High Surface Area Activated Charcoal for Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing the Raw Material
2.3. Activated Carbon Purification
2.4. Measurement of Charcoal Activated Surface Area: Iodine Number Measurement
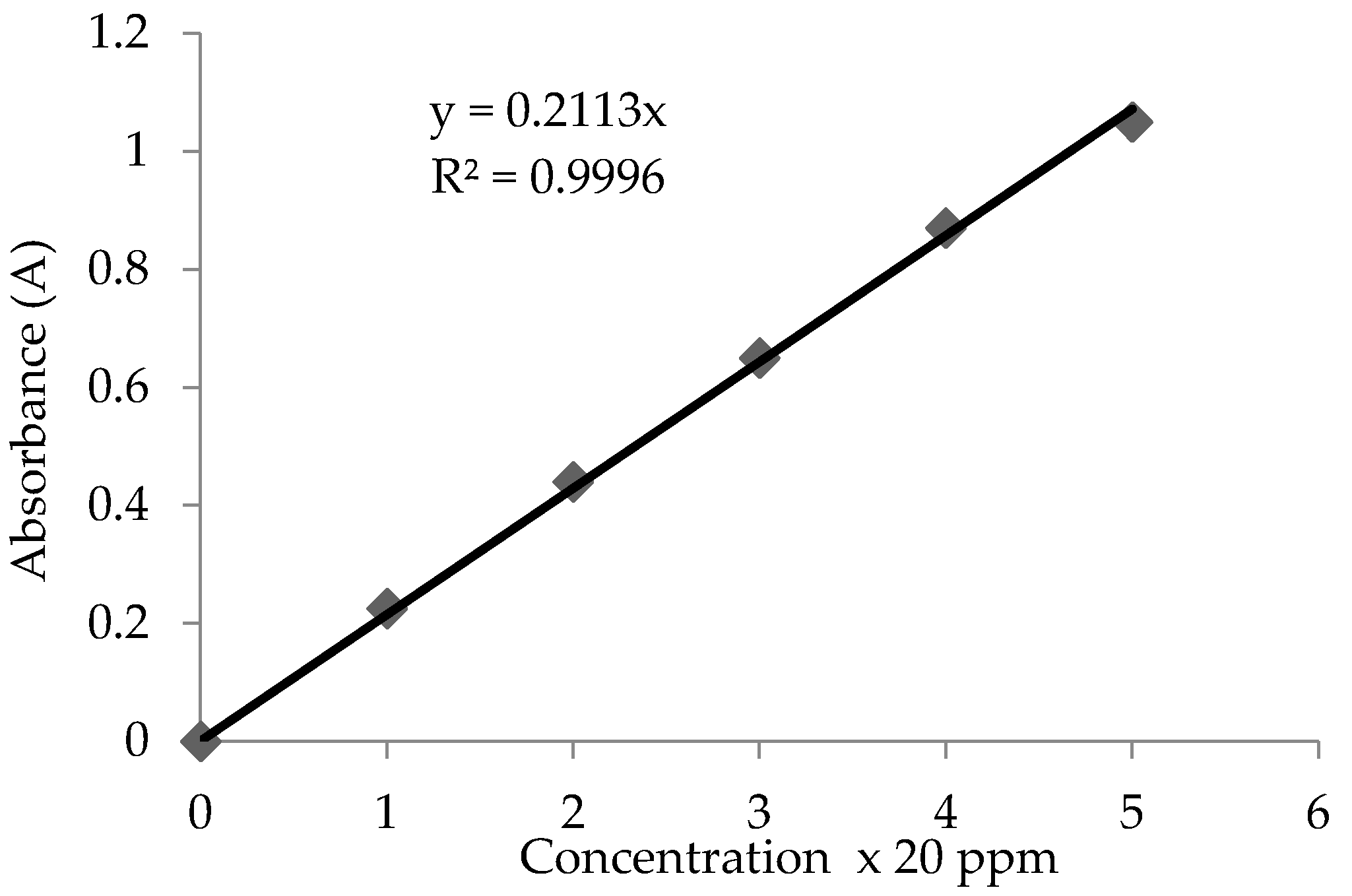
2.5. Measurement of Charcoal Activated Surface Area: Methylene Blue Dye Number Measurement
2.6. Measurement of Charcoal Activated Surface Area: BET Method
2.7. Humidity Moisture Measurement
exposure to air and moisture × 100
2.8. Ash Ratio Measurement
weight × 100
2.9. Atomic Absorption Measurements
2.10. UV Measurements
3. Results and Discussion
3.1. Preparation and Characterisation of Activated Carbon
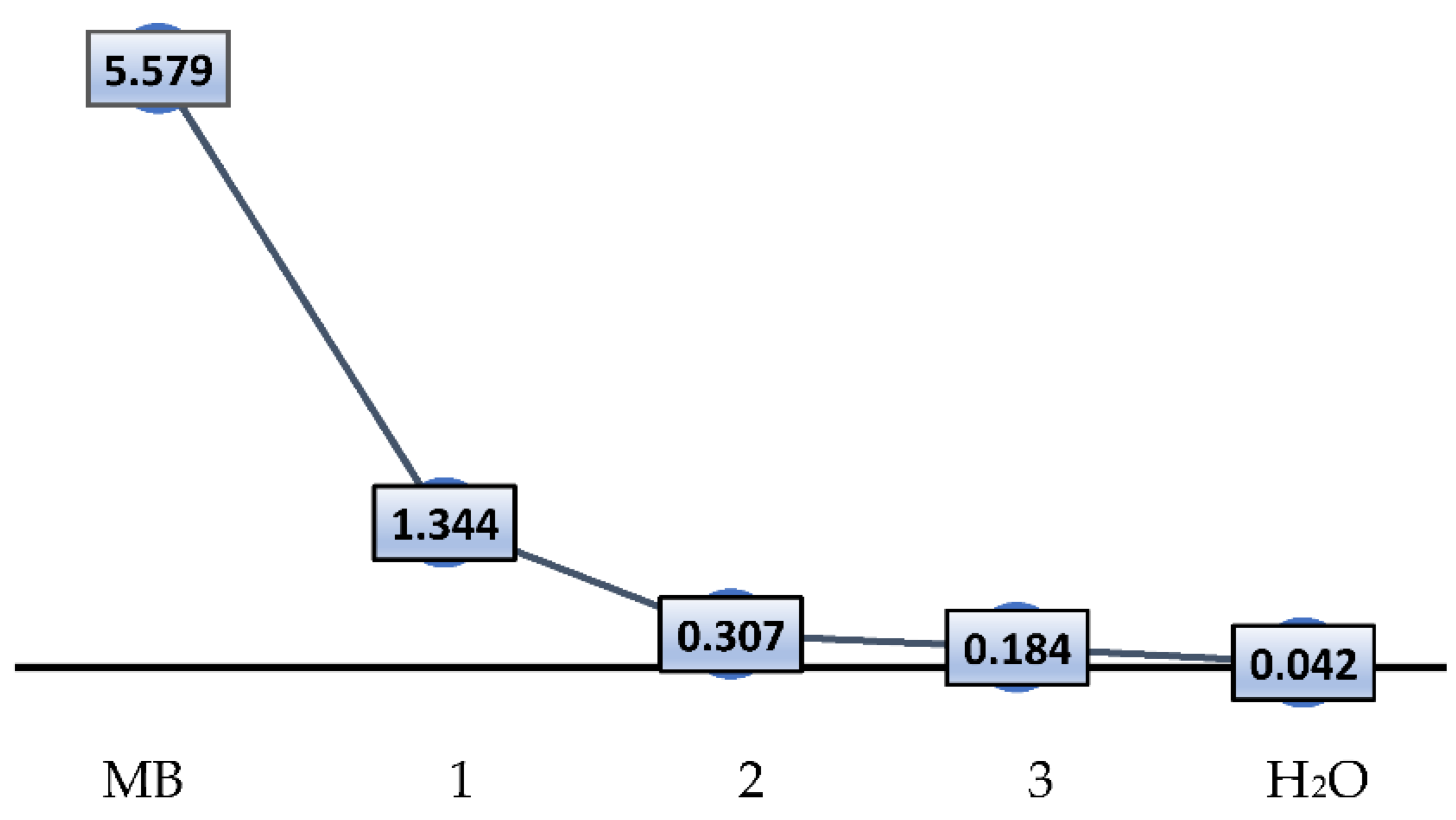
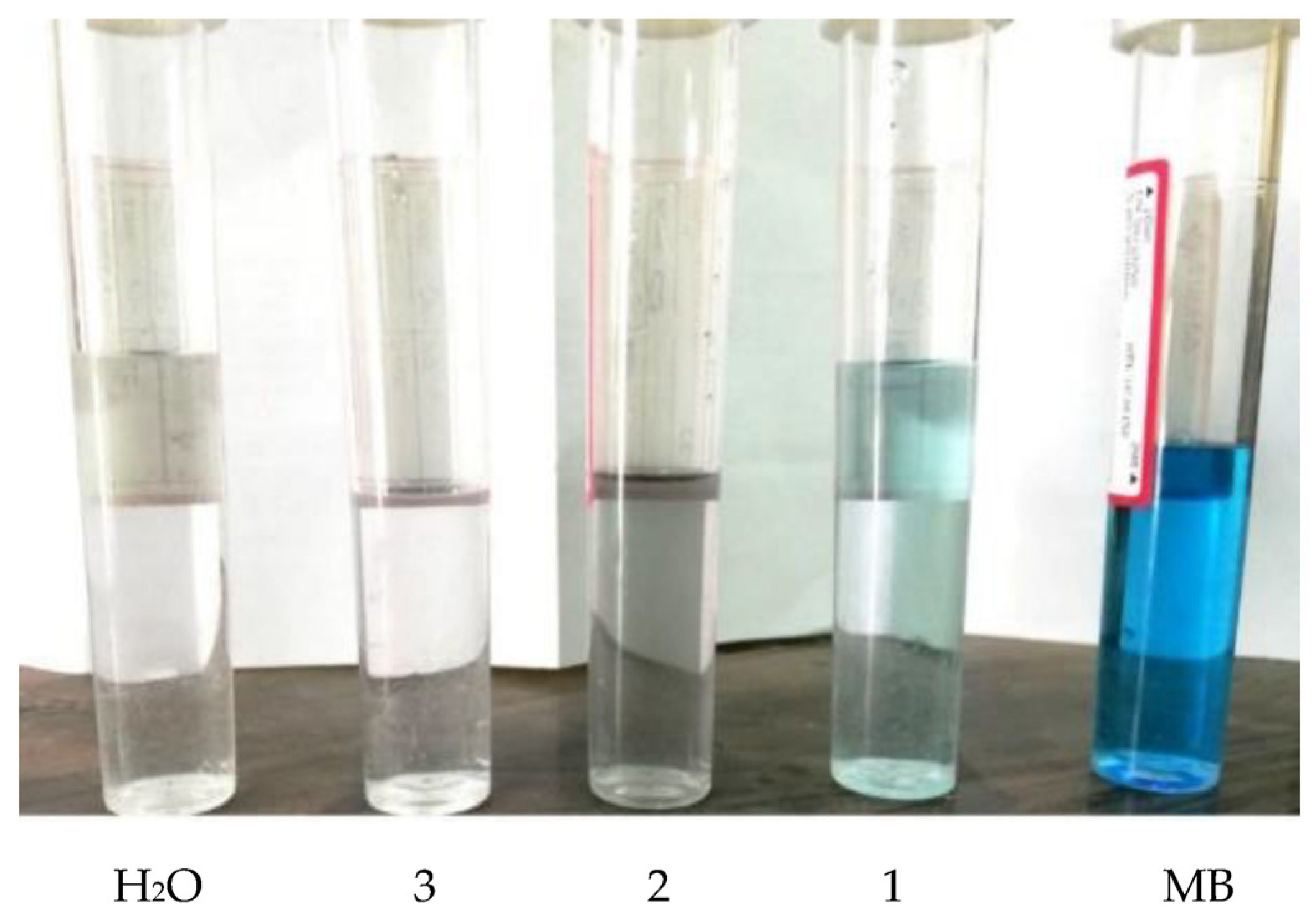
3.2. Absorption and Removal of Methylene Blue Dye from Water
Heavy Element Absorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, G.; Zheng, Y.; Zhao, Y. Preparation of activated carbon from Guhanshan coal and its effect on methane adsorption thermodynamics at different temperatures. Powder Technol. 2022, 395, 424–442. [Google Scholar] [CrossRef]
- Rösler, M.; Wedler, C. Adsorption kinetics and equilibria of two methanol samples with different water content on activated carbon. Adsorption 2021, 27, 1175–1190. [Google Scholar] [CrossRef]
