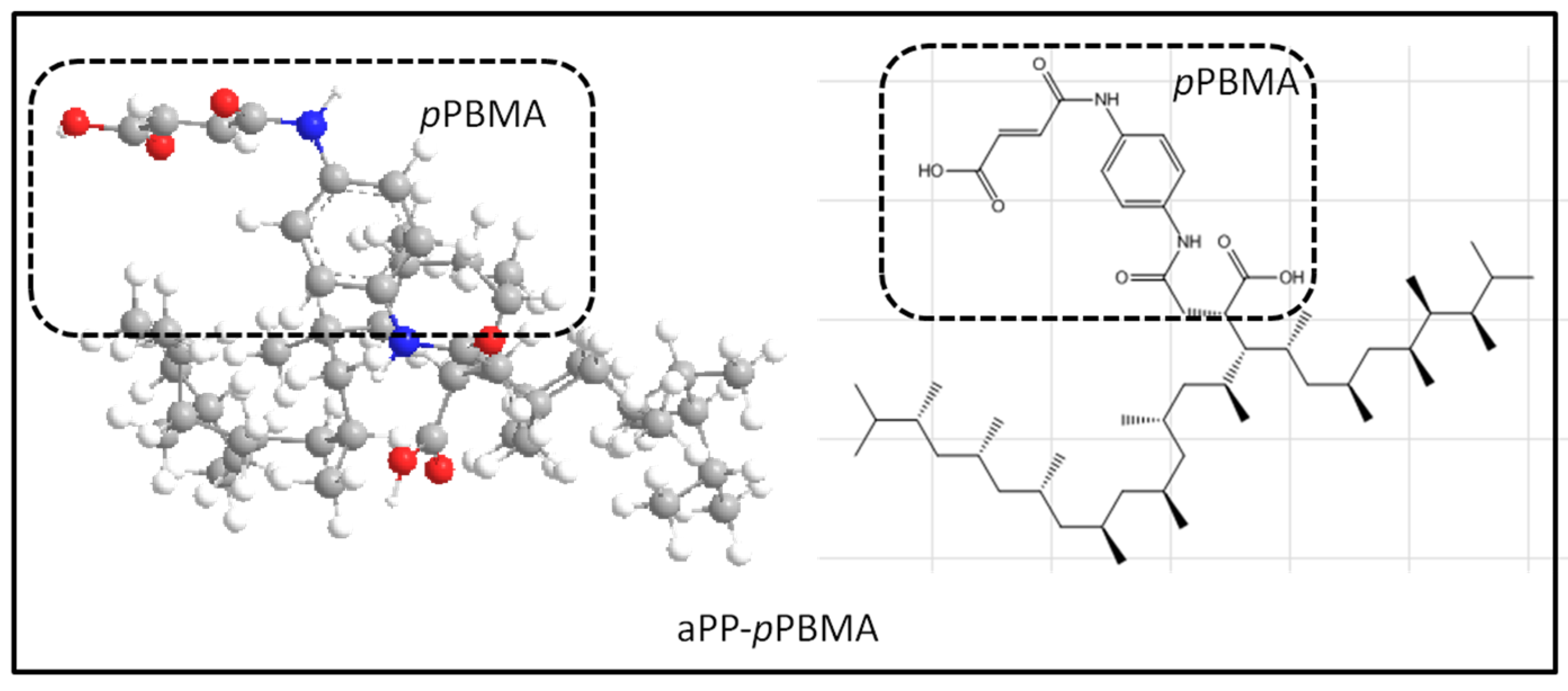
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Mathematical Model
3. Results and Discussion
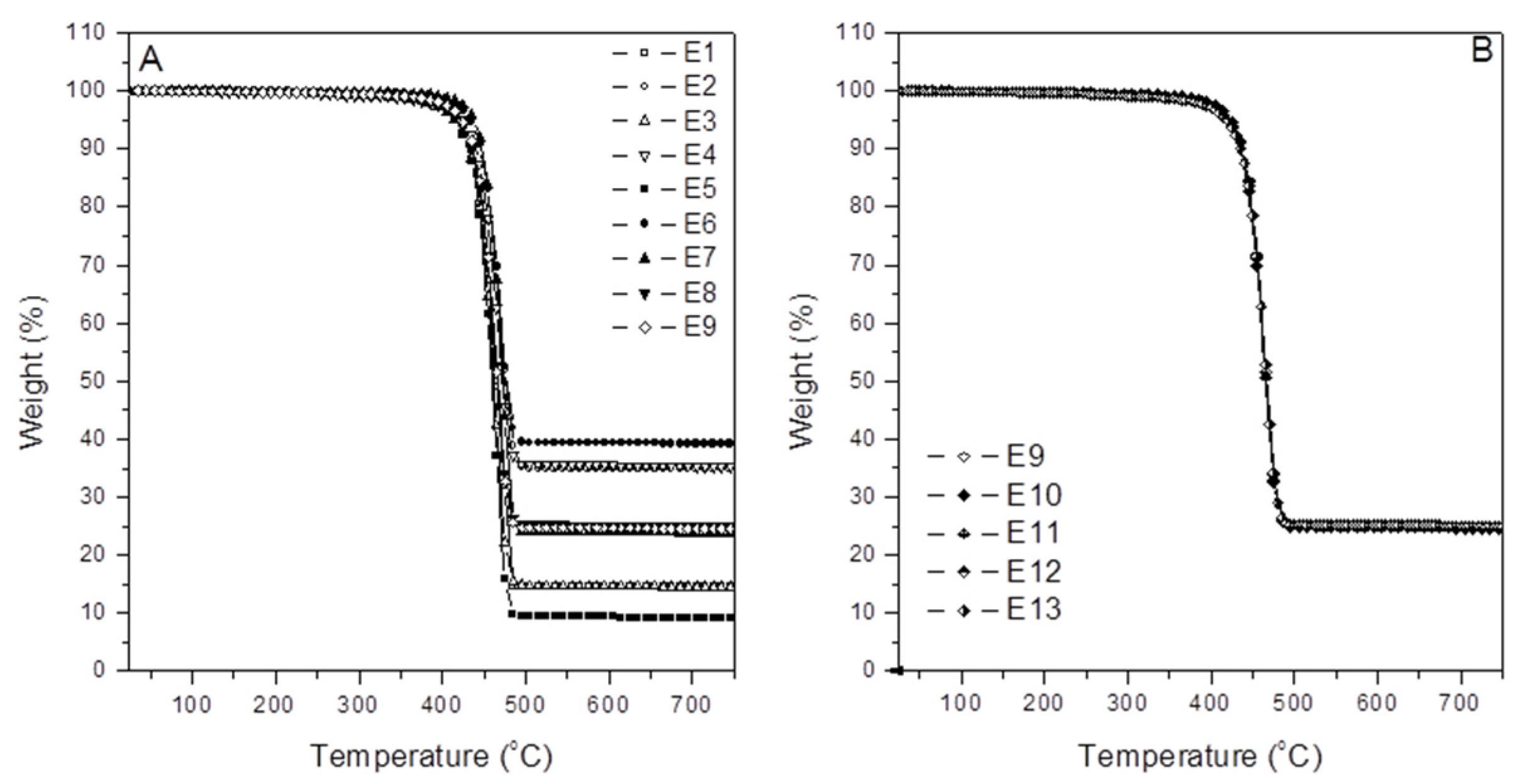
3.1. TGA Analysis
3.2. SEM Observations
3.3. Dynamic-Mechanical Analysis
3.3.1. Background
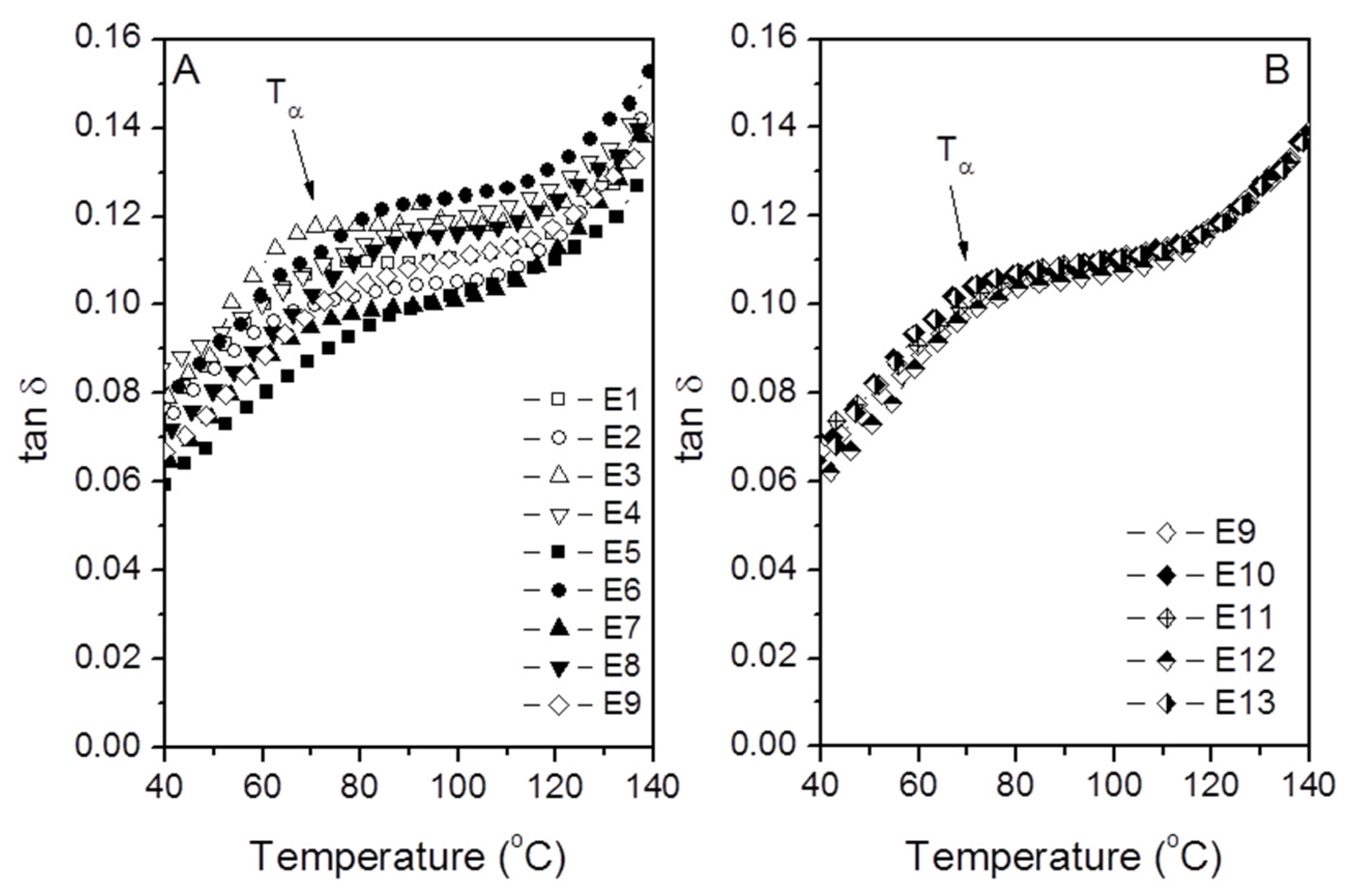
3.3.2. Dynamic Mechanical Spectra: Determination of Tα
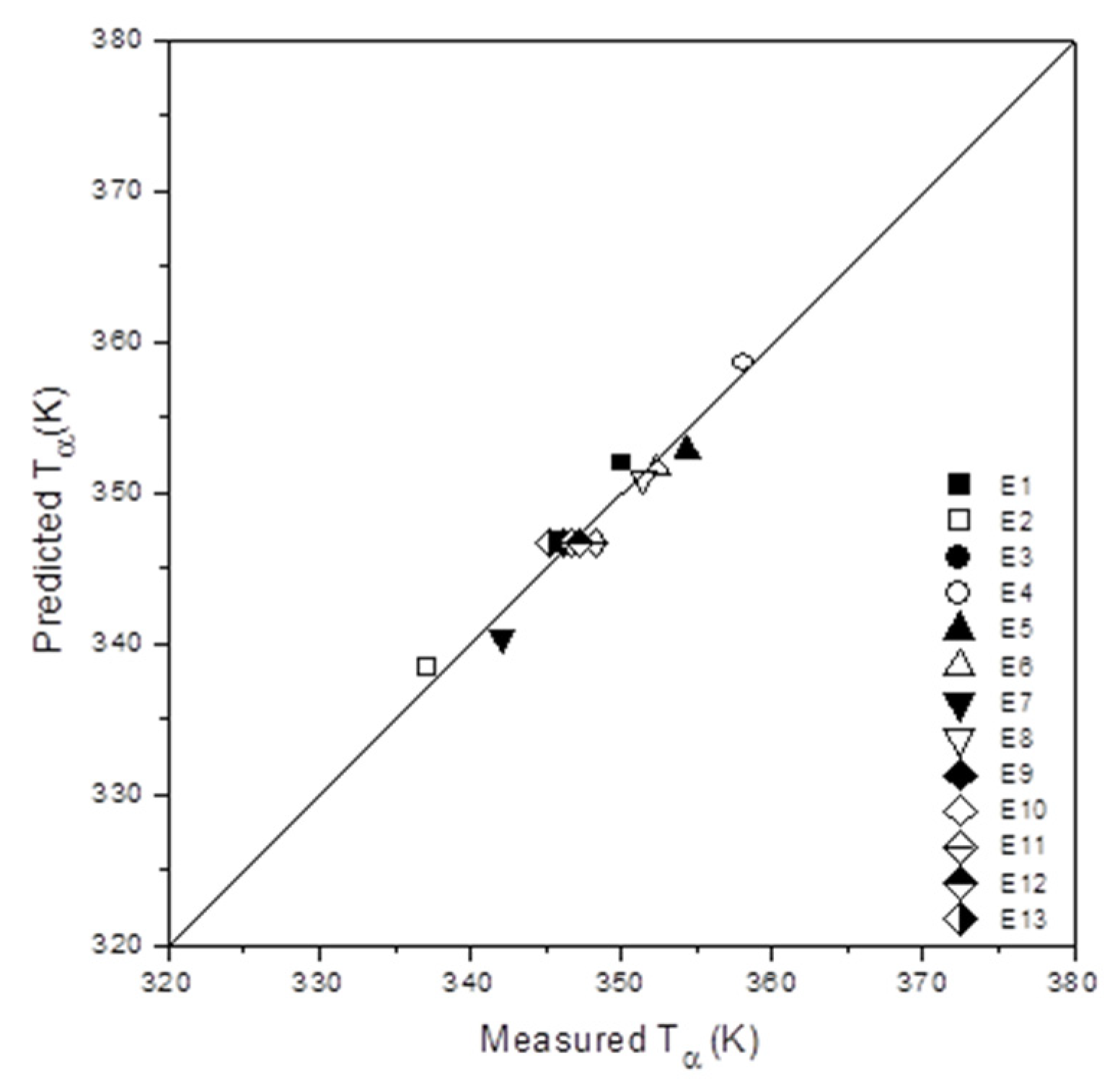
3.4. Polynomial Fits and Analysis of Variance (ANOVA)
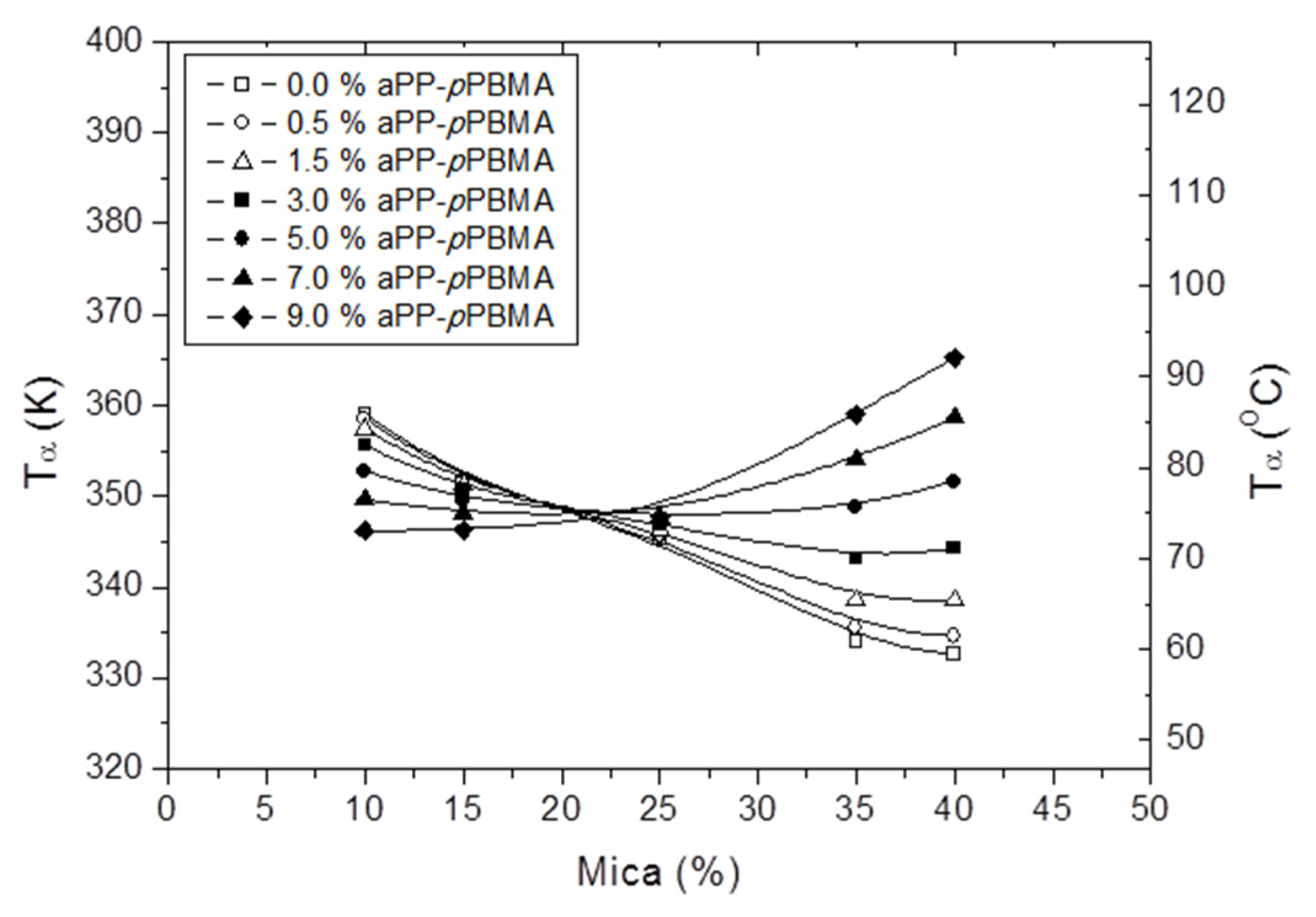
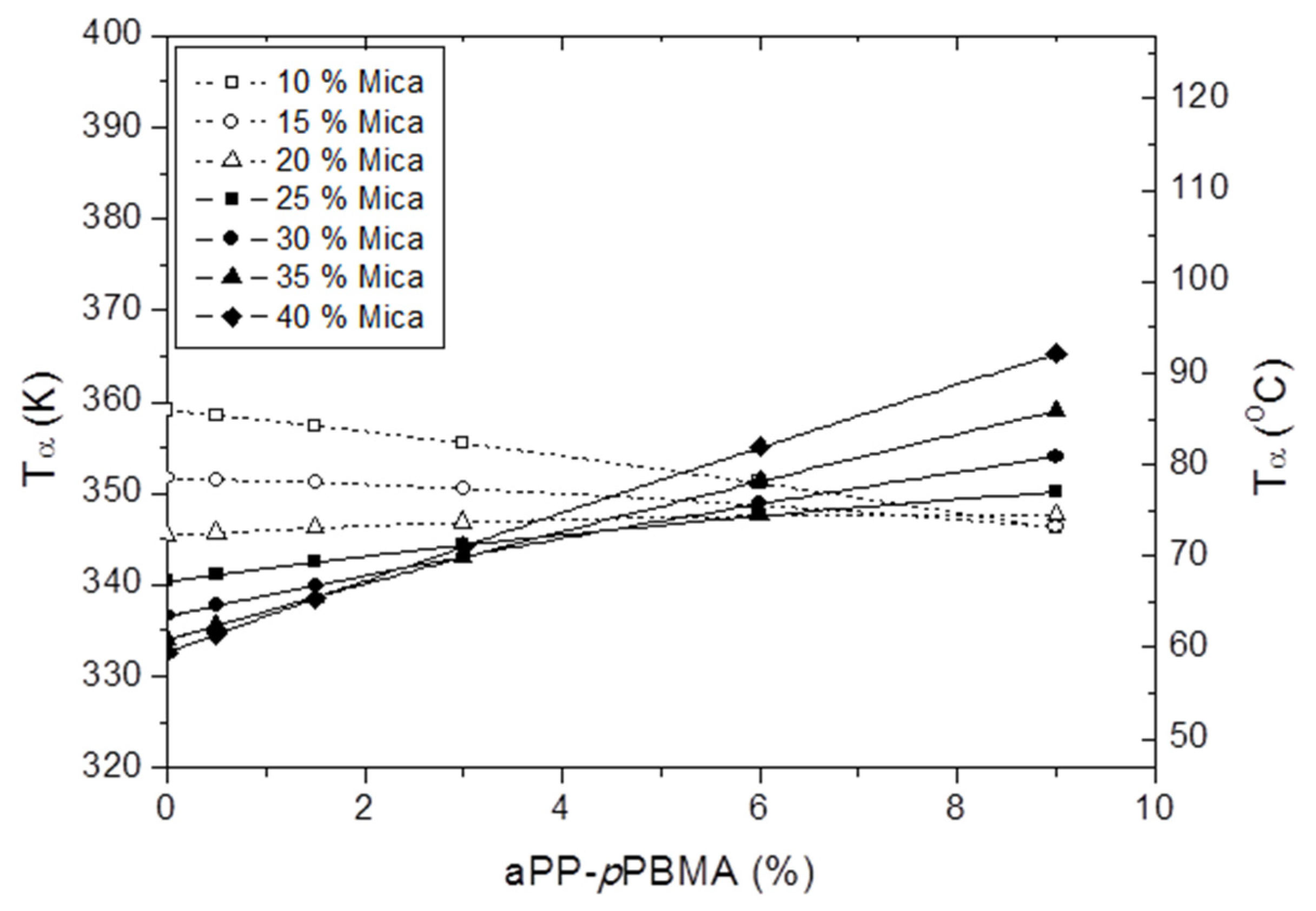
3.5. The Influence of the Composite Composition in the Tα as Determined by DMA Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galli, P.; Vecellio, G.J. Polyolefins: The most promising large—Volume materials for the 21st century. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 396–415. [Google Scholar] [CrossRef]
