In Vivo Effects of Two In-Office Vital Tooth Bleaching Systems on Enamel Permeability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Replica Technique
2.3. Evaluation of Tooth Permeability and Statistical Analysis
2.4. Evaluation of Tooth Sensitivity
3. Results
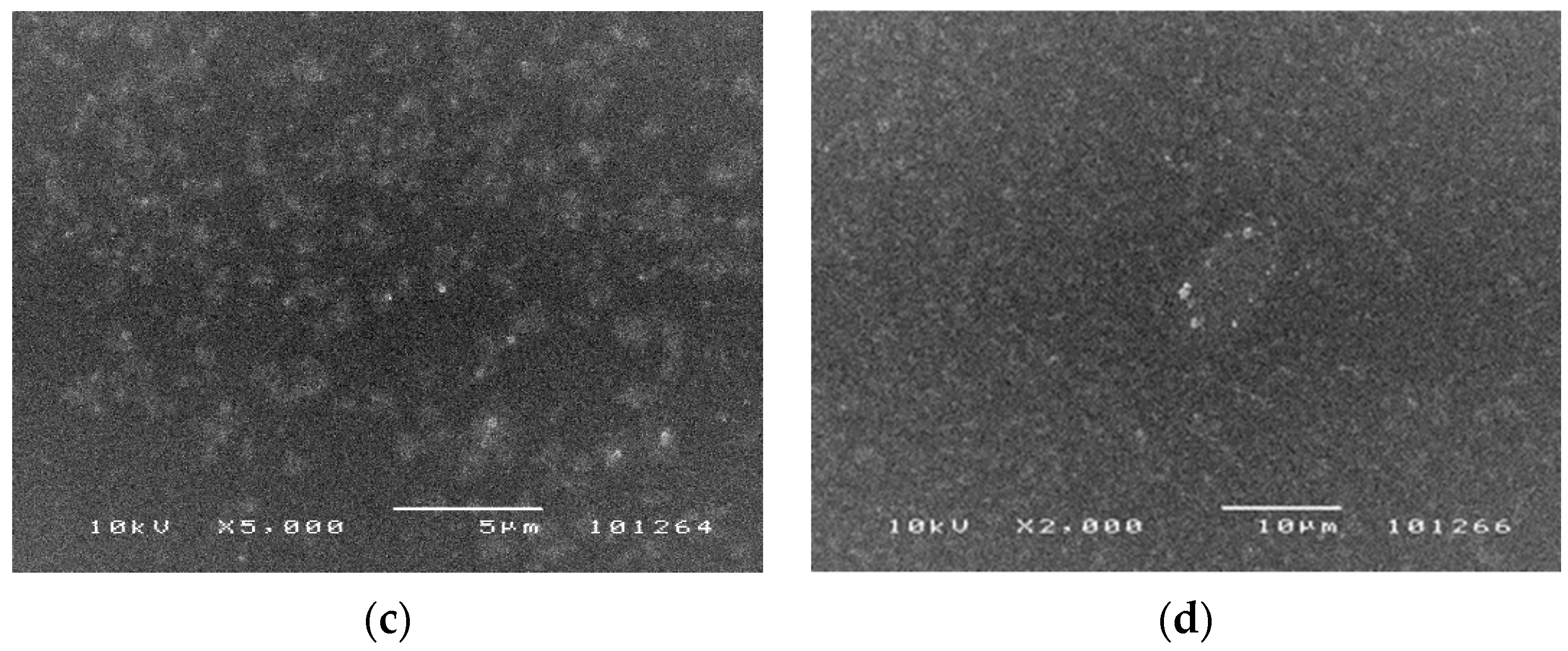
3.1. SEM Analysis
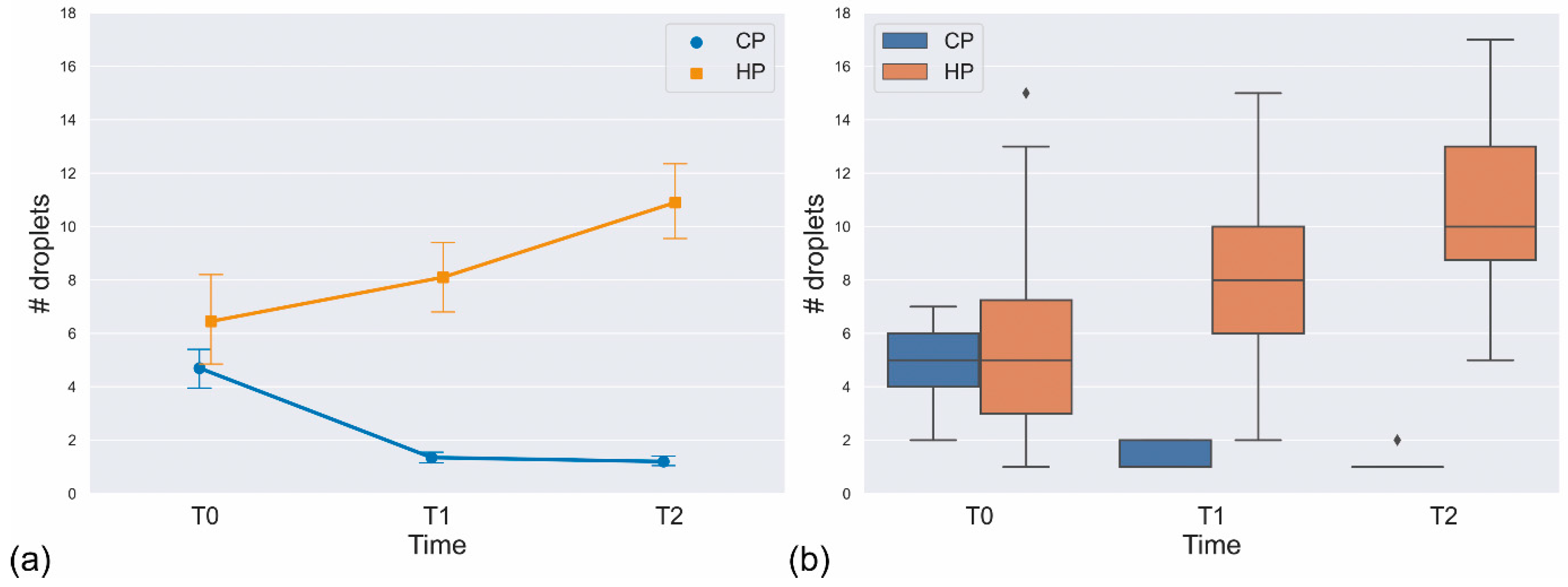
3.2. Statistical Analysis
3.3. Tooth Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blankenau, R.; Goldstein, R.E.; Haywood, V.B. The current status of vital tooth whitening techniques. Compend. Contin. Educ. Dent. 1999, 20, 781–784. [Google Scholar] [PubMed]
- Dias Ribeiro, A.P.; Sacono, N.T.; Lessa, F.C.; Nogueira, I.; Coldebella, C.R.; Hebling, J.; de Souza Costa, C.A. Cytotoxic effect of a 35% hydrogen peroxide blesching gel on odonto blast-like MDPC-23 cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 458–464. [Google Scholar] [CrossRef]
- Sun, L.; Liang, S.; Sa, Y.; Wang, Z.; Ma, X.; Jiang, T.; Wang, Y. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J. Dent. 2011, 39, 686–692. [Google Scholar] [CrossRef]
- Goldberg, M.; Grootveld, M.; Lynch, E. Undesiderable and adverse effects of tooth whitening products: A review. Clin. Oral Investig. 2010, 14, 1–10. [Google Scholar] [CrossRef]
- Dahl, J.E.; Pallesen, U. Tooth bleaching—A critical review of the biological aspects. Crit. Rev. Oral Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef]
- Cavalli, V.; Arrais, C.A.; Giannini, M.; Ambrosano, G.M. High-concentrated carbamide peroxide bleaching agents effects on enamel surface. J. Oral Rehabil. 2004, 31, 155–159. [Google Scholar] [CrossRef]
- Pinto, C.F.; Oliveira, R.; Cavalli, V.; Giannini, M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz. Oral Res. 2004, 18, 306–311. [Google Scholar] [CrossRef]
- Fu, B.; Hoth-Hannig, W.; Hannig, M. Effects of dental bleaching on micro- and nano-morphological alterations of the enamel surface. Am. J. Dent. 2007, 20, 35–40. [Google Scholar]
- Markovic, L.; Jordan, R.A.; Lakota, N.; Gaengler, P. Micromorphology of enamel surface after vital tooth bleaching. J. Endod. 2007, 33, 607–610. [Google Scholar] [CrossRef]
