A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports
Abstract
1. Introduction
2. Catalyst Support Properties and Requirements
3. Types of Reactions
4. Recent Reports, Analysis and Trends
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gawande, M.B.; Monga, Y.; Zboril, R.; Sharma, R. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015, 288, 118–143. [Google Scholar] [CrossRef]
- Lai, C.Y. Mesoporous Silica Nanomaterials Applications in Catalysis. J. Thermodyn. Catal. 2014, 5, 1–3. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: Sustainable catalysts for cross-coupling reactions. Co-ord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Qi, J.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Enhanced heterogeneous Fenton-like systems based on highly dispersed Fe0-Fe2O3 nanoparticles embedded ordered mesoporous carbon composite catalyst. Environ. Pollut. 2018, 243, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A. Incredible pace of research on mesoporous silica nanoparticles. Inorg. Chem. Front. 2014, 1, 735–739. [Google Scholar] [CrossRef]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Bedi, P.M. Silica supported Brönsted acids as catalyst in organic transformations: A comprehensive review. Chin. J. Catal. 2015, 36, 520–549. [Google Scholar] [CrossRef]
- Vincent, A.; Babu, S.; Brinley, E.; Karakoti, A.; Deshpande, A.S.; Seal, S. Role of Catalyst on Refractive Index Tunability of Porous Silica Antireflective Coatings by Sol−Gel Technique. J. Phys. Chem. C 2007, 111, 8291–8298. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of nano-fumed silica in heavy oil recovery. Pet. Sci. Technol. 2016, 34, 12–18. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloids Interf. Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hemmati, M.; Bazgir, S. Application of TiO2 and fumed silica nanoparticles and improve the performance of drilling fluids. Electron. Photonic Plasmonic Phononic Magn. Prop. Nanomater. 2014, 270, 266–270. [Google Scholar]
- Kamiński, M.; Jurkiewicz, K.; Burian, A.; Bródka, A. The structure of gold nanoparticles: Molecular dynamics modeling and its verification by X-ray diffraction. J. Appl. Crystallogr. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Scalarone, D.; Brunella, V.; Berlier, G. Poly(NIPAM-co-MPS)-grafted multimodal porous silica nanoparticles as reverse thermoresponsive drug delivery system. Asian J. Pharm. Sci. 2017, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1–21. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. Structural modification of nano bentonite by aluminum, iron pillarization and 3D growth of silica mesoporous framework for arsenic removal from gold mine wastewater. J. Hazard. Mater. 2019, 378, 120779. [Google Scholar] [CrossRef]
- Nayab, S.; Baig, H.; Ghaffar, A.; Tuncel, E.; Oluz, Z.; Duran, H.; Yameen, B. Silica based inorganic–organic hybrid materials for the adsorptive removal of chromium. RSC Adv. 2018, 8, 23963–23972. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shin, C.-K.; Ahn, C.-H.; Kim, G.-J. Syntheses and application of silica monolith with bimodal meso/macroscopic pore structure. J. Porous Mater. 2006, 13, 201–205. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Simopoulos, A.; Boukos, A.N.; Petridis, D. Magnetic Modification of the External Surfaces in the MCM-41 Porous Silica: Synthesis, Characterization, and Functionalization. J. Phys. Chem. B 2001, 105, 7432–7437. [Google Scholar] [CrossRef]
- Sen, T.; Tiddy, G.J.T.; Casci, J.L.; Anderson, M.W. Synthesis and Characterization of Hierarchically Ordered Porous Silica Materials. Chem. Mater. 2004, 16, 2044–2054. [Google Scholar] [CrossRef]
