Bioactive Calcium Phosphate-Based Composites for Bone Regeneration
Abstract
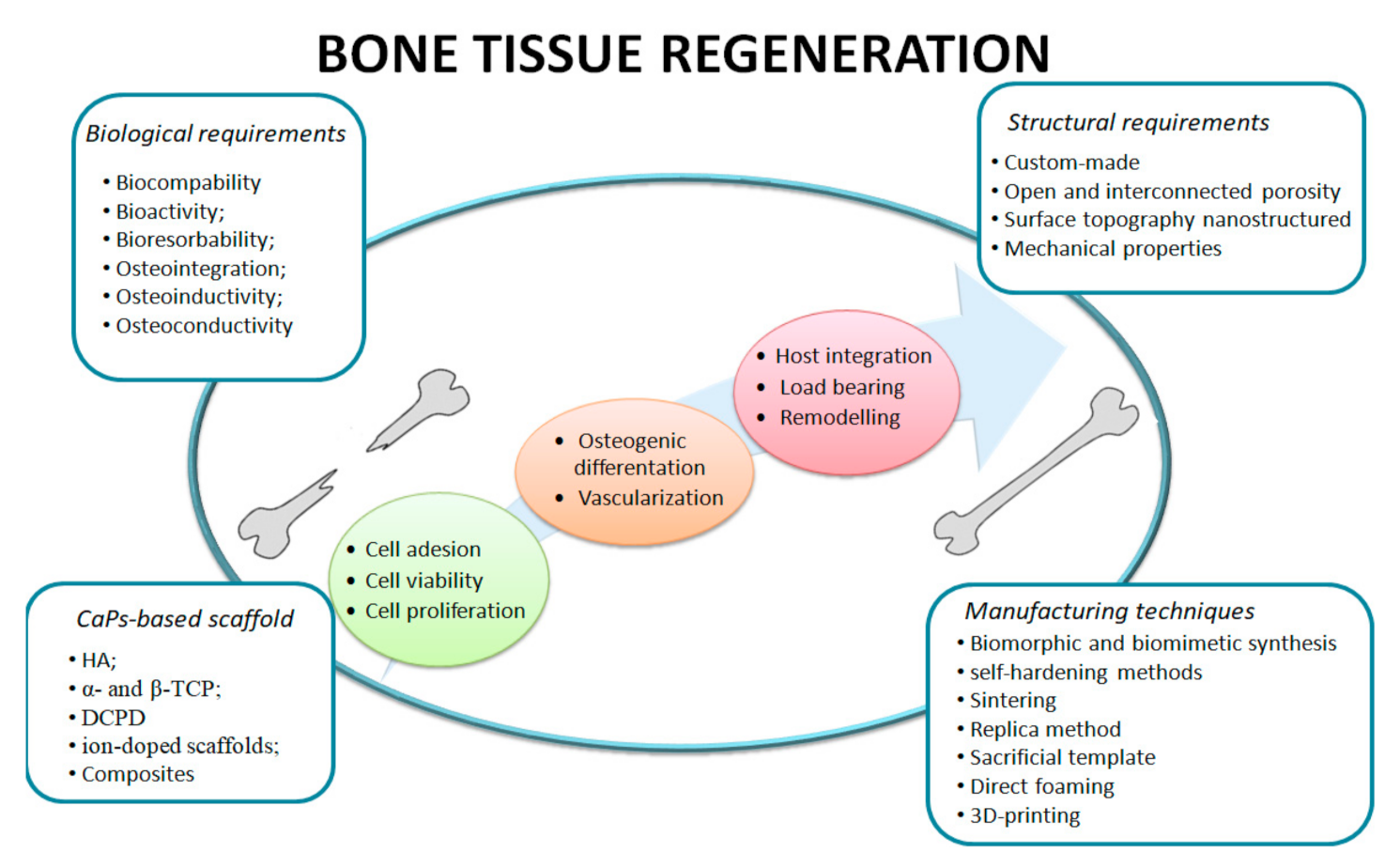
:1. Introduction
- Macrostructure: cancellous and cortical bone;
- Microstructure: (10–500 mm): Haversian channel, osteons and single trabeculae;
- Sub-microstructure (1–10 mm): lamellae;
- Nanostructure (100 nm–1 mm): fibrillar collagen and embedded mineral;
- Sub-nanostructures (<100 nm): molecular structure of constituent elements, such as minerals, collagen and non-collagenous proteins [22].
- The rapid exchange of Ca2+ with H+ or H3O+ from a body fluid solution results in the hydrolysis of silica groups, which creates silanol, according to Si-O-Ca+ +H+→Si-OH+Ca2+(aq).
- The loss of soluble silica in the form of Si(OH)4 to the body fluid, resulting from the breaking of Si-O-Si bonds and the formation of silanol (Si-OH) at the glass solution interface: Si-O-Si + H2O → 2Si-OH.
- The condensation and polymerization of a SiO2-rich layer on the surface short in alkalis and alkaline earth cations: Si-OH+HO-Si → Si-O-Si+H2O.
- The migration of Ca2+ and PO43− groups to the surface via the SiO2-rich layer forming a CaO-P2O5-rich film by the incorporation of soluble calcium and phosphates from the solution.
- The crystallization of the amorphous CaO-P2O5-rich film by the addition of OH− and CO32− anions from body fluid forms a mixed hydroxyl, carbonated apatite layer.
- The adsorption and desorption of biological growth factors on the carbonated apatite layer to activate stem cells.
- The action of macrophages to remove debris from the site allowing cells to occupy their space.
- The attachment of stem cells to the bioactive surface and its differentiation to form osteoblasts.