- Ushigome, T.; Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Influence of peroxide treatment on bovine enamel surface—Cross-sectional analysis. Dent. Mater. J. 2009, 28, 315–323. [Google Scholar] [CrossRef]
- Eimar, H.; Siciliano, R.; Abdallah, M.N.; Nader, S.A.; Amin, W.M.; Martinez, P.P.; Celemin, A.; Cerruti, M.; Tamimi, F. Hydrogen peroxide whitens teeth by oxidizing the organic structure. J. Dent. 2012, 40 (Suppl. S2), e25–e33. [Google Scholar] [CrossRef]
- Götz, H.; Duschner, H.; White, D.J.; Klukowska, A. Effects of elevated hydrogen peroxide strip bleaching on surface and subsurface enamel including subsurface histomorphology, micro-chemical composition and fluorescence changes. J. Dent. 2007, 35, 457–466. [Google Scholar] [CrossRef]
- Lewistein, I.; Hirschfeld, Z.; Stabholz, A.; Rostein, I. Effect of hydrogen peroxide and sodium perborate on the microhardness of human enamel and dentin. J. Endod. 1994, 20, 61–63. [Google Scholar] [CrossRef]
- Attin, T.; Kocabiyik, K.; Buchalla, W.; Hanning, C.; Becker, K. Susceptibility of enamel surfaces to demineralization after application of fluoridated carbamide peroxide gels. Caries Res. 2003, 37, 93–99. [Google Scholar] [CrossRef]
- Kwamoto, K.; Tsujimoto, Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef]
- Ruse, N.D.; Smith, D.C.; Torneck, C.D.; Titley, K.C. Preliminary surface analysis of etched, bleached, and normal bovine enamel. J. Dent. Res. 1990, 69, 1610–1613. [Google Scholar] [CrossRef]
- Oltu, Ü.; Gürgan, S. Effects of three concentrations of carbamide peroxide on the structure of enamel. J. Oral Rehabil. 2000, 27, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Chiatti, F.; Corno, M.; Sakhno, Y.; Martra, G.; Ugliengo, P. Affinity of hydroxyapatite (001) and (010) surfaces to formic and alendronic acids: A quantum-mechanical and infrared study. Phys. Chem. Chem. Phys. 2011, 13, 1099–1111. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. First-principles study of structural and surface properties of (001) and (010) surfaces of hydroxylapatite and carbonated hydroxylapatite. J. Appl. Cryst. 2016, 49, 1893–1903. [Google Scholar] [CrossRef]