- L Lupaşcu, T.; Petuhov, O.; Ţîmbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B12 and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095. [Google Scholar] [CrossRef] [PubMed]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Décima, M.A.; Marzeddu, S.; Barchiesi, M.; Di Marcantonio, C.; Chiavola, A.; Boni, M.R. A review on the removal of carbamazepine from aqueous solution by using activated carbon and biochar. Sustainability 2021, 13, 11760. [Google Scholar] [CrossRef]
- Xin, W.; Li, X.; Song, Y. Sludge-based mesoporous activated carbon: The effect of hydrothermal pretreatment on material preparation and adsorption of bisphenol A. J. Chem. Technol. Biotechnol. 2020, 95, 1666–1674. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: An application in methylene blue removal by RSM optimization. Int. J. Phytoremediation 2022, 24, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hirunpraditkoon, S.; Tunthong, N.; Ruangchai, A.; Nuithitikul, K. Adsorption capacities of activated carbons prepared from bamboo by KOH activation. Int. J. Chem. Mol. Eng. 2011, 5, 491–495. [Google Scholar]
- Basta, A.H.; Fierro, V.; El-Saied, H.; Celzard, A. 2-Steps KOH activation of rice straw: An efficient method for preparing high-performance activated carbons. Bioresour. Technol. 2009, 100, 3941–3947. [Google Scholar] [CrossRef]