- The generation of ECM by the osteoblast to form new bone and its crystallization in the living composite structure.
2. Fabrication of Bioceramic Composites
2.1. Macroporous Compositescaffolds
2.2. Self-Hardening Bioceramic Composites
- -
- Hydrolysis of metastable α-TCP:
- -
- Acid–base reaction between tetra calcium phosphate, TTCP (basic), and di calcium phosphate anhydrous, DCPA (acidic):
2.3. Biomorphic Transformations
2.4. 3D Printing
3. Enhancing the Biological Performance of Bioceramic Composites
3.1. Biofunctionalization
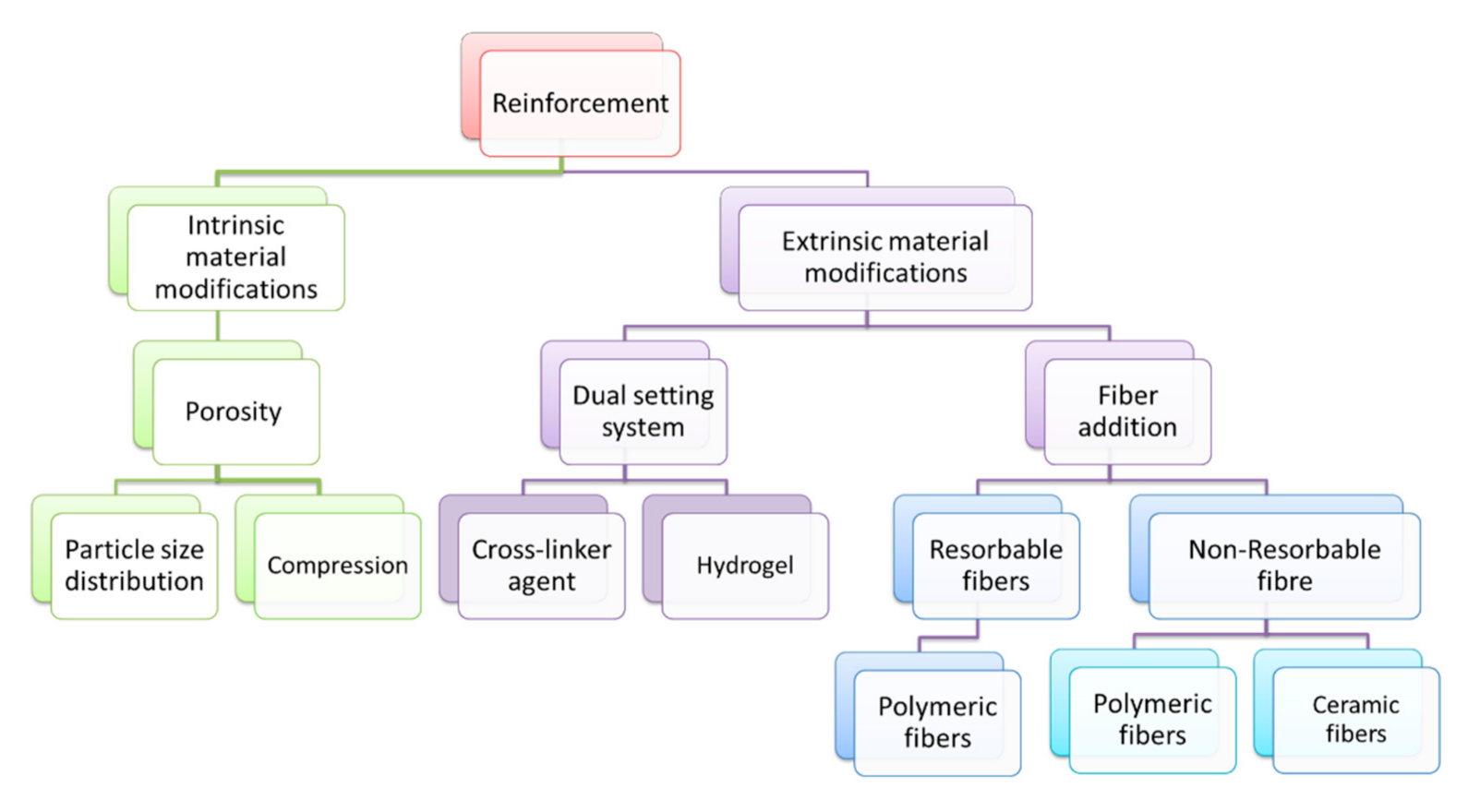
3.2. Enhancing the Mechanical Performance of Bioceramics Composites
3.2.1. Intrinsic Material Modifications
3.2.2. Extrinsic Material Modifications
- Fiber bridging: the fibers bridge the existing crack, limiting its opening and propagation;
- Crack deflection: the fibers increase the length of the crack propagation, requiring more energy in newly formed surfaces;
- Frictional sliding: the presence of intergranular fibers in the matrix increases the fracture resistance of the composite.