- Bertacci, A.; Moro, D.; Ulian, G.; Valdre, G. Development of A Nano-Apatite Based Composite Sealer for Endodontic Root Canal Filling. J. Compos. Sci. 2021, 5, 30. [Google Scholar] [CrossRef]
- Bertacci, A.; Chersoni, S.; Davidson, C.L.; Prati, C. In vivo enamel fluid movement. Eur. J. Oral Sci. 2007, 115, 169–173. [Google Scholar] [CrossRef]
- Lucchese, A.; Bertacci, A.; Chersoni, S.; Portelli, M. Primary enamel permeability: A SEM evaluation in vivo. Eur. J. Paediatr. Dent. 2012, 13, 231–235. [Google Scholar] [PubMed]
- Chersoni, S.; Bertacci, A.; Pashley, D.H.; Tay, F.R.; Montebugnoli, L.; Prati, C. In vivo effects of fluoride on enamel permeability. Clin Oral Investig. 2011, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bertacci, A.; Lucchese, A.; Taddei, P.; Gherlone, E.F.; Chersoni, S. Enamel structural changes induced by hydrochloric and phosphoric acid treatment. J. Appl. Biomater. Funct. Mater. 2014, 12, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Nanjundasetty, J.K.; Ashrafulla, M. Efficacy of desensitizing agents on postoperative sensitivity following an in-office vital tooth bleaching: A randomized controlled clinical trial. J. Conserv. Dent. 2016, 19, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gunjikar, T.; Chute, M.; Lobo, T.; Pol, D.S.; Pol, G.D. Methods for the evaluation of the pain associated with dentinal hypersensitivity. JIDA 2012, 6, 57–59. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- He, L.B.; Shao, M.Y.; Tan, K.; Xu, X.; Li, J.Y. The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: A systematic review and meta-analysis. J. Dent. 2012, 40, 644–653. [Google Scholar] [CrossRef]
- Kothari, S.; Gray, A.R.; Lyons, K.; Tan, X.W.; Brunton, P.A. Vital bleaching and oral-health-related quality of life in adults: A systematic review and meta-analysis. J. Dent. 2019, 84, 22–29. [Google Scholar] [CrossRef]
- Mounika, A.; Mandava, J.; Roopesh, B.; Karri, G. Clinical evaluation of colour change and tooth sensitivity with in-office and home bleaching treatments. Indian J. Dent. Res. 2018, 29, 423–427. [Google Scholar] [CrossRef]
- Cooper, J.S.; Bokmeyer, T.J.; Bowles, W.H. Penetration of the pulp chamber by carbamide peroxide bleaching agents. J. Endod. 1992, 18, 315–317. [Google Scholar] [CrossRef]