- Giraldo, L.F.; López, B.L.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous Silica Applications. Macromol. Symp. 2007, 258, 129–141. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, S.K.; Pathak, D.D. Selective adsorption of toxic heavy metal ions using guanine-functionalized mesoporous silica [SBA-16-g] from aqueous solution. Microporous Mesoporous Mater. 2019, 288, 109577. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Yuanab, S.; Wanga, M.; Liub, J.; Guoc, B. Recent advances of SBA-15-based composites as the heterogeneous catalysts in water decontamination: A mini-review. J. Environ. Manag. 2020, 254, 109787. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Chehimi, M.; Selmane, M. Synthesis and Structural Characterization of G-SBA-IDA, G-SBA-EDTA and G-SBA-DTPA Modified Mesoporous SBA-15 Silica and Their Application for Removal of Toxic Metal Ions Pollutants. Silicon 2018, 10, 981–993. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Synthesis and Testing of Functional Mesoporous Silica Nanoparticles for Removal of Cr(VI) Ions From Water. Biointerface Res. Appl. Chem. 2020, 11, 8599–8607. [CrossRef]
- Jadhav, S.A.; Patil, V.S.; Shinde, P.S.; Thoravat, S.S.; Patil, P.S. A short review on recent progress in mesoporous silicas for the removal of metal ions from water. Chem. Pap. 2020, 74, 4143–4157. [Google Scholar] [CrossRef]
- Shimizu, W.; Hokka, J.; Sato, T.; Usami, H.; Murakami, Y. Microstructure Investigation on Micropore Formation in Microporous Silica Materials Prepared via a Catalytic Sol–Gel Process by Small Angle X–Ray Scattering. J. Phys. Chem. B 2011, 115, 9369–9378. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 4, pp. 350–357. [Google Scholar]
- Jadhav, S.A.; Miletto, I.; Brunella, V.; Scalarone, D.; Berlier, G. Porous Silica Particles: Synthesis, Physicochemical Character-ization and Evaluation of Suspension Stability. Phys. Chem. Ind. J. 2017, 1–11. [Google Scholar]
- Kageyama, K.; Tamazawa, J.-I.; Aida, T. Extrusion Polymerization: Catalyzed Synthesis of Crystalline Linear Polyethylene Nanofibers Within a Mesoporous Silica. Science 1999, 285, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Della Pina, C.; Falletta, E. Gold-catalyzed oxidation in organic synthesis: A promise kept. Catal. Sci. Technol. 2011, 1, 1564–1571. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Li, B.; Sasaki, T.; Miyazaki, C.; Kajino, T.; Inagaki, S. Catalytic Activity in Organic Solvents and Stability of Immobilized Enzymes Depend on the Pore Size and Surface Characteristics of Mesoporous Silica. Chem. Mater. 2000, 12, 3301–3305. [Google Scholar] [CrossRef]
- Amin, M.H. Relationship between the Pore Structure of Mesoporous Silica Supports and the Activity of Nickel Nanocatalysts in the CO2 Reforming of Methane. Catalysts 2020, 10, 51. [Google Scholar] [CrossRef]
- Chu, X.; Wang, C.; Guo, L.; Chi, Y.; Gao, X.; Yang, X. Mesoporous Silica Supported Au Nanoparticles with Controlled Size as Efficient Heterogeneous Catalyst for Aerobic Oxidation of Alcohols. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Le, H.V.; Parishan, S.; Sagaltchik, A.; Ahi, H.; Trunschke, A.; Schomäcker, R.; Thomas, A. Stepwise Methane-to-Methanol Conversion on CuO/SBA-15. Chem. A Eur. J. 2018, 24, 12592–12599. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; An, D.; Wang, Y. Catalytic behavior and kinetic features of FeOx/SBA-15 catalyst for selective oxidation of methane by oxygen. Appl. Catal. A Gen. 2009, 356, 103–111. [Google Scholar] [CrossRef]
- Fu, T.; Wang, Y.; Wernbacher, A.M.; Schlögl, R.; Trunschke, A. Single-Site Vanadyl Species Isolated within Molybdenum Oxide Monolayers in Propane Oxidation. ACS Catal. 2019, 9, 4875–4886. [Google Scholar] [CrossRef]