4. Ion-Doped Bioceramics and Composite Scaffolds
4.1. Ion Doping
4.1.1. Magnesium
4.1.2. Strontium
4.1.3. Silicon
4.1.4. Silver
4.1.5. Iron
4.1.6. Fluorine
4.2. Composites with Silicates
4.3. Composites with Carbon
4.4. Composites with Titanates
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bean, A.C. Basic Science Concepts in Musculoskeletal Regenerative Medicine. In Regenerative Medicine for Spine and Joint Pain; Springer: Berlin/Heidelberg, Germany, 2020; pp. 5–27. [Google Scholar] [CrossRef]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Healthc. Mater. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeldt, D.; Rubin, C. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 2001, 10, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.R.; Hatt, L.P.; Sanchez Rosenberg, G.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 1909874, 1–41. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Herrera, J.; Kirkbride, J.; Perlman, Z. Introduction to Regenerative Medicine. In Regenerative Medicine for Spine and Joint Pain; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Vaish, A.; Murrell, W.; Vaishya, R. History of regenerative medicine in the field of orthopedics. J. Arthrosc. Surg. Sports Med. 2020, 1, 154–158. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jodati, H.; Bengi, Y.; Evis, Z. A review of bioceramic porous sca ff olds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Samuel, F.; Rui, S.; Reis, L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielty, C.M.; Grant, M.E. The Collagen Family: Structure, Assembly, and Organization in the Extracellular Matrix. In Connective Tissue and Its Heritable Disorders; Wiley: Hoboken, NJ, USA, 2003; pp. 159–221. [Google Scholar] [CrossRef]
- No, Y.J.; Holzmeister, I.; Lu, Z.; Prajapati, S.; Shi, J.; Gbureck, U.; Zreiqat, H. Effect of baghdadite substitution on the physicochemical properties of brushite cements. Materials 2019, 12, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampieri, A.; Iafisco, M.; Sprio, S.; Ruffini, A.; Panseri, S.; Montesi, M.; Adamiano, A.; Sandri, M. Hydroxyapatite: From Nanocrystals to Hybrid Nanocomposites for Regenerative Medicine. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 119–144. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Filardo, G.; Kon, E.; Marcacci, M.; Tampieri, A. Composite biomedical foams for engineering bone tissue. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Sawston, UK, 2014; pp. 249–280. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Sprio, S.; Ruffini, A.; Valentini, F.; D’Alessandro, T.; Sandri, M.; Panseri, S.; Tampieri, A. Biomimesis and biomorphic transformations: New concepts applied to bone regeneration. J. Biotechnol. 2011, 156, 347–355. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nature 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Kang, J.; Dong, E.; Li, D.; Dong, S.; Zhang, C.; Wang, L. Anisotropy characteristics of microstructures for bone substitutes and porous implants with application of additive manufacturing in orthopaedic. Mater. Des. 2020, 191, 108608. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Burdușel, A.-C. Bioactive composites for bone regeneration. Biomed. Eng. Int. 2019, 1, 9–15. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 1909539, 1–28. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration A review of ceramics and polymers. BioMatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001987259. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, T. Bioceramics. In Biomechanics and Biomaterials in Orthopedics; Springer: Berlin/Heidelberg, Germany, 2004; pp. 22–33. [Google Scholar] [CrossRef]
- Gul, H.; Khan, M.; Khan, A.S. 3—Bioceramics: Types and clinical applications. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–83. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Joiner, A.; Chang, J. The remineralisation of enamel: A review of the literature. J. Dent. 2014, 42, S12–S20. [Google Scholar] [CrossRef]
- Doremus, R.H. Review Bioceramics. J. Mater. Sci. 1992, 27, 285–297. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Kucko, N.W.; Herber, R.-P.; Leeuwenburgh, S.C.G.; Jansen, J.A. Calcium Phosphate Bioceramics and Cements. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 591–611. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and bone healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.E.; Livingston Arinzeh, T. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Maji, K.; Mondal, S. Calcium Phosphate Biomaterials for Bone Tissue Engineering: Properties and Relevance in Bone Repair. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 535–555. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements for enhanced bone tissue regeneration. Mater. Sci. Eng. C 2014, 43, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ferreira, J.M.F. Brushite-forming Mg-, Zn- and Sr-substituted bone cements for clinical applications. Materials 2010, 3, 519–535. [Google Scholar] [CrossRef] [Green Version]
- Theiss, F.; Apelt, D.; Brand, B.; Kutter, A.; Zlinszky, K.; Bohner, M.; Matter, S.; Frei, C.; Auer, J.A.; Von Rechenberg, B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Novel bioactive composite bone cements based on the β-tricalcium phosphate-monocalcium phosphate monohydrate composite cement system. Acta Biomater. 2009, 5, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Sepantafar, M.; Mohammadi, H.; Maheronnaghsh, R.; Tayebi, L.; Baharvand, H. Single phased silicate-containing calcium phosphate bioceramics: Promising biomaterials for periodontal repair. Ceram. Int. 2018, 44, 11003–11012. [Google Scholar] [CrossRef]