- ASTM. Standard test method for determination of iodine number of activated carbon. ASTM Annu. Book 1999, 4, 30–65. [Google Scholar] [CrossRef]
- Anzalone, G.C.; Diaz-Loya, I.; Minkara, R.Y.; Sutter, L.L. Comparison of Methods to Measure Adsorptive Capacity of Coal Fly Ash. ACI Mater. J. 2019, 116, 107–112. [Google Scholar] [CrossRef]
- Ahuja, V.; Mehta, S.; Rathour, R.K.; Sharma, V.; Rana, N.; Sheetal, S.; Bhatt, A.K.; Kieliszek, M. Elucidation of new xylose metabolizing pathway in Pseudomonas gessardii VXlt-16 and its correlation with xylitol production from sugarcane bagasse hydrolysate. 2021. [Google Scholar] [CrossRef]
- Bae, Y.S.; Yazaydın, A.O.; Snurr, R.Q. Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain ultra-micropores. Langmuir 2010, 26, 5475–5483. [Google Scholar] [CrossRef]
- Katona, R.M.; Tokuda, S.; Perry, J.; Kelly, R.G. Design, construction, and validation for in-situ water layer thickness determination during accelerated corrosion testing. Corros. Sci. 2020, 175, 108849. [Google Scholar] [CrossRef]
- Kuchta, B.; Firlej, L.; Mohammadhosseini, A.; Boulet, P.; Beckner, M.; Romanos, J.; Pfeifer, P. Hypothetical high-surface-area carbons with exceptional hydrogen storage capacities: Open carbon frameworks. J. Am. Chem. Soc. 2012, 134, 15130–15137. [Google Scholar] [CrossRef] [PubMed]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Beyan, S.M.; Ambio, T.A.; Sundramurthy, V.P.; Gomadurai, C.; Getahun, A.A. Adsorption