Aligned Collagen-CNT Nanofibrils and the Modulation Effect on Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Matrix Preparation
2.2. Cell Culture and Characterization
2.3. Immunofluorescent Imaging
2.4. AFM Measurements
2.5. RT-qPCR Analysis
3. Results
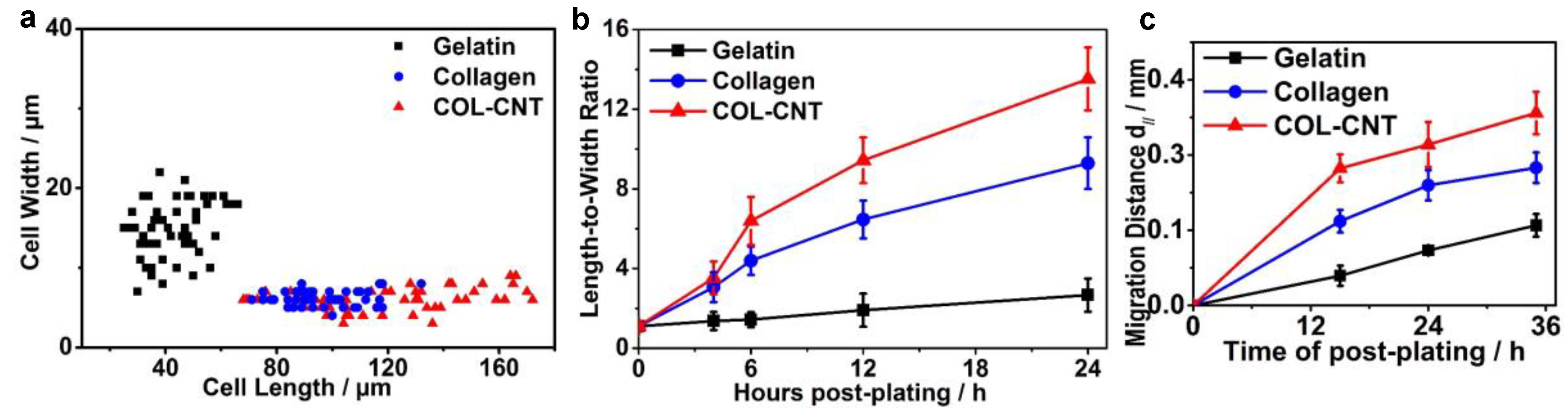
3.1. Aligned Collagen and COL-CNT Fibrils with Matrix-Mediated Cell Polarization and Migration
3.2. Characterization of Fibril Stiffness and Cell Stiffness
3.3. Cell Adhesion
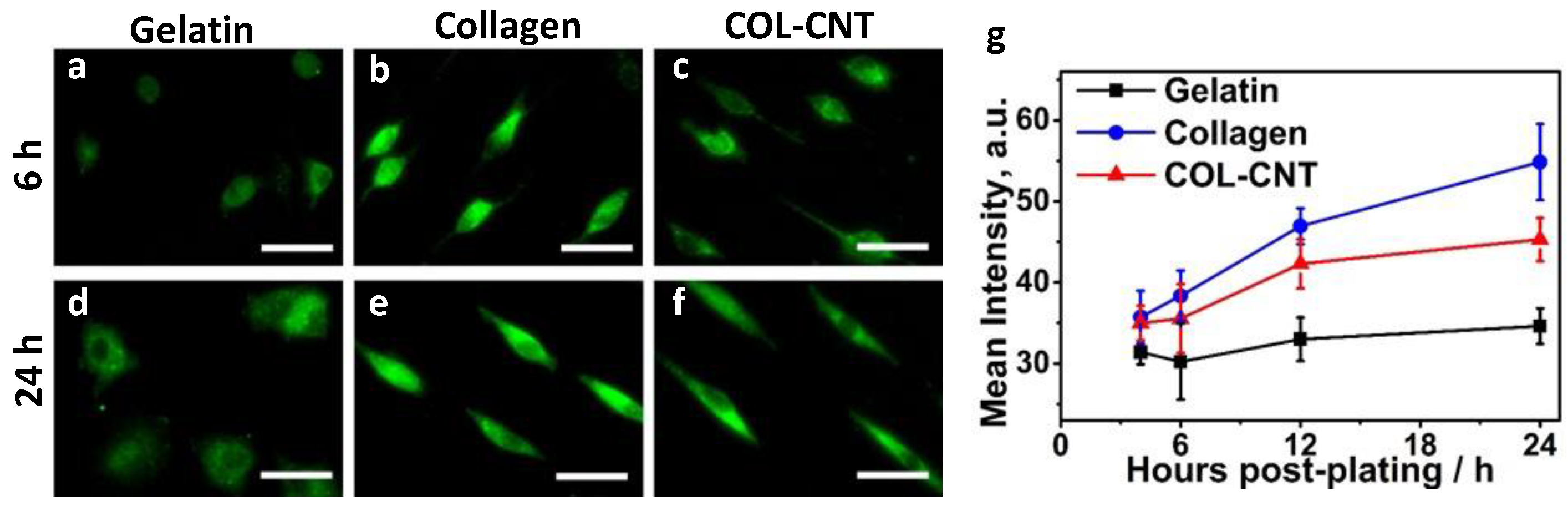
3.4. Collagen and COL-CNT Induced Cell Transformation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In Vivo Biodistribution and Highly Efficient Tumour Targeting of Carbon Nanotubes in Mice. Nat. Nanotechnol. 2007, 2, 47–52. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.; Laurenzi, B.; Viswanathan, G.; Ajayan, P.; Stegemann, J. Collagen-Carbon Nanotube Composite Materials as Scaffolds in Tissue Engineering. J. Biomed. Mater. Res. A 2005, 74, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Veetil, J.V.; Ye, K. Tailored Carbon Nanotubes for Tissue Engineering Applications. Biotechnol. Prog. 2009, 25, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef]
- Ravanbakhsh, H.; Bao, G.; Mongeau, L. Carbon Nanotubes Promote Cell Migration in Hydrogels. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.P.; Santos, A.K.; Gomes, K.N.; Lorençon, E.; Guatimosim, S.; Ladeira, L.O.; Resende, R.R. Carbon Nanotube Interaction with Extracellular Matrix Proteins Producing Scaffolds for Tissue Engineering. Int. J. Nanomed. 2012, 7, 4511–4529. [Google Scholar] [CrossRef]
- Chi, N.; Wang, R. Electrospun Protein-CNT Composite Fibers and the Application in Fibroblast Stimulation. Biochem. Biophys. Res. Commun. 2018, 504, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.; Zheng, S.; Clutter, E.; Wang, R. Silk-CNT Mediated Fibroblast Stimulation toward Chronic Wound Repair. Recent Prog. Mater. 2019, 1. [Google Scholar] [CrossRef]
- Sridharan, I.; Kim, T.; Wang, R. Adapting Collagen/CNT Matrix in Directing HESC Differentiation. Biochem. Biophys. Res. Commun. 2009, 381, 508–512. [Google Scholar] [CrossRef]
- Sridharan, I.; Kim, T.; Strakova, Z.; Wang, R. Matrix-Specified Differentiation of Human Decidua Parietalis Placental Stem Cells. Biochem. Biophys. Res. Commun. 2013, 437, 489–495. [Google Scholar] [CrossRef][Green Version]
- Kim, T.; Sridharan, I.; Zhu, B.; Orgel, J.; Wang, R. Effect of CNT on Collagen Fiber Structure, Stiffness Assembly Kinetics and Stem Cell Differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 281–289. [Google Scholar] [CrossRef]
- Bottini, M.; Rosato, N.; Bottini, N. PEG-Modified Carbon Nanotubes in Biomedicine: Current Status and Challenges Ahead. Biomacromolecules 2011, 12, 3381–3393. [Google Scholar] [CrossRef]
