Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nanocomposites for Targeted Photo-/Magneto-Thermal Cancer Treatments
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Preparation of the Fe(Salen)-PPy Core–Shell Nanoassemblies (CSNAs)
2.3. Preparation of Tf-MTX-Coated CSNAs and Tf-Coated CSNAs
2.4. Characterizations
2.5. Cell Culture
2.6. Photo-Thermal Cancer Treatments
2.7. Magneto-Thermal Cancer Treatments
2.8. Cell Viability Imaging and Assay
3. Results and Discussions
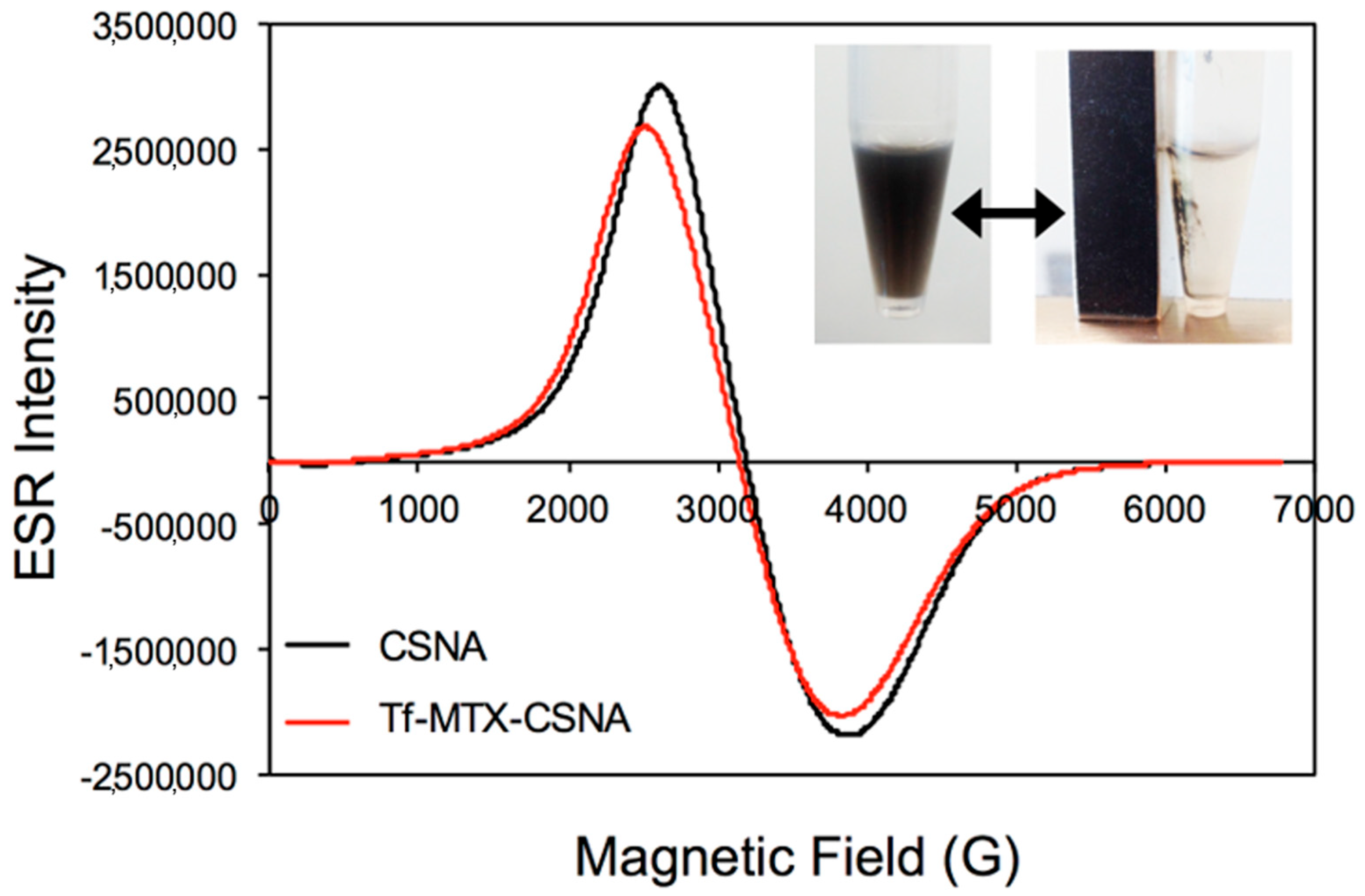
3.1. Preparation and Characterization of Tf-MTX-CSNAs
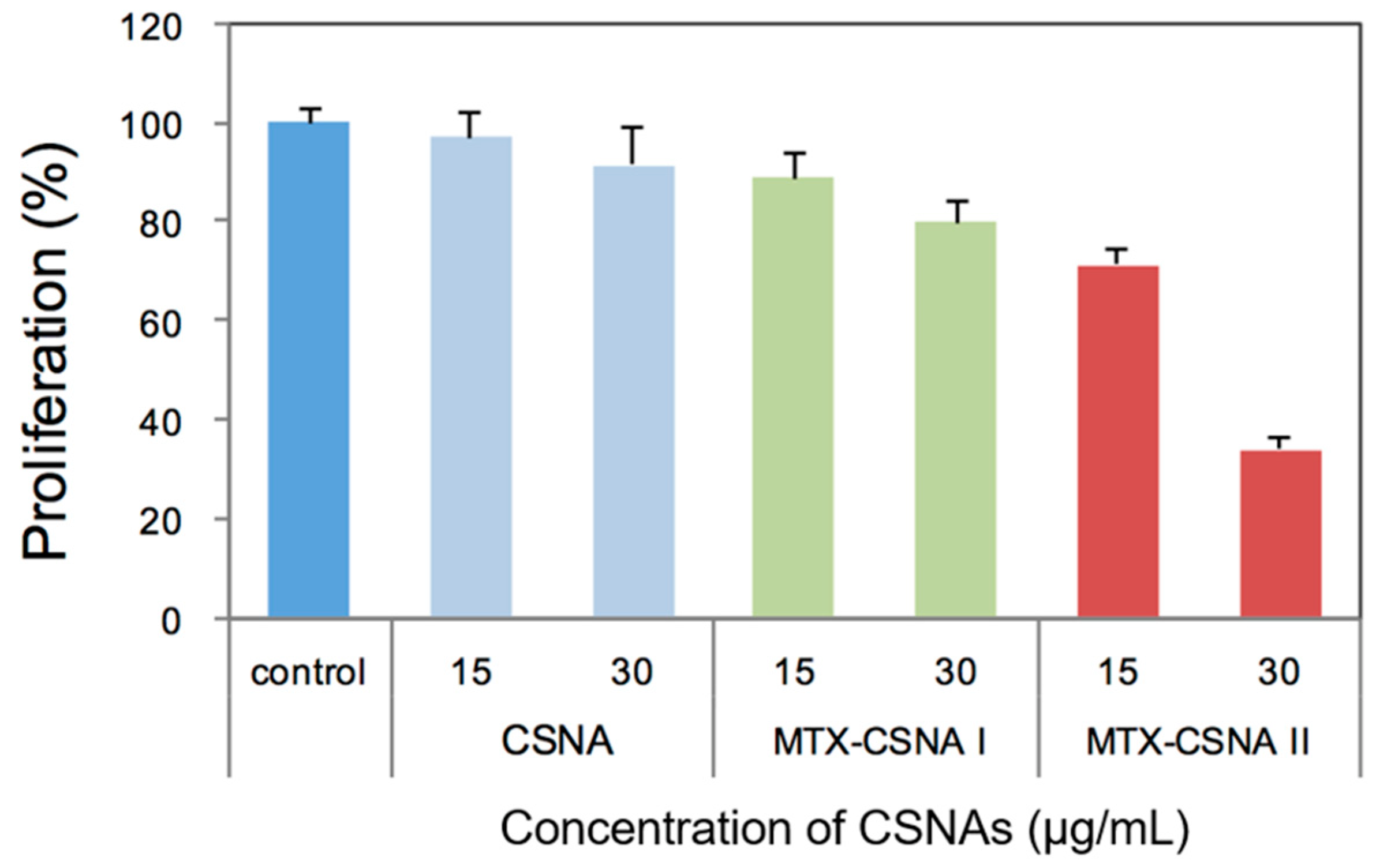
3.2. Biological Activities
3.3. Dual-Modal Hyperthermia Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.; Serrano Garcia, R.; Gun’ko, Y.K. Multimodal Magnetic-plasmonic nanoparticles for biomedical applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Eguchi, H.; Umemura, M.; Ishikawa, Y. Hybrid metal complex nanocomposites for targeted cancer diagnosis and therapeutics. In Materials for Biomedical Engineering: Inorganic Micro- and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–461. [Google Scholar]
- Cianciaruso, C.; Pagani, A.; Martelli, C.; Bacigaluppi, M.; Squadrito, M.L.; Lo Dico, A.; De Palma, M.; Furlan, R.; Lucignani, G.; Falini, A.; et al. Cellular magnetic resonance with iron oxide nanoparticles: Long-term persistence of SPIO signal in the CNS after transplanted cell death. Nanomedicine 2014, 9, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Oh, S.Y.; Kim, Y.; Yoon, C.; Lee, B.S.; Kim, S.D.; Kim, J.S. Distribution and immunotoxicity by intravenous injection of iron nanoparticles in a murine model. J. Appl. Toxicol. 2016, 36, 414–423. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef]