- Nozaki, C.; Lugmair, C.G.; Bell, A.T.; Tilley, T.D. Synthesis, Characterization, and Catalytic Performance of Single-Site Iron(III) Centers on the Surface of SBA-15 Silica. J. Am. Chem. Soc. 2002, 124, 13194–13203. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qu, Z.; Dong, C.; Wang, Y.; Huang, N. Highly catalytic activity of Mn/SBA-15 catalysts for toluene combustion improved by adjusting the morphology of supports. J. Environ. Sci. 2019, 76, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Wang, J.A.; Chen, L.; Manriquez, M.E.; Dominguez, J.M. Structural Defects, Lewis Acidity, and Catalysis Properties of Mesostructured WO3/SBA-15 Nanocatalysts. J. Phys. Chem. C 2017, 121, 23988–23999. [Google Scholar] [CrossRef]
- Barroso-Martín, I.; Infantes-Molina, A.; Talon, A.; Storaro, L.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E.; Moretti, E. CO Preferential Photo-Oxidation in Excess of Hydrogen in Dark and Simulated Solar Light Irradiation over AuCu-Based Catalysts on SBA-15 Mesoporous Silica-Titania. Materials 2018, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martell, A.; Pawelec, B.; Nava, R.; Mota, N.; Escamilla-Perea, L.; Navarro, R.M.; Fierro, J.L. CO Oxidation at 20 °C on Au Catalysts Supported on Mesoporous Silica: Effects of Support Structural Properties and Modifiers. Materials 2018, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Al Soubaihi, R.M.; Saoud, K.M.; Ye, F.; Myint, M.T.Z.; Saeed, S.; Dutta, J. Synthesis of hierarchically porous silica aerogel supported Palladium catalyst for low-temperature CO oxidation under ignition/extinction conditions. Microporous Mesoporous Mater. 2020, 292, 109758. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; Shkir, M.; Khder, A.S.; Kaushik, A.; Hamdy, M.S. Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide. Nanomaterials 2020, 10, 1067. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, D.; Liu, J.; Kang, X.; Yan, H.; Wu, A.; Gu, Y.; Tian, C.; Fu, H. Ultra-small Mo2N on SBA-15 as a highly efficient promoter of low-loading Pd for catalytic hydrogenation. Nanoscale 2018, 10, 22348–22356. [Google Scholar] [CrossRef]
- Finocchio, E.; Gonzalez-Prior, J.; Gutierrez-Ortiz, J.I.; Lopez-Fonseca, R.; Busca, G.; De Rivas, B. Surface Characterization of Mesoporous CoOx/SBA-15 Catalyst upon 1,2-Dichloropropane Oxidation. Materials 2018, 11, 912. [Google Scholar] [CrossRef]
- Barroso-Martín, I.; Moretti, E.; Talon, A.; Storaro, L.; Rodríguez-Castellón, E.; Infantes-Molina, A. Au and AuCu Nanoparticles Supported on SBA-15 Ordered Mesoporous Titania-Silica as Catalysts for Methylene Blue Photodegradation. Materials 2018, 11, 890. [Google Scholar] [CrossRef]
- Sánchez-Zambrano, K.S.; Duarte, L.L.; Maia, D.A.S.; Vilarrasa-García, E.; Bastos-Neto, M.; Rodríguez-Castellón, E.; De Azevedo, D.C.S. CO2 Capture with Mesoporous Silicas Modified with Amines by Double Functionalization: Assessment of Adsorption/Desorption Cycles. Materials 2018, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kang, D.-Y.; Copeland, J.R.; Brunelli, N.A.; Didas, S.A.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Dramatic Enhancement of CO2 Uptake by Poly(ethyleneimine) Using Zirconosilicate Supports. J. Am. Chem. Soc. 2012, 134, 10757–10760. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kang, D.-Y.; Copeland, J.R.; Bollini, P.; Sievers, C.; Kamegawa, T.; Yamashita, H.; Jones, C.W. Enhanced CO2 Adsorption over Polymeric Amines Supported on Heteroatom-Incorporated SBA-15 Silica: Impact of Heteroatom Type and Loading on Sorbent Structure and Adsorption Performance. Chem. A Eur. J. 2012, 18, 16649–16664. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.S.; Phan, T.N.; Chung, I.S.; Lee, I.-G.; Park, Y.-K.; Ko, C.H. Metallic nickel supported on mesoporous silica as catalyst for hydrodeoxygenation: Effect of pore size and structure. Res. Chem. Intermed. 2018, 44, 3723–3735. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Fujitani, T.; Yamashita, H. Esterification of levulinic acid with ethanol over sulfated mesoporous zirconosilicates: Influences of the preparation conditions on the structural properties and catalytic performances. Catal. Today 2014, 237, 18–28. [Google Scholar] [CrossRef]
- Silva, Â.; Wilson, K.; Lee, A.F.; dos Santos, V.C.; Bacilla, A.C.C.; Mantovani, K.M.; Nakagaki, S. Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B Environ. 2017, 205, 498–504. [Google Scholar] [CrossRef]
- Manga, J.; Ahmad, A.; Taba, P. Firdaus Synthesis and modification of mesoporous silica with sulfated titanium dioxide as a heterogeneous catalyst for biodiesel production from palm fatty acid distillate. J. Phys. Conf. Ser. 2019, 1341, 032014. [Google Scholar] [CrossRef]
- Fei, L.; Reddy, H.K.; Hill, J.; Lin, Q.; Yuan, B.; Xu, Y.; Dailey, P.; Deng, S.; Luo, H. Preparation of Mesoporous Silica-Supported Palladium Catalysts for Biofuel Upgrade. J. Nanotechnol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Zapelini, I.W.; Silva, L.L.; Cardoso, D. Effect of Hydrothermal Treatment on Structural and Catalytic Properties of [CTA]-MCM-41 Silica. Materials 2018, 11, 860. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Jiménez-Gómez, C.P.; Moreno-Tost, R.; Maireles-Torres, P. Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products. Materials 2018, 11, 1569. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Nava, R.; Peza-Ledesma, C.L.; Lara-Romero, J.; Alonso-Núñez, G.; Pawelec, B.; Rivera-Muñoz, E.M. SBA-15 Mesoporous Silica as Catalytic Support for Hydrodesulfurization Catalysts—Review. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.-F.; Wang, A.-Q.; Li, L.; Liu, X.-Y.; Zhang, T.; Mou, C.-Y.; Li, J. Bimetallic Au–Pd Alloy Catalysts for N2O Decomposition: Effects of Surface Structures on Catalytic Activity. J. Phys. Chem. C 2012, 116, 6222–6232. [Google Scholar] [CrossRef]
- Jin, M.-H.; Oh, D.-K.; Park, J.-H.; Lee, C.-B.; Lee, S.-W.; Park, J.-S.; Lee, K.-Y.; Lee, D.-W. Mesoporous Silica Supported Pd-MnOx Catalysts with Excellent Catalytic Activity in Room-Temperature Formic Acid Decomposition. Sci. Rep. 2016, 6, 33502. [Google Scholar] [CrossRef]
- Verho, O.; Gao, F.; Johnston, E.V.; Wan, W.; Nagendiran, A.; Zheng, H.; Bäckvall, J.-E.; Zou, X. Mesoporous silica nanoparticles applied as a support for Pd and Au nanocatalysts in cycloisomerization reactions. APL Mater. 2014, 2, 113316. [Google Scholar] [CrossRef]
- Macquarrie, D.J.; Gotov, B.; Toma, S. Silica-supported palladium-based catalysts for clean synthesis. Platin. Met. Rev. 2001, 45, 102–110. [Google Scholar]
- Crudden, C.M.; Sateesh, M.; Lewis, R. Mercaptopropyl-Modified Mesoporous Silica: A Remarkable Support for the Preparation of a Reusable, Heterogeneous Palladium Catalyst for Coupling Reactions. J. Am. Chem. Soc. 2005, 127, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Erigoni, A.; Hernández-Soto, M.C.; Rey, F.; Segarra, C.; Díaz, U. Highly active hybrid mesoporous silica-supported base organocatalysts for Csingle bondC bond formation. Catal. Today 2020, 345, 227–236. [Google Scholar] [CrossRef]