- Pritchard, J. Polyolefin Reaction Engineering; Soares, J.B.P., McKenna, T.F.I., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2012; Chapter 1. [Google Scholar]
- García-Martínez, J.M.; Taranco, J.; Areso, S.; Collar, E.P. A DMA study of the interfacial changes on injection-molded iPP/mica composites modified by a p-phenylen-bis-maleamic acid grafted atactic polypropylene. J. Appl. Polym. Sci. 2017, 134, 45366. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, J.M.; Collar, E.P. On the combined effect of both the reinforcement and a waste based interfacial modifier on the matrix glass transition in iPP/aPP-pPBMA/mica composites. Polymers 2020, 12, 2606. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.M.; Collar, E.P. Organic-Inorganic hybrid materials. Polymers 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.M.; Collar, E.P. Organic–Inorganic Hybrid Materials II: Some Additional Contributions to the Topic. Polymers 2021, 13, 2390. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Areso, S.; Collar, E.P. The effect of a p-phenylen-bis-maleamic acid grafted atactic polypropylene interface agent on the dynamic-mechanical properties of iPP/mica composites measured at the polymer transitions. Polym. Eng. Sci. 2017, 57, 746–755. [Google Scholar] [CrossRef]
- Karger-Kocsis, J. Polypropylene, Structure, Blends and Composites, 1st ed.; Chapman & Hall: London, UK, 1995. [Google Scholar]
- García-Martínez, J.M.; Areso, S.; Taranco, J.; Collar, E.P. The Role of a Novel p-phenylen-bis-maleamic acid grafted atactic polypropylene interfacial modifier in Polypropylene/Mica Composites as evidenced by Tensile Properties. J. Appl. Polym. Sci. 2009, 113, 3929–3943. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Laguna, O.; Areso, S.; Collar, E.P. Polypropylene/Mica Composites Modified by Succinic Anhydride Grafted Atactic Polypropylene. A Thermal and Mechanical Study under Dynamical Conditions. J. Appl. Polym. Sci. 2001, 81, 625–636. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Laguna, O.; Areso, S.; Collar, E.P. A thermal and mechanical study under dynamical conditions of Polypropylene/Mica composites containing atactic polypropylene with succinil-fluoresceine grafted groups as interfacial modifier from the matrix side. J. Polym. Sci. Polym. Phys. 2000, 38, 1564–1574. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Polym. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Pegoretti, A.; Karger-Kocsis, J. Interphase engineering in polymer composites: Challenging the devil…. Express Polym. Lett. 2015, 9, 838. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Cofrades, A.G.; Areso, S.; Collar, E.P. On the Chemical Modification Process of Atactic Polypropylene by p-Phenylen-bis-Maleamic Acid in the Melt. J. Appl. Polym. Sci. 2003, 88, 2202–2209. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic-Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M. Nanocoatings Nanosystems Nanotechnologies; Bentham Books: Sharjah, United Arab Emirates, 2012. [Google Scholar] [CrossRef] [Green Version]
- Verbeeck, J.; Chistopher, M. Mica-Reinforced Polymer Composites. In Polymer Composites. Vol I: Macro and Microcomposites, 1st ed.; Thomas, S., Ed.; Wiley: Weinheim, Germany, 2012; pp. 673–714. [Google Scholar]
- Collar, E.P.; Areso, S.; Taranco, J.; García-Martínez, J.M. Heterogeneous Materials based on Polypropylene. In Polyolefin Blends, 1st ed.; Nwabunma, D., Kyu, T., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 379–410. [Google Scholar]
- Ciardelli, F.; Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites based on thermoplastic materials. Polym. Int. 2008, 57, 805–836. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mat. 1998, 8, 29–35. [Google Scholar] [CrossRef]