phenomenon for removal of Pb (II) via teff straw based activated carbon prepared by microwave-assisted pyrolysis: Process modelling, statistical optimisation, isotherm, kinetics, and thermodynamic studies. Int. J. Environ. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Qu, J.; Qu, B. Adsorption of Hg (II) in an aqueous solution by activated carbon prepared from rice husk using KOH activation. ACS Omega 2020, 5, 29231–29242. [Google Scholar] [CrossRef]
- Dwiyaniti, M.; Barruna, A.E.; Naufal, R.M.; Subiyanto, I.; Setiabudy, R.; Hudaya, C. Extremely high surface area of activated carbon originated from sugarcane bagasse. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 909, p. 012018. [Google Scholar] [CrossRef]




| BET (m2/g) | IN (m2/g) | Ash (%) | Humidity (%) | |
|---|---|---|---|---|
| Physical properties data | 4320 | 3943 | 0.245 | 11.33 |
| Property | |
|---|---|
| Family | Thiazine (basic dye) |
| Chemical formula | C16H18N3SCl |
| Scientific name (IUPAC) | 3,7-bis-(dimethylamino)phenazathionium |
| Molar mass | 319.85 g/mol |
| Colour point remark number (CI) | 52,011 |
| Maximum absorbance wavelength | 665 nm |
| Chemical structure formula | 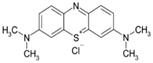 |
| Symbol | MB | 1 | 2 | 3 | H2O |
|---|---|---|---|---|---|