- Kim, J.-H.; Eguchi, H.; Umemura, M.; Sato, I.; Yamada, S.; Hoshino, Y.; Masuda, T.; Aoki, I.; Sakurai, K.; Yamamoto, M.; et al. Magnetic metal-complex-conducting copolymer core–shell nanoassemblies for a single-drug anticancer platform. NPG Asia Mater. 2017, 9, e367. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, H.; Umemura, M.; Kurotani, R.; Fukumura, H.; Sato, I.; Kim, J.-H.; Hoshino, Y.; Lee, J.; Amemiya, N.; Sato, M.; et al. A magnetic anti-cancer compound for magnet-guided delivery and magnetic resonance imaging. Sci. Rep. 2015, 5, 9194. [Google Scholar] [CrossRef] [Green Version]
- Sato, I.; Umemura, M.; Mitsudo, K.; Fukumura, H.; Kim, J.-H.; Hoshino, Y.; Nakashima, H.; Kioi, M.; Nakakaji, R.; Sato, M.; et al. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci. Rep. 2016, 6, 24629. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, M.; Umemura, M.; Sato, I.; Akimoto, T.; Oda, K.; Nagasako, A.; Kim, J.-H.; Fujita, T.; Yokoyama, U.; Nakayama, T.; et al. Hyperthermia and chemotherapy using Fe(Salen) nanoparticles might impact glioblastoma treatment. Sci. Rep. 2017, 7, 42783. [Google Scholar] [CrossRef]
- Nakakaji, R.; Umemura, M.; Mitsudo, K.; Kim, J.-H.; Hoshino, Y.; Sato, I.; Masuda, T.; Yamamoto, M.; Kioi, M.; Koizumi, T.; et al. Treatment of oral cancer using magnetized paclitaxel. Oncotarget 2018, 9, 15591–15605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, M.; Kim, J.-H.; Aoyama, H.; Hoshino, Y.; Fukumura, H.; Nakakaji, R.; Sato, I.; Ohtake, M.; Akimoto, T.; Narikawa, M. The iron chelating agent, deferoxamine detoxifies Fe(Salen)-induced cytotoxicity. J. Pharmacol. Sci. 2017, 134, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Tripathi, R.; Mishra, B. Methotrexate: A detailed review on drug delivery and clinical aspects. Expert. Opin. Drug Deliv. 