- Glotzer, S.C. Complex rules for soft systems. Nat. Mater. 2003, 2, 713–714. [Google Scholar] [CrossRef]
- Ellis, G.; Marco, C.; Gómez, M.A.; Collar, E.P.; García-Martínez, J.M. The study of heterogeneous polymer systems by synchrotron infrared microscopy. J. Macromol. Sci. Phys. 2004, B43, 253–266. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Collar, E.P. Flexural behavior of PP/Mica composites interfacial modified by a p-phenylen-bis-maleamic acid grafted atactic polypropylene modifier obtained from industrial wastes. J. Appl. Polym. Sci. 2015, 34, 42437. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, J.M.; Taranco, J.; Collar, E.P. Effect of a p-phenylen-bis-maleamic acid grafted atactic polypropylene interfacial agent on the impact properties of iPP/mica composites. J. Appl. Polym. Sci. 2017, 134, 44619. [Google Scholar]
- Passaglia, E.; Martin, G.M. Dependence of mechanical relaxation on morphology in isotactic polypropylene. J. Res. Natl. Bur. Stand. 1964, 68, 519–527. [Google Scholar] [CrossRef] [PubMed]
- McCrum, N.G.; Read, B.E.; Williams, G. Anelastic and Dielectric effects in Polymeric Solids, 1st ed.; Wiley: London, UK, 1967. [Google Scholar]
- Ottino, J.M. Complex Systems. AICHE J. 2003, 49, 292–299. [Google Scholar] [CrossRef]
- Ottino, J.M. Engineering Complex Systems. Nature 2004, 427, 399. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Response surface methods. In Statistics for Experimenters, 1st ed.; Wiley: New York, NY, USA, 1978; pp. 510–539. [Google Scholar]
- Fisher, R.A. The Desing of Experiments, 1st ed.; Hafner: New York, NY, USA, 1960. [Google Scholar]
- ISO 527-2:2012. Plastics Determination of Tensile Properties. Part 2: Test Conditions for Moulding and Extrusion Plastics. Available online: https://www.iso.org/standard/56046.html (accessed on 2 January 2022).