- Jablonská, E.; Horkavcová, D.; Rohanová, D.; Brauer, D.S. A review of: In vitro cell culture testing methods for bioactive glasses and other biomaterials for hard tissue regeneration. J. Mater. Chem. B 2020, 8, 10941–10953. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Gremillard, L.; Chevalier, J.; Chazeau, L.; Gauthier, C.; Hamaide, T. Toughening of bio-ceramics scaffolds by polymer coating. J. Eur. Ceram. Soc. 2007, 27, 2679–2685. [Google Scholar] [CrossRef]
- Steinbrech, R.W. Toughening mechanisms for ceramic materials. J. Eur. Ceram. Soc. 1992, 10, 131–142. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Eom, J.H.; Kim, Y.W.; Raju, S. Processing and properties of macroporous silicon carbide ceramics: A review. J. Asian Ceram. Soc. 2013, 1, 220–242. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.J.; Park, J.G.; Han, Y.H.; Kim, S.Y.; Shackelford, J.F. Wet foam stability from colloidal suspension to porous ceramics: A review. J. Korean Ceram. Soc. 2019, 56, 211–232. [Google Scholar] [CrossRef] [Green Version]
- Ohji, T.; Fukushima, M. Macro-porous ceramics: Processing and properties. Int. Mater. Rev. 2012, 57, 115–131. [Google Scholar] [CrossRef]
- Dapporto, M.; Sprio, S.; Fabbi, C.; Figallo, E.; Tampieri, A. A novel route for the synthesis of macroporous bioceramics for bone regeneration. J. Eur. Ceram. Soc. 2016, 36, 2383–2388. [Google Scholar] [CrossRef]
- Deville, S. Freeze-casting of porous ceramics: A review of current achievements and issues. Adv. Eng. Mater. 2008, 10, 155–169. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P. Cements as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 271–308. [Google Scholar] [CrossRef]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 456–468. [Google Scholar] [CrossRef]
- Şahin, E.; Kalyon, D.M. The rheological behavior of a fast-setting calcium phosphate bone cement and its dependence on deformation conditions. J. Mech. Behav. Biomed. Mater. 2017, 72, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Canal, C.; Ginebra, M.P. Fibre-reinforced calcium phosphate cements: A review. J. Mech. Behav. Biomed. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E. Functionalized biomimetic calcium phosphates for bone tissue repair. J. Appl. Biomater. Funct. Mater. 2017, 15, e313–e325. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, M.; Gardini, D.; Tampieri, A.; Sprio, S. Nanostructured Strontium-Doped Calcium Phosphate Cements: A Multifactorial Design. Appl. Sci. 2021, 11, 2075. [Google Scholar] [CrossRef]
- International Organization for Standardization. Implants for Surgery—Calcium Phosphate Bioceramics—Characterization of Hardening Bone Paste Materials—ISO/DIS 18531(en); ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Baino, F.; Ferraris, M. Learning from Nature: Using bioinspired approaches and natural materials to make porous bioceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 507–520. [Google Scholar] [CrossRef]
- Sprio, S.; Panseri, S.; Montesi, M.; Dapporto, M.; Ruffini, A.; Dozio, S.M.; Cavuoto, R.; Misseroni, D.; Paggi, M.; Bigoni, D.; et al. Hierarchical porosity inherited by natural sources affects the mechanical and biological behaviour of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 1717–1727. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Ruffini, A.; Celotti, G.; Lesci, I.G.; Roveri, N. From wood to bone: Multi-step process to convert wood hierarchical structures into biomimetic hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Chem. 2009, 19, 4973–4980. [Google Scholar] [CrossRef]
- Lucas-Aparicio, J.; Manchón, Á.; Rueda, C.; Pintado, C.; Torres, J.; Alkhraisat, M.H.; López-Cabarcos, E. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: Substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. Mater. Sci. Eng. C 2020, 106, 110173. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Adamiano, A.; Montesi, M.; Campodoni, E.; Tampieri, A. Bio-inspired assembling/mineralization process as a flexible approach to develop new smart scaffolds for the regeneration of complex anatomical regions. J. Eur. Ceram. Soc. 2016, 36, 2857–2867. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [Green Version]
- White, R.A.; Weber, J.N.; White, E.W. Replamineform: A new process for preparing porous ceramic, metal, and polymer prosthetic materials. Science 1972, 176, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Ruffini, A.; Ballardini, A.; Montesi, M.; Panseri, S.; Salamanna, F.; Fini, M.; Sprio, S. Heterogeneous chemistry in the 3-D state: An original approach to generate bioactive, mechanically-competent bone scaffolds. Biomater. Sci. 2019, 7, 307–321. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Hart, L.R.; He, Y.; Ruiz-Cantu, L.; Zhou, Z.; Irvine, D.; Wildman, R.; Hayes, W. 3D and 4D printing of biomaterials and biocomposites, bioinspired composites, and related transformers. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Elsevier: Amsterdam, The Netherlands, 2019; pp. 467–504. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Yue, P.; Qi, H.; Liu, J.; Li, L.; Guo, C.; Xie, H.; Zhou, X.; Yu, X. A novel CPC composite cement reinforced by dopamine coated SCPP fibers with improved physicochemical and biological properties. Mater. Sci. Eng. C 2020, 109. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Ghorbani, F.; Li, D.; Ni, S.; Zhou, Y.; Yu, B. 3D printing of acellular scaffolds for bone defect regeneration: A review. Mater. Today Commun. 2020, 22, 100979. [Google Scholar] [CrossRef]