2012, 9, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, J.; Wu, H.; Jia, M.; Yuan, C.; Chang, Y.; Hou, Z.; Dai, L. Novel methotrexate prodrug-targeted drug delivery system based on PEG-lipid-PLA hybrid nanoparticles for enhanced anticancer efficacy and reduced toxicity of mitomycin C. J. Mater. Chem. B 2014, 2, 6534–6548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Bhargava, P.; Bahadur, D. Methotrexate conjugated magnetic nanoparticle for targeted drug delivery and thermal therapy. J. Appl. Phys. 2014, 115, 17B516. [Google Scholar] [CrossRef]
- Alizadeh, N.; Shamaeli, E. Electrochemically controlled release of anticancer drug methotrexate using nanostructured polypyrrole modified with cetylpyridinium: Release kinetics Investigation. Electrochim. Acta 2014, 130, 488–496. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lu, T.M. Bio-inspired Janus composite nanoscroll for on-demand tumor targeting. RSC Adv. 2016, 6, 17179–17187. [Google Scholar] [CrossRef]
- Ardani, H.K.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd leaves using polyvinyl alcohol. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012056. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef]
- Osman, N.M. Polypyrrole as a thermoplasmonic. In Proceedings of the 2015 International Conference on Computing, Control, Networking, Electronics and Embedded Systems Engineering (ICCNEE), Khartoum, Sudan, 7–9 September 2015; pp. 475–478. [Google Scholar]