- McLane, J.S.; Rivet, C.J.; Gilbert, R.J.; Ligon, L.A. A Biomaterial Model of Tumor Stromal Microenvironment Promotes Mesenchymal Morphology but Not Epithelial to Mesenchymal Transition in Epithelial Cells. Acta Biomater. 2014, 10, 4811–4821. [Google Scholar] [CrossRef]
- Kim, T.; Sridharan, I.; Ma, Y.; Zhu, B.; Chi, N.; Kobak, W.; Rotmensch, J.; Schieber, J.D.; Wang, R. Identifying Distinct Nanoscopic Features of Native Collagen Fibrils towards Early Diagnosis of Pelvic Organ Prolapse. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 667–675. [Google Scholar] [CrossRef]
- Sridharan, I.; Ma, Y.; Kim, T.; Kobak, W.; Rotmensch, J.; Wang, R. Structural and Mechanical Profiles of Native Collagen Fibers in Vaginal Wall Connective Tissues. Biomaterials 2012, 33, 1520–1527. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Leung, P.C.K. Type I Collagen Down-Regulates E-Cadherin Expression by Increasing PI3KCA in Cancer Cells. Cancer Lett. 2011, 304, 107–116. [Google Scholar] [CrossRef]
- Imamichi, Y.; Menke, A. Signaling Pathways Involved in Collagen-Induced Disruption of the E-Cadherin Complex during Epithelial-Mesenchymal Transition. Cells Tissues Organs 2007, 185, 180–190. [Google Scholar] [CrossRef]
- Jordan, N.V.; Johnson, G.L.; Abell, A.N. Tracking the Intermediate Stages of Epithelial-Mesenchymal Transition in Epithelial Stem Cells and Cancer. Cell Cycle Georget. Tex 2011, 10, 2865–2873. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and BHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Kievit, F.M.; Cooper, A.; Jana, S.; Leung, M.C.; Wang, K.; Edmondson, D.; Wood, D.; Lee, J.S.H.; Ellenbogen, R.G.; Zhang, M. Aligned Chitosan-Polycaprolactone Polyblend Nanofibers Promote the Migration of Glioblastoma Cells. Adv. Healthc. Mater. 2013, 2, 1651–1659. [Google Scholar] [CrossRef]
- Saha, S.; Duan, X.; Wu, L.; Lo, P.-K.; Chen, H.; Wang, Q. Electrospun Fibrous Scaffolds Promote Breast Cancer Cell Alignment and Epithelial-Mesenchymal Transition. Langmuir 2012, 28, 2028–2034. [Google Scholar] [CrossRef]
- Foroni, L.; Vasuri, F.; Valente, S.; Gualandi, C.; Focarete, M.L.; Caprara, G.; Scandola, M.; D’Errico-Grigioni, A.; Pasquinelli, G. The Role of 3D Microenvironmental Organization in MCF-7 Epithelial-Mesenchymal Transition after 7 Culture Days. Exp. Cell Res. 2013, 319, 1515–1522. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix Stiffness Drives Epithelial–Mesenchymal Transition and Tumour Metastasis through a TWIST1–G3BP2 Mechanotransduction Pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Li, W.; Zhu, B.; Strakova, Z.; Wang, R. Two-Way Regulation between Cells and Aligned Collagen Fibrils: Local 3D Matrix Formation and Accelerated Neural Differentiation of Human Decidua Parietalis Placental Stem Cells. Biochem. Biophys. Res. Commun. 2014, 450, 1377–1382. [Google Scholar] [CrossRef]