- Sinha, S.K. Additive manufacturing (AM) of medical devices and scaffolds for tissue engineering based on 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–160. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. International J. Oral Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tarafder, S.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 679–690. [Google Scholar] [CrossRef]
- Touri, M.; Moztarzadeh, F.; Abu Osman, N.A.; Dehghan, M.M.; Brouki Milan, P.; Farzad-Mohajeri, S.; Mozafari, M. Oxygen-Releasing Scaffolds for Accelerated Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2985–2994. [Google Scholar] [CrossRef]
- Sun, H.; Hu, C.; Zhou, C.; Wu, L.; Sun, J.; Zhou, X.; Xing, F.; Long, C.; Kong, Q.; Liang, J.; et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 108540. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement strategies for load-bearing calcium phosphate biocements. Materials 2015, 8, 2700–2717. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.H.K.; Quinn, J.B.; Takagi, S.; Chow, L.C.; Eichmiller, F.C. Strong and macroporous calcium phosphate cement: Effects of porosity and fiber reinforcement on mechanical properties. J. Biomed. Mater. Res. 2001, 57, 457–466. [Google Scholar] [CrossRef]
- Boehm, A.V.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F.A. The mechanical properties of biocompatible apatite bone cement reinforced with chemically activated carbon fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef] [Green Version]
- Holthaus, M.G.; Stolle, J.; Treccani, L.; Rezwan, K. Orientation of human osteoblasts on hydroxyapatite-based microchannels. Acta Biomater. 2012, 8, 394–403. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; He, Z.; Han, F.; Shi, C.; Zhou, P.; Ling, F.; Zhu, X.; Yang, H.; Li, B. Reinforcement of calcium phosphate cement using alkaline-treated silk fibroin. Int. J. Nanomed. 2018, 13, 7183–7193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, F.; Wolke, J.G.C.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Sitasuwan, P.; Feng, S.; Wang, Q. Effect of Roughness on in Situ Biomineralized CaP-Collagen Coating on the Osteogenesis of Mesenchymal Stem Cells. Langmuir 2016, 32, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Tan, Z.; Liu, Y.; Tao, J.; Cai, Y.; Zhang, M.; Pan, H.; Xu, X.; Tang, R. Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. J. Mater. Chem. 2007, 17, 4690–4698. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Mirström, M.M.; Li, D. Cellulose-reinforced bioglass composite as flexible bioactive bandage to enhance bone healing. Ceram. Int. 2021, 47, 416–423. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; De Oliveira, L.C.; da Silva Rigo, E.C.; Carrodéguas, R.G.; Boschi, A.O.; Fonseca de Arruda, A.C. Fiber Reinforced Calcium Phosphate Cement. J. Mech. Behav. Biomed. Mater. 2000, 4, 1658–1671. [Google Scholar] [CrossRef] [Green Version]
- Sprio, S.; Ruffini, A.; Dapporto, M.; Tampieri, A. New biomimetic strategies for regeneration of load-bearing bones. In Bio-Inspired Regenerative Medicine: Materials, Processes and Clinical Applications; Pan Stanford Publishing: Singapore, 2016; Volume 6, pp. 85–117. [Google Scholar]
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetti, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.; Yin, Y. C-fibre reinforced hydroxyapatite bioceramics. Ceram. Int. 2003, 29, 113–116. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Pei, Z.; Liu, P.; Chang, J.; Zhang, K.; Zhao, S.; Zhang, K.; Jiang, N.; Liang, G. Basalt fibre reinforced calcium phosphate cement with enhanced toughness. Mater. Technol. 2020, 35, 152–158. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, J.; Zhang, W.; Chen, X.; Gui, Z. Preparation of silicon coated-carbon fiber reinforced HA bio-ceramics for application of load-bearing bone. Ceram. Int. 2020, 46, 7903–7911. [Google Scholar] [CrossRef]
- Dorner-Reisel, A.; Müller, E.; Tomandl, G. Short fiber reinforced hydroxyapatite-based bioceramics. Adv. Eng. Mater. 2004, 6, 572–577. [Google Scholar] [CrossRef]
- Krüger, R.; Groll, J. Fiber reinforced calcium phosphate cements e On the way to degradable load bearing bone substitutes? Biomaterials 2012, 33, 5887–5900. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.A.; Motisuke, M.; Bertran, C.A.; Hausen, M.A.; De Rezende Duek, E.A.; Camilli, J.A. Addition of Wollastonite Fibers to Calcium Phosphate Cement Increases Cell Viability and Stimulates Differentiation of Osteoblast-Like Cells. Sci. World J. 2017, 2017, 5260106. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S.; Mozafari, M. Synergistically reinforcement of a self-setting calcium phosphate cement with bioactive glass fibers. Ceram. Int. 2011, 37, 927–934. [Google Scholar] [CrossRef]
- Petersen, R. Carbon fiber biocompatibility for implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A review on the use of hydroxyapatite- carbonaceous structure composites in bone replacement materials for strengthening purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [Green Version]
- Motisuke, M.; Santos, V.R.; Bazanini, N.C.; Bertran, C.A. Apatite bone cement reinforced with calcium silicate fibers. J. Mater. Sci. Mater. Med. 2014, 25, 2357–2363. [Google Scholar] [CrossRef]
- Müller, F.A.; Gbureck, U.; Kasuga, T.; Mizutani, Y.; Barralet, J.E.; Lohbauer, U. Whisker-reinforced calcium phosphate cements. J. Am. Ceram. Soc. 2007, 90, 3694–3697. [Google Scholar] [CrossRef]