- Wang, Y.; Meng, H.M.; Song, G.; Li, Z.; Zhang, X.B. Conjugated-polymer-based nanomaterials for photothermal therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Kim, J.-H.; Eguchi, H.; Ishikawa, Y. Anticancer luminescent gold quantum clusters for in situ cancer-selective marking-imaging-targeting. Nanoscale 2017, 9, 9071–9082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, L.; Wong, W.Y. Energy materials based on metal Schiff base complexes. Coord. Chem. Rev. 2018, 355, 180. [Google Scholar] [CrossRef]
- Freire, C.; Nunes, M.; Pereira, C.; Fernandes, D.M.; Peixoto, A.F.; Rocha, M. Metallo(salen) complexes as versatile building blocks for the fabrication of molecular materials and devices with tuned properties. Coord. Chem. Rev. 2019, 394, 104. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Umemura, M.; Eguchi, H.; Ishikawa, Y. Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nanocomposites for Targeted Photo-/Magneto-Thermal Cancer Treatments. J. Compos. Sci. 2022, 6, 136. https://doi.org/10.3390/jcs6050136
Kim J-H, Umemura M, Eguchi H, Ishikawa Y. Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nanocomposites for Targeted Photo-/Magneto-Thermal Cancer Treatments. Journal of Composites Science. 2022; 6(5):136. https://doi.org/10.3390/jcs6050136
Chicago/Turabian StyleKim, Jeong-Hwan, Masanari Umemura, Haruki Eguchi, and Yoshihiro Ishikawa. 2022. "Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nanocomposites for Targeted Photo-/Magneto-Thermal Cancer Treatments" Journal of Composites Science 6, no. 5: 136. https://doi.org/10.3390/jcs6050136
APA StyleKim, J.-H., Umemura, M., Eguchi, H., & Ishikawa, Y. (2022). Methotrexate-Transferrin-Functionalized Fe(Salen)-Polypyrrole Nanocomposites for Targeted Photo-/Magneto-Thermal Cancer Treatments. Journal of Composites Science, 6(5), 136. https://doi.org/10.3390/jcs6050136







