Sparsely Cross-Linked Hydrogel with Starch Fragments as a Multifunctional Soil Conditioner
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis and Characterization of the Anionic Cross-Linked Copolymer
3.2. Swelling of the Cross-Linked Copolymer in Different Environments
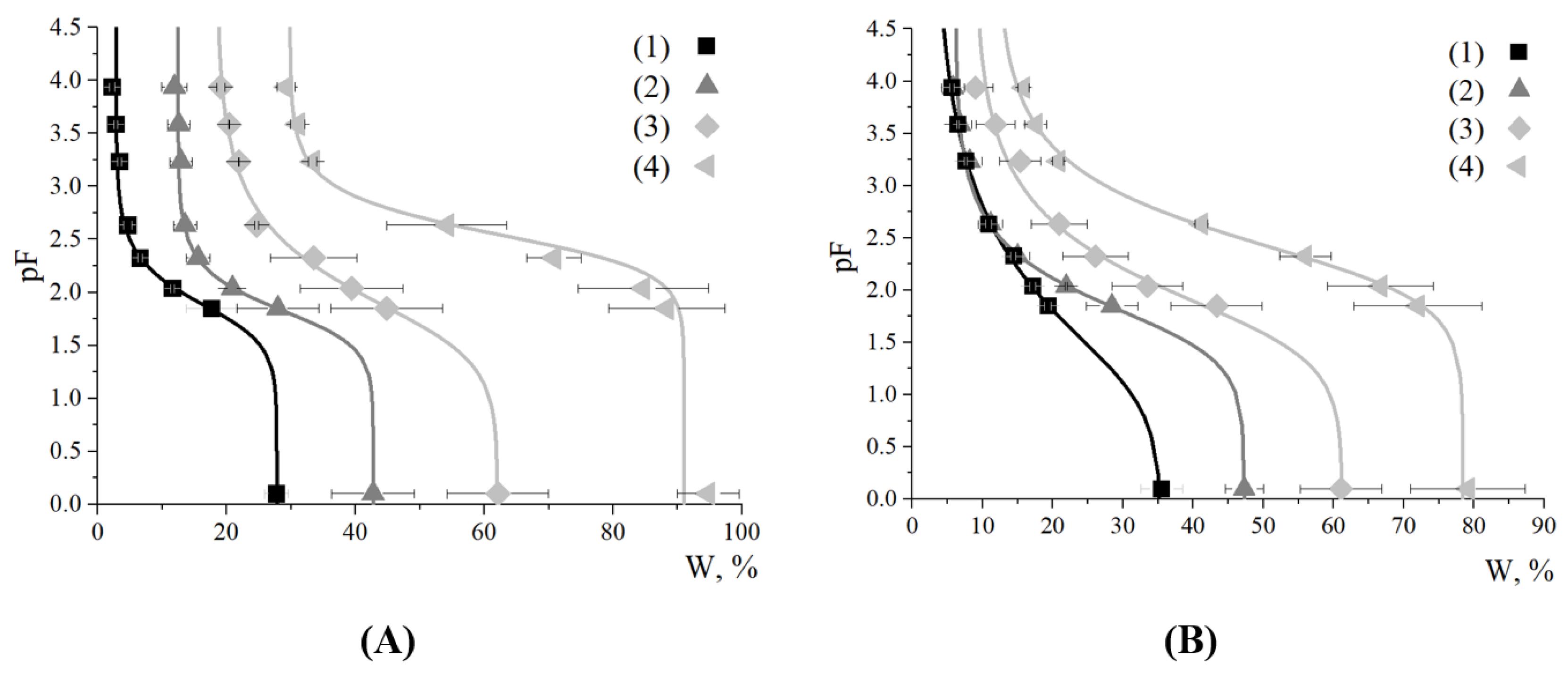
3.3. Effect of the Cross-Linked Copolymer on Water Retention Capacity of Sand and Soil
3.4. Stabilization of Sand and Soil with the Cross-Linked Copolymer
3.5. Biological Testing of Polycomplex Formulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Tian, X.; Fan, H.; Wang, J.; Ippolito, J.; Li, Y.; Feng, S.; An, M.; Zhang, F.; Wang, K. Effect of polymer materials on soil structure and organic carbon under drip irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Yakupoglu, T.; Rodrigo-Comino, J.; Cerdà, A. Potential Benefits of Polymers in Soil Erosion Control for Agronomical Plans: A Laboratory Experiment. Agronomy 2019, 9, 276. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.S.; Kim, K.H.; Ok, Y.S.; Kuzyakov, Y. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: Effects of biochar, oyster shells, and polymers. Chemosphere 2018, 198, 40–48. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, Y. Efficacy of Natural Polymer Derivatives on Soil Physical Properties and Erosion on an Experimental Loess Hillslope. Int. J. Environ. Res. Public Health 2017, 15, 9. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Huang, C.h.; Aliev, F.A.; Levy, G.J. Aggregate Stability and Water Retention Near Saturation Characteristics as Affected by Soil Texture, Aggregate Size and Polyacrylamide Application. Land Degrad. Dev. 2016, 28, 543–552. [Google Scholar] [CrossRef]
- Tingle, J.S.; Newman, J.K.; Larson, S.L.; Weiss, C.A.; Rushing, J.F. Stabilization Mechanisms of Nontraditional Additives. Transp. Res. Rec. J. Transp. Res. Board 2007, 1989, 59–67. [Google Scholar] [CrossRef]
- Zezin, A.B.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv. Colloid Interface Sci. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Bimendina, L.A.; Yashkarova, M.G.; Orazzhanova, L.K.; Kudaibergenov, S.E. Application of Interpolymer Complexes of Novel Poly(ampholyteelectrolyte) as Soil Structuring Agents and for Extraction of Radioactive Strontium. Eurasian Chem.-Technol. J. 2017, 7, 139–145. [Google Scholar] [CrossRef]
- Panova, I.G.; Khaydapova, D.D.; Ilyasov, L.O.; Umarova, A.B.; Yaroslavov, A.A. Polyelectrolyte complexes based on natural macromolecules for chemical sand/soil stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124504. [Google Scholar] [CrossRef]
- Panova, I.G.; Ilyasov, L.O.; Khaidapova, D.D.; Bashina, A.S.; Smagin, A.V.; Ogawa, K.; Adachi, Y.; Yaroslavov, A.A. Soil conditioners based on anionic polymer and anionic micro-sized hydrogel: A comparative study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125635. [Google Scholar] [CrossRef]
- Smagin, A.; Panova, I.; Ilyasov, L.; Ogawa, K.; Adachi, Y.; Yaroslavov, A. Water retention in sandy substrates modified by cross-linked polymeric microgels and their complexes with a linear cationic polymer. J. Appl. Polym. Sci. 2021, 138, 50754. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. Open Access 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent polymers in agriculture and other applications: A review. Polym.-Plast. Technol. Mater. 2019, 59, 341–356. [Google Scholar] [CrossRef]