- Kobylinski, T.P.; Hammel, J.J.; Swift, H.E. Porous Silica Beads-A Catalyst Support for Removal of Exhaust Gas Pollutants. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 147–150. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, X. Mesoporous Materials as Catalyst support for Wastewater Treatment. Madridge J. Nanotechnol. Nanosci. 2019, 4, 160–167. [Google Scholar] [CrossRef]
- Pullukat, T.J.; Patterson, R.E. Porous Silica in Transition Metal Polymerization Catalysts. Handb. Transit. Metal Polym. Catal. 2018, 31–55. [Google Scholar] [CrossRef]
- Lee, J.S.; Yim, J.-H.; Jeon, J.-K.; Ko, Y.S. Polymerization of olefins with single-site catalyst anchored on amine-functionalized surface of SBA-15. Catal. Today 2012, 185, 175–182. [Google Scholar] [CrossRef]
- McKittrick, M.W.; Jones, C.W. Toward Single-Site, Immobilized Molecular Catalysts: Site-Isolated Ti Ethylene Polymerization Catalysts Supported on Porous Silica. J. Am. Chem. Soc. 2004, 126, 3052–3053. [Google Scholar] [CrossRef]
- Wilson, B.C. Silica-Supported Organic Catalysts for the Synthesis of Biodegradable Polymers. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2004; pp. 1–72. [Google Scholar]
- Kusumastuti, H.; Trisunaryanti, W.; Falah, I.I.; Marsuki, M.F. Synthesis of mesoporous silica-alumina from lapindo mud as a support of ni and mo metals catalysts for hydrocracking of pyrolyzed α- cellulose. Rasayan J. Chem. 2018, 11, 522–530. [Google Scholar] [CrossRef]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B Environ. 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Masoud, N.; Delannoy, L.; Calers, C.; Gallet, J.-J.; Bournel, F.; De Jong, K.P.; Louis, C.; De Jongh, P.E. Silica-Supported Au-Ag Catalysts for the Selective Hydrogenation of Butadiene. ChemCatChem 2017, 9, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Großmann, D.; Klementiev, K.; Sinev, I.; Grünert, W. Surface Alloy or Metal-Cation Interaction-The State of Zn Promoting the Active Cu Sites in Methanol Synthesis Catalysts. ChemCatChem 2017, 9, 365–372. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Dai, X.; Qin, Y.; Duan, A.; Yu, Y.; Zhuo, H.; Zhao, H.; Zhang, P.; Jiang, Y.; et al. Morphology-selective synthesis of active and durable gold catalysts with high catalytic performance in the reduction of 4-nitrophenol. Nano Res. 2016, 9, 3099–3115. [Google Scholar] [CrossRef]
- Qian, X.; Kuwahara, Y.; Mori, K.; Yamashita, H. Silver Nanoparticles Supported on CeO2-SBA-15 by Microwave Irradiation Possess Metal-Support Interactions and Enhanced Catalytic Activity. Chem. A Eur. J. 2014, 20, 15746–15752. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Verma, P.; Hayashi, R.; Fuku, K.; Yamashita, H. Color-Controlled Ag Nanoparticles and Nanorods within Confined Mesopores: Microwave-Assisted Rapid Synthesis and Application in Plasmonic Catalysis under Visible-Light Irradiation. Chem. A Eur. J. 2015, 21, 11885–11893. [Google Scholar] [CrossRef] [PubMed]
- Berro, Y.; Gueddida, S.; Bouizi, Y.; Bellouard, C.; Bendeif, E.-E.; Gansmuller, A.; Celzard, A.; Fierro, V.; Ihiawakrim, D.; Ersen, O.; et al. Imprinting isolated single iron atoms onto mesoporous silica by templating with metallosurfactants. J. Colloid Interface Sci. 2020, 573, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of Ag-Based Plasmonic Photocatalysis in Hydrogen Production from Ammonia Borane by the Assistance of Single-Site Ti-Oxide Moieties within a Silica Framework. Chem. A Eur. J. 2017, 23, 3616–3622. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Synthesis and characterization of a Pd/Ag bimetallic nanocatalyst on SBA-15 mesoporous silica as a plasmonic catalyst. J. Mater. Chem. A 2015, 3, 18889–18897. [Google Scholar] [CrossRef]