- Iannotti, V.; Adamiano, A.; Ausanio, G.; Lanotte, L.; Aquilanti, G.; Coey, J.M.D.; Lantieri, M.; Spina, G.; Fittipaldi, M.; Margaris, G.; et al. Fe-Doping-Induced Magnetism in Nano-Hydroxyapatites. Inorg. Chem. 2017, 56, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, M.; Corsi, A.; Francioso, E.; Di Comite, M.; Monetti, F.; Scaglione, S.; Favia, A.; Crovace, A.; Bianco, P.; Cancedda, R. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 2006, 12, 1261–1273. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Synthesis and spectral characterization of silver/magnesium co-substituted hydroxyapatite for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic magnesium-carbonate-apatite nanocrystals endowed with strontium ions as anti-osteoporotic trigger. Mater. Sci. Eng. C 2014, 35, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Teo, E.Y.; Ho, B.; Tay, B.Y.; Thian, E.S. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2013, 101 A, 2456–2464. [Google Scholar] [CrossRef]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C 2015, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jadalannagari, S.; Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Antimicrobial activity of hemocompatible silver doped hydroxyapatite nanoparticles synthesized by modified sol–gel technique. Appl. Nanosci. 2014, 4, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface Phenomena Enhancing the Antibacterial and Osteogenic Ability of Nanocrystalline Hydroxyapatite, Activated by Multiple-Ion Doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959. [Google Scholar] [CrossRef] [PubMed]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstić, A.; Jovanović, D.; Raičević, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowders for bone tissue engineering. Appl. Surf. Science 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Ge, X.; Leng, Y.; Bao, C.; Xu, S.L.; Wang, R.; Ren, F. Antibacterial coatings of fluoridated hydroxyapatite for percutaneous implants. J. Biomed. Mater. Res. Part A 2010, 95 A, 588–599. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2010, 6, 928–935. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Markovic, S.; Ignjatovic, N. Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: A novel route to produce hydroxyapatite bioceramics. J. Mater. Chem. B 2021, 10. [Google Scholar] [CrossRef]
- Yin, X.; Bai, Y.; Zhou, S.j.; Ma, W.; Bai, X.; Chen, W.d. Solubility, Mechanical and Biological Properties of Fluoridated Hydroxyapatite/Calcium Silicate Gradient Coatings for Orthopedic and Dental Applications. J. Therm. Spray Technol. 2020, 29, 471–488. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef]
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal Growth of Calcium Phosphates in the Presence of Magnesium Ions. Langmuir 1985, 1, 119–122. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Harris, W.G.; Josan, M.S.; Nair, V.D. Inhibition of calcium phosphate precipitation under environmentally-relevant conditions. Sci. Total Environ. 2007, 383, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Diallo-Garcia, S.; Laurencin, D.; Krafft, J.M.; Casale, S.; Smith, M.E.; Lauron-Pernot, H.; Costentin, G. Influence of magnesium substitution on the basic properties of hydroxyapatites. J. Phys. Chem. C 2011, 115, 24317–24327. [Google Scholar] [CrossRef]
- Bianchi, M.; Degli Esposti, L.; Ballardini, A.; Liscio, F.; Berni, M.; Gambardella, A.; Leeuwenburgh, S.C.G.; Sprio, S.; Tampieri, A.; Iafisco, M. Strontium doped calcium phosphate coatings on poly(etheretherketone) (PEEK) by pulsed electron deposition. Surf. Coat. Technol. 2017, 319, 191–199. [Google Scholar] [CrossRef]
- Guo, D.; Xu, K.; Zhao, X.; Han, Y. Development of a strontium-containing hydroxyapatite bone cement. Biomaterials 2005, 26, 4073–4083. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Dapporto, M.; Tampieri, A.; Sprio, S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS ONE 2017, 12, e0172100. [Google Scholar] [CrossRef] [Green Version]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel Osteointegrative Sr-Substituted Apatitic Cements Enriched with Alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef]
- Biris, A.R.; Mahmood, M.; Lazar, M.D.; Dervishi, E.; Watanabe, F.; Mustafa, T.; Baciut, G.; Baciut, M.; Bran, S.; Ali, S.; et al. Novel multicomponent and biocompatible nanocomposite materials based on few-layer graphenes synthesized on a gold/hydroxyapatite catalytic system with applications in bone regeneration. J. Phys. Chem. C 2011, 115, 18967–18976. [Google Scholar] [CrossRef]
- Liang, W.; Gao, M.; Lou, J.; Bai, Y.; Zhang, J.; Lu, T.; Sun, X.; Ye, J.; Li, B.; Sun, L.; et al. Integrating silicon/zinc dual elements with PLGA microspheres in calcium phosphate cement scaffolds synergistically enhances bone regeneration. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Spark plasma sintered hydroxyapatite/graphite nanosheet and hydroxyapatite/multiwalled carbon nanotube composites: Mechanical and in vitro cellular properties. Adv. Eng. Mater. 2011, 13, 336–341. [Google Scholar] [CrossRef]
- Dong, G.; Zheng, Y.; He, L.; Wu, G.; Deng, C. The effect of silicon doping on the transformation of amorphous calcium phosphate to silicon-substituted α-tricalcium phosphate by heat treatment. Ceram. Int. 2016, 42, 883–890. [Google Scholar] [CrossRef]