- Leow, W.W.; Hwang, W. Epitaxially Guided Assembly of Collagen Layers on Mica Surfaces. Langmuir ACS J. Surf. Colloids 2011, 27, 10907–10913. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.-M.; Kim, I.A.; Park, K.-D.; Shin, J.-W. Nanofiber Alignment and Direction of Mechanical Strain Affect the ECM Production of Human ACL Fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Li, W.; Chi, N.; Rathnayake, R.A.C.; Wang, R. Distinctive Roles of Fibrillar Collagen I and Collagen III in Mediating Fibroblast-Matrix Interaction: A Nanoscopic Study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71. [Google Scholar] [CrossRef]
- Zhu, B.; Li, W.; Lewis, R.V.; Segre, C.U.; Wang, R. E-Spun Composite Fibers of Collagen and Dragline Silk Protein: Fiber Mechanics, Biocompatibility, and Application in Stem Cell Differentiation. Biomacromolecules 2015, 16, 202–213. [Google Scholar] [CrossRef]
- Touhami, A.; Nysten, B.; Dufrêne, Y.F. Nanoscale Mapping of the Elasticity of Microbial Cells by Atomic Force Microscopy. Langmuir 2003, 19, 4539–4543. [Google Scholar] [CrossRef]
- Heim, A.; Matthews, W.; Koob, T. Determination of the Elastic Modulus of Native Collagen Fibrils via Radial Indentation. Appl. Phys. Lett. 2006, 89, 181902. [Google Scholar] [CrossRef]
- Sariisik, E.; Docheva, D.; Padula, D.; Popov, C.; Opfer, J.; Schieker, M.; Clausen-Schaumann, H.; Benoit, M. Probing the Interaction Forces of Prostate Cancer Cells with Collagen I and Bone Marrow Derived Stem Cells on the Single Cell Level. PLoS ONE 2013, 8, e57706. [Google Scholar] [CrossRef]
- Zamir, E.; Geiger, B. Molecular Complexity and Dynamics of Cell-Matrix Adhesions. J. Cell Sci. 2001, 114, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Brakebusch, C.; Fässler, R. The Integrin-Actin Connection, an Eternal Love Affair. EMBO J. 2003, 22, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Ruff, L.E.; Skubitz, A.P. Beta 1-Integrins Regulate the Formation and Adhesion of Ovarian Carcinoma Multicellular Spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Jiao, J.; Huang, L.; Ye, F.; Shi, M.; Cheng, X.; Wang, X.; Hu, D.; Xie, X.; Lu, W. Cyclin D1 Affects Epithelial-Mesenchymal Transition in Epithelial Ovarian Cancer Stem Cell-like Cells. OncoTargets Ther. 2013, 6, 667–677. [Google Scholar] [CrossRef]
- Radisky, D.C.; LaBarge, M.A. Epithelial-Mesenchymal Transition and the Stem Cell Phenotype. Cell Stem Cell 2008, 2, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, Y.S.; Kim, H.J.; Kim, C.H.; Lim, S.W.; Huh, J.W.; Lee, J.H.; Kim, H.R. CD44 Enhances the Epithelial-Mesenchymal Transition in Association with Colon Cancer Invasion. Int. J. Oncol. 2012, 41, 211–218. [Google Scholar] [CrossRef]