- Tasfy, S.F.H.; Shaharun, M.S.; Zabidi, N.A.M.; Subbarao, D. Effect of Mesoporous Silica Support on the Performance of Copper/Zinc Oxide Catalyst in Hydrogenation of CO2 to Methanol. Adv. Mater. Res. 2015, 1109, 128–132. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, H.; Yang, M.; Wang, G.; Li, J.; Zhang, H.; Tao, Z. NiO promoted CuO–NiO/SBA-15 composites as highly active catalysts for epoxidation of olefins. New J. Chem. 2016, 40, 8543–8548. [Google Scholar] [CrossRef]
- Jarupatrakorn, J.; Tilley, T.D.; Tantirungrotechai, J. Silica-Supported, Single-Site Titanium Catalysts for Olefin Epoxidation. A Molecular Precursor Strategy for Control of Catalyst Structure. J. Am. Chem. Soc. 2002, 124, 8380–8388. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, Y.; Qiu, Z.; Zhao, L.; Zhu, Y. Synthesis and characterization of cobalt-substituted SBA-15 and its high activity in epoxidation of styrene with molecular oxygen. Appl. Catal. B Environ. 2010, 101, 45–53. [Google Scholar] [CrossRef]
- Avenier, P.; Taoufik, M.; Lesage, A.; Solans-Monfort, X.; Baudouin, A.; De Mallmann, A.; Veyre, L.; Basset, J.-M.; Eisenstein, O.; Emsley, L.; et al. Dinitrogen Dissociation on an Isolated Surface Tantalum Atom. Science 2007, 317, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, L.; Zhang, L.; Zhang, H.; Ren, J. Function of well-established mesoporous layers of recrystallized ZSM-22 zeolites in the catalytic performance of n-alkane isomerization. New J. Chem. 2020, 44, 4744–4754. [Google Scholar] [CrossRef]
- Zheng, M.; Ding, Y.; Cao, X.; Tian, T.; Lin, J. Homogeneous and heterogeneous photocatalytic water oxidation by polyoxometalates containing the most earth-abundant transition metal, iron. Appl. Catal. B Environ. 2018, 237, 1091–1100. [Google Scholar] [CrossRef]
- Chang, F.; Jiao, M.; Xu, Q.; Deng, B.; Hu, X. Facile fabrication of mesoporous Fe-Ti-SBA15 silica with enhanced visible-light-driven simultaneous photocatalytic degradation and reduction reactions. Appl. Surf. Sci. 2018, 435, 708–717. [Google Scholar] [CrossRef]
- Jeong, S.; Chung, K.-H.; Lee, H.; Park, H.; Jeon, K.-J.; Park, Y.-K.; Jung, S.-C. Enhancement of Hydrogen Evolution from Water Photocatalysis Using Liquid Phase Plasma on Metal Oxide-Loaded Photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 3659–3666. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Fujitani, T. Catalytic Conversion of Levulinic Acid and Its Esters to γ-Valerolactone over Silica-Supported Zirconia Catalysts. Bull. Chem. Soc. Jpn. 2014, 87, 1252–1254. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic transfer hydrogenation of biomass-derived levulinic acid and its esters to γ-valerolactone over ZrO 2 catalyst supported on SBA-15 silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Kou, J.; Sun, L.-B. Fabrication of Metal–Organic Frameworks inside Silica Nanopores with Significantly Enhanced Hydrostability and Catalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 12051–12059. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, J.W.; Rioux, R.M. Structural elucidation of supported Rh complexes derived from RhCl(PPh3)3 immobilized on surface-functionalized SBA-15 and their catalytic performance for C-heteroatom (S, O) bond formation. J. Catal. 2018, 365, 43–54. [Google Scholar] [CrossRef]
- Wen, Z.; Duan, X.; Hu, M.; Cao, Y.; Ye, L.; Jiang, L.; Yuan, Y. Efficient low-temperature soot combustion by bimetallic Ag–Cu/SBA-15 catalysts. J. Environ. Sci. 2018, 64, 122–129. [Google Scholar] [CrossRef]