| Bleaching Agents | Composition |
|---|---|
| Pola Office 35% | Liquid: 35% hydrogen peroxide, distilled water, and stabilizers. Powder: thickener, catalyst, pigments, and potassium nitrate. pH ~7. |
| Vivastyle | Gel ready-for-use: glycerine, aqua, urea peroxide, potassium nitrate, carbomer, sodium hydroxide, disodium EDTA, and flavour. Active ingredients: 30% carbamide peroxide. |
| A | B | mean(A) | σ(A) | mean(B) | σ(B) | T | df | p |
|---|---|---|---|---|---|---|---|---|
| HP group | ||||||||
| T0 | T1 | 6.45 | 4.148 | 8.10 | 3.194 | −1.313 | 19 | 2.050 × 10−1 |
| T0 | T2 | 6.45 | 4.148 | 10.90 | 3.275 | −4.783 | 19 | 1.290 × 10−4 |
| T1 | T2 | 8.10 | 3.194 | 10.90 | 3.275 | −4.729 | 19 | 1.460 × 10−4 |
| CP group | ||||||||
| T0 | T1 | 4.70 | 1.658 | 1.35 | 0.489 | 9.571 | 19 | 1.061 × 10−8 |
| T0 | T2 | 4.70 | 1.658 | 1.20 | 0.410 | 9.200 | 19 | 1.982 × 10−8 |
| T1 | T2 | 1.35 | 0.489 | 1.20 | 0.410 | 0.900 | 19 | 3.793 × 10−1 |
| Absent | Mild | Moderate | Severe | |
|---|---|---|---|---|
| n% | n% | n% | n% | |
| 35% HP (n = 20) | 0 | 4–20 | 10–50 | 6–30 |
| 30% CP (n = 20) | 5–25 | 10–50 | 5–25 | 00 |
| Total (n = 40) | 5–12.5 | 14–35 | 15–37 | 6–15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertacci, A.; Ulian, G.; Moro, D.; Chersoni, S.; Valdrè, G. In Vivo Effects of Two In-Office Vital Tooth Bleaching Systems on Enamel Permeability. J. Compos. Sci. 2021, 5, 98. https://doi.org/10.3390/jcs5040098
Bertacci A, Ulian G, Moro D, Chersoni S, Valdrè G. In Vivo Effects of Two In-Office Vital Tooth Bleaching Systems on Enamel Permeability. Journal of Composites Science. 2021; 5(4):98. https://doi.org/10.3390/jcs5040098
Chicago/Turabian StyleBertacci, Angelica, Gianfranco Ulian, Daniele Moro, Stefano Chersoni, and Giovanni Valdrè. 2021. "In Vivo Effects of Two In-Office Vital Tooth Bleaching Systems on Enamel Permeability" Journal of Composites Science 5, no. 4: 98. https://doi.org/10.3390/jcs5040098
APA StyleBertacci, A., Ulian, G., Moro, D., Chersoni, S., & Valdrè, G. (2021). In Vivo Effects of Two In-Office Vital Tooth Bleaching Systems on Enamel Permeability. Journal of Composites Science, 5(4), 98. https://doi.org/10.3390/jcs5040098