- Mestres, G.; Le Van, C.; Ginebra, M.P. Silicon-stabilized α-tricalcium phosphate and its use in a calcium phosphate cement: Characterization and cell response. Acta Biomater. 2012, 8, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Manchón, A.; Hamdan Alkhraisat, M.; Rueda-Rodriguez, C.; Prados-Frutos, J.C.; Torres, J.; Lucas-Aparicio, J.; Ewald, A.; Gbureck, U.; López-Cabarcos, E. A new iron calcium phosphate material to improve the osteoconductive properties of a biodegradable ceramic: A study in rabbit calvaria. Biomed. Mater. 2015, 10, 055012. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Patel, K.D.; Kim, H.W. Novel magnetic nanocomposite injectables: Calcium phosphate cements impregnated with ultrafine magnetic nanoparticles for bone regeneration. RSC Adv. 2015, 5, 13411–13419. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Y.; Gao, W.; Xu, J.; Zuo, G.; Chen, Y.; Zhao, M.; Li, J.; Song, J.; Zhang, N.; et al. Magnetic Hyperthermia Ablation of Tumors Using Injectable Fe3O4/Calcium Phosphate Cement. ACS Appl. Mater. Interfaces 2015, 7, 13866–13875. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like cell behaviour. J. Nanobiotechnol. 2012, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramirez-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivanchenko, P.; Martra, G.; Lin, F.H.; Delgado-López, J.M.; et al. Combined Effect of Citrate and Fluoride Ions on Hydroxyapatite Nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Guo, H.; Wei, J.; Yuan, Y.; Liu, C. Development of calcium silicate/calcium phosphate cement for bone regeneration. Biomed. Mater. 2007, 2, S153–S159. [Google Scholar] [CrossRef]
- Sprio, S.; Tampieri, A.; Celotti, G.; Landi, E. Development of hydroxyapatite/calcium silicate composites addressed to the design of load-bearing bone scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hesaraki, S.; Alizadeh, M.; Borhan, S.; Pourbaghi-Masouleh, M. Polymerizable nanoparticulate silica-reinforced calcium phosphate bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1627–1635. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hesaraki, S.; Hafezi-Ardakani, M. Investigation of biocompatible nanosized materials for development of strong calcium phosphate bone cement: Comparison of nano-titania, nano-silicon carbide and amorphous nano-silica. Ceram. Int. 2014, 40, 8377–8387. [Google Scholar] [CrossRef]
- Golzar, H.; Mohammadrezaei, D.; Yadegari, A.; Rasoulianboroujeni, M.; Hashemi, M.; Omidi, M.; Yazdian, F.; Shalbaf, M.; Tayebi, L. Incorporation of functionalized reduced graphene oxide/magnesium nanohybrid to enhance the osteoinductivity capability of 3D printed calcium phosphate-based scaffolds. Compos. Part B Eng. 2020, 185, 107749. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan–hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci. Mater. Med. 2016, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Wang, Y.; Sun, X.; Sun, K. Reduced graphene oxide/carbon nanotubes reinforced calcium phosphate cement. Ceram. Int. 2017, 43, 13083–13088. [Google Scholar] [CrossRef]
- Medvecky, L.; Stulajterova, R.; Giretova, M.; Sopcak, T.; Faberova, M. Properties of CaO-SiO2-P2O5 reinforced calcium phosphate cements and in vitro osteoblast response. Biomed. Mater. 2017, 12, aa5b3b. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Zhao, K.; Tang, Y.; Cheng, Z.; Chen, J.; Zang, Y.; Wu, J.; Kong, L.; Liu, S.; et al. A Novel Injectable Calcium Phosphate Cement-Bioactive Glass Composite for Bone Regeneration. PLoS ONE 2013, 8, e62570. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, J.L.; Rueda, C.; Manchón, Á.; Ewald, A.; Gbureck, U.; Alkhraisat, M.H.; Jerez, L.B.; Cabarcos, E.L. Effect of physicochemical properties of a cement based on silicocarnotite/calcium silicate on in vitro cell adhesion and in vivo cement degradation. Biomed. Mater. 2016, 11, 045005. [Google Scholar] [CrossRef]
- Sopcak, T.; Medvecky, L.; Giretova, M.; Stulajterova, R.; Molcanova, Z.; Podobova, M.; Girman, V. Physical, mechanical and in vitro evaluation of a novel cement based on akermantite and dicalcium phosphate dihydrate phase. Biomed. Mater. 2019, 14, ab216d. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 201909045. [Google Scholar] [CrossRef] [Green Version]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D printing of piezoelectric barium titanate-hydroxyapatite scaffiolds with interconnected porosity for bone tissue engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Tanaka, M.; Aoki, K.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Okamoto, M.; Kato, H.; Saito, N. Applications of carbon nanotubes in bone regenerative medicine. Nanomaterials 2020, 10, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabás, R.; de Souza Ávila, E.; Ladeira, L.O.; Antônio, L.M.; Tötös, R.; Simedru, D.; Bizo, L.; Cadar, O. Graphene Oxides/Carbon Nanotubes–Hydroxyapatite Nanocomposites for Biomedical Applications. Arab. J. Sci. Eng. 2020, 45, 219–227. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Gao, R.; Bajpai, I.; Kim, B.N.; Yoshida, H.; Nieto, A.; Son, H.W.; Yun, J.; Jang, B.K.; Jhung, S.; et al. Spark plasma sintered bioceramics–from transparent hydroxyapatite to graphene nanocomposites: A review. Adv. Appl. Ceram. 2020, 119, 57–74. [Google Scholar] [CrossRef]
- Jakus, A.E.; Shah, R.N. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 274–283. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Mata, D.; Oliveira, F.J.; Neto, M.A.; Belmonte, M.; Bastos, A.C.; Lopes, M.A.; Gomes, P.S.; Fernandes, M.H.; Silva, R.F. Smart electroconductive bioactive ceramics to promote in situ electrostimulation of bone. J. Mater. Chem. B 2015, 3, 1831–1845. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Yue, C.; Zhang, T.; Li, P.; Xing, Z.; Chen, Y. A tough graphene nanosheet/hydroxyapatite composite with improved in vitro biocompatibility. Carbon 2013, 61, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sun, X.; Wang, Y.; Sun, K.; Bi, J. Properties of reduced graphene/carbon nanotubes reinforced calcium phosphate bone cement in a microwave environment. J. Mater. Sci. Mater. Med. 2019, 30, 37. [Google Scholar] [CrossRef]