- Sacks, J.D.; Barbolina, M.V. Expression and Function of CD44 in Epithelial Ovarian Carcinoma. Biomolecules 2015, 5, 3051–3066. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, L.; Sancho-Torres, I.; Mesonero, C.; Gibbon, D.G.; Shih, W.J.; Zotalis, G. The CD44 Receptor Is a Molecular Predictor of Survival in Ovarian Cancer. Med. Oncol. Northwood Lond. Engl. 2003, 20, 255–263. [Google Scholar] [CrossRef]
- Jeleniewicz, W.; Cybulski, M.; Nowakowski, A.; Stenzel-Bembenek, A.; Guz, M.; Marzec-Kotarska, B.; Kotarski, J.; Stepulak, A. MMP-2 MRNA Expression in Ovarian Cancer Tissues Predicts Patients’ Response to Platinum-Taxane Chemotherapy. Anticancer Res. 2019, 39, 1821–1827. [Google Scholar] [CrossRef]
- Al-Alem, L.; Curry, T.E. Ovarian Cancer: Involvement of the Matrix Metalloproteinases. Reprod. Camb. Engl. 2015, 150, R55–R64. [Google Scholar] [CrossRef]
- Parry, D.A.; Barnes, G.R.; Craig, A.S. A Comparison of the Size Distribution of Collagen Fibrils in Connective Tissues as a Function of Age and a Possible Relation between Fibril Size Distribution and Mechanical Properties. Proc. R. Soc. Lond. B Biol. Sci. 1978, 203, 305–321. [Google Scholar] [CrossRef]
- Parry, D.A. The Molecular and Fibrillar Structure of Collagen and Its Relationship to the Mechanical Properties of Connective Tissue. Biophys. Chem. 1988, 29, 195–209. [Google Scholar] [CrossRef]
- San Antonio, J.D.; Lander, A.D.; Karnovsky, M.J.; Slayter, H.S. Mapping the Heparin-Binding Sites on Type I Collagen Monomers and Fibrils. J. Cell Biol. 1994, 125, 1179–1188. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-Cell Invasion and Migration: Diversity and Escape Mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Pathak, A.; Kumar, S. Biophysical Regulation of Tumor Cell Invasion: Moving beyond Matrix Stiffness. Integr. Biol. Quant. Biosci. Nano Macro 2011, 3, 267–278. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of Cell Migration: A Multiscale Tuning Model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chi, N.; Clutter, E.D.; Zhu, B.; Wang, R.R. Aligned Collagen-CNT Nanofibrils and the Modulation Effect on Ovarian Cancer Cells. J. Compos. Sci. 2021, 5, 148. https://doi.org/10.3390/jcs5060148
Li W, Chi N, Clutter ED, Zhu B, Wang RR. Aligned Collagen-CNT Nanofibrils and the Modulation Effect on Ovarian Cancer Cells. Journal of Composites Science. 2021; 5(6):148. https://doi.org/10.3390/jcs5060148
Chicago/Turabian StyleLi, Wen, Naiwei Chi, Elwin D. Clutter, Bofan Zhu, and Rong R. Wang. 2021. "Aligned Collagen-CNT Nanofibrils and the Modulation Effect on Ovarian Cancer Cells" Journal of Composites Science 5, no. 6: 148. https://doi.org/10.3390/jcs5060148
APA StyleLi, W., Chi, N., Clutter, E. D., Zhu, B., & Wang, R. R. (2021). Aligned Collagen-CNT Nanofibrils and the Modulation Effect on Ovarian Cancer Cells. Journal of Composites Science, 5(6), 148. https://doi.org/10.3390/jcs5060148