| MB conc. in ppm | 5.579 | 1.344 | 0.307 | 0.184 | 0.042 |
| UV-Abs. | 1.176 | 0.284 | 0.092 | 0.039 | 0.009 |
| Symbol | Wastewater | Mosul East Well | Mosul West Well | Batteries Workshop—Washing Area (Waste) | Natural Spring Water (East Mosul) |
|---|---|---|---|---|---|
| Pb conc. (ppm; before AC treatment) | 6.00 | 0 | 0 | 2.000 | 1.00 |
| Pb conc. (ppm; after AC treatment) | 0.43 | 0 | 0 | 0.153 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.S.; Alsultan, M.; Hameed, R.T.; Assim, Y.F.; Swiegers, G.F. High Surface Area Activated Charcoal for Water Purification. J. Compos. Sci. 2022, 6, 311. https://doi.org/10.3390/jcs6100311
Ahmed AS, Alsultan M, Hameed RT, Assim YF, Swiegers GF. High Surface Area Activated Charcoal for Water Purification. Journal of Composites Science. 2022; 6(10):311. https://doi.org/10.3390/jcs6100311
Chicago/Turabian StyleAhmed, Ahmed. S., Mohammed Alsultan, Rowaa Tareq Hameed, Yamama F. Assim, and Gerhard F. Swiegers. 2022. "High Surface Area Activated Charcoal for Water Purification" Journal of Composites Science 6, no. 10: 311. https://doi.org/10.3390/jcs6100311
APA StyleAhmed, A. S., Alsultan, M., Hameed, R. T., Assim, Y. F., & Swiegers, G. F. (2022). High Surface Area Activated Charcoal for Water Purification. Journal of Composites Science, 6(10), 311. https://doi.org/10.3390/jcs6100311