- Shen, D.; Chen, L.; Yang, J.; Zhang, R.; Wei, Y.; Li, X.; Li, W.; Sun, Z.; Zhu, H.; Abdullah, A.M.; et al. Ultradispersed Palladium Nanoparticles in Three-Dimensional Dendritic Mesoporous Silica Nanospheres: Toward Active and Stable Heterogeneous Catalysts. ACS Appl. Mater. Interfaces 2015, 7, 17450–17459. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; Chen, B.; Wang, L.; He, G. Rapidly Constructing Multiple AuPt Nanoalloy Yolk@Shell Hollow Particles in Ordered Mesoporous Silica Microspheres for Highly Efficient Catalysis. ACS Sustain. Chem. Eng. 2016, 4, 2780–2788. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: A magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel 2021, 283, 118893. [Google Scholar] [CrossRef]
- Daneshjou, S.; Dabirmanesh, B.; Rahimi, F.; Jabbari, S.; Khajeh, K. Catalytic parameters and thermal stability of chondroitinase ABCI on red porous silicon nanoparticles. J. Biotechnol. 2020, 324, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, e2001818. [Google Scholar] [CrossRef]
- Martín, N.; Cirujano, F.G. Organic synthesis of high added value molecules with MOF catalysts. Org. Biomol. Chem. 2020, 18, 8058–8073. [Google Scholar] [CrossRef]
- Haynes, T.; Bougnouch, O.; Dubois, V.; Hermans, S. Preparation of mesoporous silica nanocapsules with a high specific surface area by hard and soft dual templating approach: Application to biomass valorization catalysis. Microporous Mesoporous Mater. 2020, 306, 110400. [Google Scholar] [CrossRef]
- Lee, J.; Dubbu, S.; Kumari, N.; Kumar, A.; Lim, J.; Kim, S.; Lee, I.S. Magnetothermia-Induced Catalytic Hollow Nanoreactor for Bioorthogonal Organic Synthesis in Living Cells. Nano Lett. 2020, 20, 6981–6988. [Google Scholar] [CrossRef]
- Norouzi, N.; Das, M.K.; Richard, A.J.; Ibrahim, A.A.; El-Kaderi, H.M.; El-Shall, M.S. Heterogeneous catalysis by ultra-small bimetallic nanoparticles surpassing homogeneous catalysis for carbon–carbon bond forming reactions. Nanoscale 2020, 12, 19191–19202. [Google Scholar] [CrossRef]
- Yang, G.; Yang, H.; Zhang, X.; Lqbal, K.; Feng, F.; Ma, J.; Qin, J.; Yuan, F.; Cai, Y.; Ma, J. Surfactant-free self-assembly to the synthesis of MoO3 nanoparticles on mesoporous SiO2 to form MoO3/SiO2 nanosphere networks with excellent oxidative desulfurization catalytic performance. J. Hazard. Mater. 2020, 397, 122654. [Google Scholar] [CrossRef]
- Diaz, C.; Valenzuela, M.; Cifuentes-Vaca, O.; Segovia, M.; Laguna-Bercero, M. Iridium nanostructured metal oxide, its inclusion in silica matrix and their activity toward photodegradation of methylene blue. Mater. Chem. Phys. 2020, 252, 123276. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Wang, Y.; Luan, Y.; Li, X.; Du, X. Growth of Cu-BTC MOFs on dendrimer-like porous silica nanospheres for the catalytic aerobic epoxidation of olefins. New J. Chem. 2020, 44, 14350–14357. [Google Scholar] [CrossRef]
- Heravi, M.M.; Heidari, B.; Zadsirjan, V.; Mohammadi, L. Applications of Cu(0) encapsulated nanocatalysts as superior catalytic systems in Cu-catalyzed organic transformations. RSC Adv. 2020, 10, 24893–24940. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Chernyshevcd, V.V.; Fomkin, A.A.; Shkolin, A.V.; Veselovsky, V.V.; Kapustin, G.I.; Sokolova, N.A.; Kustov, L.M. Preparation of novel hybrid catalyst with an hierarchical micro-/mesoporous structure by direct growth of the HKUST-1 nanoparticles inside mesoporous silica matrix (MMS). Microporous Mesoporous Mater. 2020, 300, 110136. [Google Scholar] [CrossRef]
- Hemming, E.B.; Masters, A.F.; Maschmeyer, T. Exploring Opportunities for Platinum Nanoparticles Encapsulated in Porous Liquids as Hydrogenation Catalysts. Chem. A Eur. J. 2020, 26, 7059–7064. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, F.-Q.; Liao, X.; Lai, S.-L.; Wang, S.-B.; Xiong, X.-Q.; Zheng, J.; Liu, Y.-G. Customized three-dimensional porous catalyst for Knoevenagel reaction. J. Porous Mater. 2020, 27, 779–788. [Google Scholar] [CrossRef]