- El-Asmar, J.; Jaafar, H.; Bashour, I.; Farran, M.T.; Saoud, I.P. Hydrogel Banding Improves Plant Growth, Survival, and Water Use Efficiency in Two Calcareous Soils. CLEAN-Soil Air Water 2017, 45, 1700251. [Google Scholar] [CrossRef]
- Alam, M.N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent Hydrogels That Are Robust and Highly Stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Banedjschafie, S.; Durner, W. Water retention properties of a sandy soil with superabsorbent polymers as affected by aging and water quality. J. Plant Nutr. Soil Sci. 2015, 178, 798–806. [Google Scholar] [CrossRef]
- Yu, J.; Shi, J.G.; Dang, P.F.; Mamedov, A.I.; Shainberg, I.; Levy, G.J. Soil and Polymer Properties Affecting Water Retention by Superabsorbent Polymers under Drying Conditions. Soil Sci. Soc. Am. J. 2012, 76, 1758–1767. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Moia, T.A.; Mattoso, L.H.C.; Tambourgi, E.B. Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J. Appl. Polym. Sci. 2010, 117, 3146–3154. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Xing, S.; Du, Z.; Ma, B.; Jing, D. Effects of super-absorbent polymer on dry matter accumulation and nutrient uptake of Pinus pinaster container seedlings. J. For. Res. 2017, 18, 220–227. [Google Scholar] [CrossRef]
- Urbano-Juan, M.M.; Socías-Viciana, M.M.; Ureña-Amate, M.D. Evaluation of nitrate controlled release systems based on (acrylamide-co-itaconic acid) hydrogels. React. Funct. Polym. 2019, 141, 82–90. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Coello, J.; Ameztegui, A.; Rovira, P.; Fuentes, C.; Piqué, M. Innovative soil conditioners and mulches for forest restoration in semiarid conditions in northeast Spain. Ecol. Eng. 2018, 118, 52–65. [Google Scholar] [CrossRef]
- Wilske, B.; Bai, M.; Lindenstruth, B.; Bach, M.; Rezaie, Z.; Frede, H.G.; Breuer, L. Biodegradability of a polyacrylate superabsorbent in agricultural soil. Environ. Sci. Pollut Res. Int. 2014, 21, 9453–9460. [Google Scholar] [CrossRef]
- Gokmen, V.; Senyuva, H.Z.; Acar, J.; Sarioglu, K. Determination of acrylamide in potato chips and crisps by high-performance liquid chromatography. J. Chromatogr. A 2005, 1088, 193–199. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in Agriculture and Environmental Land Management. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 92, pp. 75–162. [Google Scholar]
- Nikitin, B.A. Specification of Procedure for the Determination of Humus in Soil. Agrokhimiya 1983, 8, 18–26. [Google Scholar]
- Vanchikova, E.V.; Shamrikova, E.V.; Sytar, T.S.; Kazakov, V.G. A new method to determine the carbon content of water-soluble organic compounds in soils. Eurasian Soil Sci. 2006, 39, 1084–1088. [Google Scholar] [CrossRef]
- Voronin, A.D. Energy Concept of the Physical State of Soils. Eurasian Soil Sci. 1990, 23, 7–19. [Google Scholar]
- Khaidapova, D.D.; Pestonova, E.A. Strength of interparticle bonds in soil pastes and aggregates. Eurasian Soil Sci. 2007, 40, 1187–1192. [Google Scholar] [CrossRef]
- Panova, I.G.; Khaidapova, D.D.; Ilyasov, L.O.; Kiushov, A.A.; Umarova, A.B.; Sybachin, A.V.; Yaroslavov, A.A. Polyelectrolyte Complexes of Potassium Humates and Poly(dialyldimethylammonium chloride) for Fixing Sand Soil. Polym. Sci. Ser. B 2020, 61, 698–703. [Google Scholar] [CrossRef]