| Coded Factors * | Controlled Factors * | |||||
|---|---|---|---|---|---|---|
| Exp | [Mica] | [aPP-pPBMA] | [Mica] (%) | [aPP-pPBMA] (%) | Tα (°C) | Tα (K) |
| E1 | −1 | −1 | 14.4 | 1.465 | 76.9 | 350.05 |
| E2 | 1 | −1 | 35.6 | 1.465 | 64.0 | 337.15 |
| E3 | −1 | 1 | 14.4 | 8.535 | 72.6 | 345.75 |
| E4 | 1 | 1 | 35.6 | 8.535 | 85.0 | 358.15 |
| E5 | −√2 | 0 | 10.0 | 5.000 | 81.2 | 354.35 |
| E6 | √2 | 0 | 40.0 | 5.000 | 79.2 | 352.35 |
| E7 | 0 | −√2 | 25.0 | 0.001 | 69.0 | 342.15 |
| E8 | 0 | √2 | 25.0 | 9.999 | 78.3 | 351.45 |
| E9 | 0 | 0 | 25.0 | 5.000 | 73.0 | 346.15 |
| E10 | 0 | 0 | 25.0 | 5.000 | 73.5 | 346.65 |
| E11 | 0 | 0 | 25.0 | 5.000 | 75.2 | 348.35 |
| E12 | 0 | 0 | 25.0 | 5.000 | 74.1 | 347.25 |
| E13 | 0 | 0 | 25.0 | 5.000 | 71.1 | 345.25 |
| Exp | Nominal Mica Content (%) | Real Mica Content (%) |
|---|---|---|
| E1 | 14.4 | 14.5 ± 0.1 |
| E2 | 35.6 | 34.9 ± 0.2 |
| E3 | 14.4 | 14.5 ± 0.1 |
| E4 | 35.6 | 35.4 ± 0.3 |
| E5 | 10.0 | 9.8 ± 0.3 |
| E6 | 40.0 | 39.6 ± 0.5 |
| E7 | 25.0 | 24.9 ± 0.2 |
| E8 | 25.0 | 25.0 ± 0.1 |
| E9 | 25.0 | 24.8 ± 0.3 |
| E10 | 25.0 | 24.7 ± 0.3 |
| E11 | 25.0 | 24.8 ± 0.2 |
| E12 | 25.0 | 24.9 ± 0.2 |
| E13 | 25.0 | 25.0 ± 0.1 |
| <r2> (%) | LF (%) | CF (%) | Linear Terms | Interaction Term | Quadratic Terms | ||||
|---|---|---|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | a4 | a5 | ||||
| Tα[K] | 94.86 | 14.9 | 99.7 | 377.7 | −2.104 | −2.741 | 0.1688 | 0.02441 | −0.004234 |
| Linear Parameters | Interaction Parameter | Quadratic Parameters | |||
|---|---|---|---|---|---|
| x1 | x2 | x1·x2 | x12 | x22 | |
| Tα[K] | 7.136 (99.9%) | 3.710 (99.1%) | 7.909 (99.9%) | 4.528 (99.5%) | 0.872 (57.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, J.-M.; Collar, E.P. The Variance of the Polypropylene α Relaxation Temperature in iPP/a-PP-pPBMA/Mica Composites. J. Compos. Sci. 2022, 6, 57. https://doi.org/10.3390/jcs6020057
García-Martínez J-M, Collar EP. The Variance of the Polypropylene α Relaxation Temperature in iPP/a-PP-pPBMA/Mica Composites. Journal of Composites Science. 2022; 6(2):57. https://doi.org/10.3390/jcs6020057
Chicago/Turabian StyleGarcía-Martínez, Jesús-María, and Emilia P. Collar. 2022. "The Variance of the Polypropylene α Relaxation Temperature in iPP/a-PP-pPBMA/Mica Composites" Journal of Composites Science 6, no. 2: 57. https://doi.org/10.3390/jcs6020057
APA StyleGarcía-Martínez, J.-M., & Collar, E. P. (2022). The Variance of the Polypropylene α Relaxation Temperature in iPP/a-PP-pPBMA/Mica Composites. Journal of Composites Science, 6(2), 57. https://doi.org/10.3390/jcs6020057