- Dapporto, M.; Tampieri, A.; Sprio, S. Composite Calcium Phosphate/Titania Scaffolds in Bone Tissue Engineering. In Application of Titanium Dioxide; InTechOpen: London, UK, 2017. [Google Scholar]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate-TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Cunha, C.; Sprio, S.; Panseri, S.; Dapporto, M.; Marcacci, M.; Tampieri, A. High biocompatibility and improved osteogenic potential of novel Ca-P/titania composite scaffolds designed for regeneration of load-bearing segmental bone defects. J. Biomed. Mater. Res. Part A 2013, 101 A, 1612–1619. [Google Scholar] [CrossRef]
- Sprio, S.; Guicciardi, S.; Dapporto, M.; Melandri, C.; Tampieri, A. Synthesis and mechanical behavior of β-tricalcium phosphate/titania composites addressed to regeneration of long bone segments. J. Mech. Behav. Biomed. Mater. 2013 17, 1–10. [CrossRef]
- Liu, H.; Guan, Y.; Wei, D.; Gao, C.; Yang, H.; Yang, L. Reinforcement of injectable calcium phosphate cement by gelatinized starches. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cations | Biological Effects |
|---|---|
| Magnesium | Enhancing skeletal metabolism and bone growth |
| Strontium | Increasing bone mass: stimulating bone formation and reducing bone resorption (anti-osteoporotic agent) |
| Silicon | Stimulating extracellular matrix formation and mineralization |
| Zinc | Stimulating osteoblastic activity in vitro and inhibiting bone resorption in vivo |
| Macrostructure | Cortical bone Spongy bone |
| Microstructure | Osteons (100 μm) Haversian canals (10 μm) Collagen fibrils (25–500 nm) |
| Nanostructure | Tropocollagen triple helix Collagen molecule Hydroxyapatite nanocrystals (30 nm) |
| Name | Abbreviation | Chemical Formula | Ca/P Ratio | Solubility at 25 °C, mg/L |
|---|---|---|---|---|
| Hydroxyapatite | HA | Ca10(PO4)6(OH)2 | 1.67 | ~0.3 |
| Calcium-deficient hydroxyapatite | CDHA | Ca10−x(PO4)6−x(HPO4 or CO3)x(OH or ½ CO3)2−x | 1.5–1.67 | ~9.4 |
| Dicalcium phosphate dihydrate | DCPD | CaHPO4∙2H2O | 1 | ~88 |
| α-Tricalcium phosphate | α-TCP | α-Ca3(PO4)2 | 1.5 | ~2.5 |
| β-Tricalcium phosphate | β-TCP | β-Ca3(PO4)2 | 1.5 | ~0.5 |
| Properties | Proposed Improving Strategies |
|---|---|
| Open and interconnected porosity |
|
| Mechanical properties |
|
| Biofunctionality |
|
| Bioactivity |
|
| Template-Assisted Techniques | Processing |
|---|---|
| Replica |
|
| Sacrificial template |
|
| Template-Free Technique | |
| Direct foaming |
|
| Apatitic CPC | Brushitic CPC | ||
|---|---|---|---|
| Single Component | Multiple Components | ||
| Reactives | α-TCP | TTCP + DCPA/DCPD | Β-TCP + MCPM/MCPA |
| Reaction type | Hydrolysis | Acid-Base | |
| Reaction | |||
| Particle Size | Liquid-to-Powder Ratio | |||
|---|---|---|---|---|
| Fine Particles | Coarse Particles | Low L/P | High L/P | |
| Final crystal morphology | Needle-like crystals | Plate-like crystals | Low inter-aggregate distance | High inter-aggregate distance |
| Pore size distribution | Fine | Coarse | Fine | Coarse |
| Surface Structure | Parameters (Size, Morphology, Roughness) | Biological Function | |
|---|---|---|---|
| Enhance | Decrease | ||
| Micro/nano size (CaP) | Microgroove width: From 20–40 um to 60–100 um | Cell number inside the pattern | Cell alignment/orientation |
| Microgroove depth From 3 um to 5.5 um | Cell adhesion force | ||
| Microgroove depth pattern: From nano-hybrid to micro-hybrid | Cell adhesion, proliferation, osteogenesis | ||
| Micro-/nano-morphology (CaP) | Micro-morphology: Plate-like and net-like | Cell attachment expansion | |
| Nano-morphology: Plate-like and wire-like | Osteogenesis | ||
| Micro-/nano-roughness (CaP) | Micro-roughness: Ra from 1 um to 2 um | Cell attachment osteogenesis | |
| Nano-roughness: Ra from 5.3 nm to 9.8 nm | Focal adhesion osteogenesis | ||
| Natural Fibers | Man-Made Fibers | |||
|---|---|---|---|---|
| Resorbable | Non-Resorbable | |||
| Natural Polymer | Synthetic Polymer | Polymeric | Ceramics | |
| Silk fibroin [107] | Polylactide [109] Cellulose [112] | Poly-caprolactone [109] | Polyamide [103,113] | Carbon [104,115,116,118] Silicate based [117,121,122,126] HA whiskers [122,127] |
| Ion | Biological Effects | References |
|---|---|---|
| Si4+ |
| [39,78,114] |
| Sr2+ |
| [39,132,141] |
| Mg2+ |
| [39,132,139,141] |
| Zn2+ |
| [142,143,144] |
| Ag+ |
| [137,138,140] |
| Mn2+ |
| [131,136] |
| Cu2+ |
| [131,136] |
| Co2+ |
| [131,136] |
| Fe2+/3+ |
| [39,128,131,145] |
| F− |
| [136,142,144,146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. https://doi.org/10.3390/jcs5090227
Tavoni M, Dapporto M, Tampieri A, Sprio S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. Journal of Composites Science. 2021; 5(9):227. https://doi.org/10.3390/jcs5090227
Chicago/Turabian StyleTavoni, Marta, Massimiliano Dapporto, Anna Tampieri, and Simone Sprio. 2021. "Bioactive Calcium Phosphate-Based Composites for Bone Regeneration" Journal of Composites Science 5, no. 9: 227. https://doi.org/10.3390/jcs5090227
APA StyleTavoni, M., Dapporto, M., Tampieri, A., & Sprio, S. (2021). Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. Journal of Composites Science, 5(9), 227. https://doi.org/10.3390/jcs5090227