- Evans, C.G.T.; Herbert, D.; Tempest, D.W. The Continuous Cultivation of Micro-organisms: 2. Construction of a Chemostat. Methods Microbiol. 1970, 2, 277–327. [Google Scholar] [CrossRef]
- Belov, A.A.; Cheptsov, V.S.; Manucharova, N.A.; Ezhelev, Z.S. Bacterial Communities of Novaya Zemlya Archipelago Ice and Permafrost. Geosciences 2020, 10, 67. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Selemenev, V.F.; Semenov, V.N.; Bakalova, M.V. Method for Obtaining Hydrophilic Cross-Linked Polymer with Superabsorbent Properties. RU 2574722 C1, 10 February 2016. [Google Scholar]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Smith, A.L. Applied Infrared Spectroscopy: Fundamentals, Techniques, and Analytical Problem-Solving; Wiley: New York, NY, USA, 1979; p. 336. [Google Scholar]
- van Genuchten, M.T. A Closed-form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Shein, E.V.; Karpachevskii, L.O. Theory and Methods of Soil Physics; Grif i K: Moscow, Russia, 2007; p. 614. [Google Scholar]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry; Elsevier: London, UK, 2015; p. 604. [Google Scholar]






| Size, μm | 0–1 | 1–2 | 2–5 | 5–10 | 10–50 | 50–100 | 100–250 | 250–1000 |
| Content, % | 0.7± 0.03 | 1.4 ± 0.6 | 2.9 ± 0.14 | 1.8 ± 0.12 | 7.5 ± 0.3 | 2.7 ± 0.14 | 37 ± 2.5 | 46 ± 2 |
| Parameter | ACP#, wt.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Sand | Soil | |||||||
| 0 | 0.2 | 0.5 | 1 | 0 | 0.2 | 0.5 | 1 | |
| Wmax | 27 | 43 | 62 | 91 | 35 | 47 | 61 | 79 |
| FWC | 4 | 16 | 29 | 52 | 12 | 15 | 25 | 43 |
| WP | 1 | 12 | 19 | 30 | 3 | 6 | 10 | 14 |
| AWR = FWC − WP | 3 | 4 | 10 | 21 | 9 | 9 | 15 | 29 |
| 1 | MO | Bacteria | Yeasts | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus subtilis | Pseudomonas putida | Saitozyma podzolica | Lipomyces lipofer | Candida albicans | ||||||||||||
| 2 | Repeat | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| 3 | Survival/growth | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyasov, L.O.; Panova, I.G.; Kushchev, P.O.; Belov, A.A.; Maksimova, I.A.; Smagin, A.V.; Yaroslavov, A.A. Sparsely Cross-Linked Hydrogel with Starch Fragments as a Multifunctional Soil Conditioner. J. Compos. Sci. 2022, 6, 347. https://doi.org/10.3390/jcs6110347
Ilyasov LO, Panova IG, Kushchev PO, Belov AA, Maksimova IA, Smagin AV, Yaroslavov AA. Sparsely Cross-Linked Hydrogel with Starch Fragments as a Multifunctional Soil Conditioner. Journal of Composites Science. 2022; 6(11):347. https://doi.org/10.3390/jcs6110347
Chicago/Turabian StyleIlyasov, Leonid O., Irina G. Panova, Petr O. Kushchev, Andrey A. Belov, Irina A. Maksimova, Andrey V. Smagin, and Alexander A. Yaroslavov. 2022. "Sparsely Cross-Linked Hydrogel with Starch Fragments as a Multifunctional Soil Conditioner" Journal of Composites Science 6, no. 11: 347. https://doi.org/10.3390/jcs6110347
APA StyleIlyasov, L. O., Panova, I. G., Kushchev, P. O., Belov, A. A., Maksimova, I. A., Smagin, A. V., & Yaroslavov, A. A. (2022). Sparsely Cross-Linked Hydrogel with Starch Fragments as a Multifunctional Soil Conditioner. Journal of Composites Science, 6(11), 347. https://doi.org/10.3390/jcs6110347