- Kumari, E.; Görlich, S.; Poulsen, N.; Kröger, N. Genetically Programmed Regioselective Immobilization of Enzymes in Biosilica Microparticles. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Azizi, S.; Soleymani, J.; Shojaei, S.; Shadjou, N. Synthesize of folic acid functionalized dendritic fibrous nanosilica and its application as an efficient nanocatalyst for access to direct amidation of carboxylic acids with amines. J. Nanostructures 2020, 10, 671–681. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, X.; Wang, X.; Zhang, X. Controllable Preparation of Monodisperse Mesoporous Silica from Microspheres to Microcapsules and Catalytic Loading of Au Nanoparticles. Langmuir 2020, 36, 5271–5279. [Google Scholar] [CrossRef]
- Casas-Luna, M.; Rodriguez, J.T.; Valdés-Martínez, O.U.; Obradović, N.; Slámečka, K.; Maca, K.; Kaiser, J.; Montúfar, E.B.; Čelko, L. Robocasting of controlled porous CaSiO3–SiO2 structures: Architecture–Strength relationship and material catalytic behavior. Ceram. Int. 2020, 46, 8853–8861. [Google Scholar] [CrossRef]
- Diaz, C.; Valenzuela, M.L.; Cifuentes-Vaca, O.; Segovia, M.; Laguna-Bercero, M.A. Incorporation of Nanostructured ReO3 in Silica Matrix and Their Activity Toward Photodegradation of Blue Methylene. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1726–1734. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pillai, H.S.; Lattimer, J.; Adli, N.M.; Karakalos, S.G.; Chen, M.; Guo, L.; Xu, H.; Yang, J.; et al. Ternary PtIrNi Catalysts for Efficient Electrochemical Ammonia Oxidation. ACS Catal. 2020, 10, 3945–3957. [Google Scholar] [CrossRef]
- Li, M.; Lv, J.; Wang, S.; Wang, J.; Lin, Y. Expanded mesoporous silica-encapsulated ultrasmall Pt nanoclusters as artificial enzymes for tracking hydrogen peroxide secretion from live cells. Anal. Chim. Acta 2020, 1104, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Toprakcioglu, Z.; Hakala, T.A.; Levin, A.; Becker, C.F.W.; Bernardes, G.J.L.; Knowles, T.P.J. Multi-scale microporous silica microcapsules from gas-in water-in oil emulsions. Soft Matter 2020, 16, 3082–3087. [Google Scholar] [CrossRef]
- Elimbinzi, E.; Nyandoro, S.S.; Mubofu, E.B.; Manayil, J.C.; Lee, A.F.; Wilson, K. Valorization of rice husk silica waste: Organo-amine functionalized castor oil templated mesoporous silicas for biofuels synthesis. Microporous Mesoporous Mater. 2020, 294, 109868. [Google Scholar] [CrossRef]
- Schulze, J.S.; Migenda, J.; Becker, M.; Schuler, S.M.M.; Wende, R.C.; Schreiner, P.R.; Smarsly, B.M. TEMPO-functionalized mesoporous silica particles as heterogeneous oxidation catalysts in flow. J. Mater. Chem. A 2020, 8, 4107–4117. [Google Scholar] [CrossRef]
- Yang, J.; Fang, X.; Xu, Y.; Liu, X. Investigation of the deactivation behavior of Co catalysts in Fischer–Tropsch synthesis using encapsulated Co nanoparticles with controlled SiO2 shell layer thickness. Catal. Sci. Technol. 2020, 10, 1182–1192. [Google Scholar] [CrossRef]
- Purohit, G.; Rawat, D.S.; Reiser, O. Palladium Nanocatalysts Encapsulated on Porous Silica @ Magnetic Carbon-Coated Cobalt Nanoparticles for Sustainable Hydrogenation of Nitroarenes, Alkenes and Alkynes. ChemCatChem 2020, 12, 569–575. [Google Scholar] [CrossRef]
- Tran, M.H.; Park, B.J.; Kim, B.H.; Yoon, H.H. Mesoporous silica template-derived nickel-cobalt bimetallic catalyst for urea oxidation and its application in a direct urea/H2O2 fuel cell. Int. J. Hydrogen Energy 2020, 45, 1784–1792. [Google Scholar] [CrossRef]
- Kong, L.; Guo, Y.; Wang, X.; Zhang, X. Double-walled hierarchical porous silica nanotubes loaded Au nanoparticles in the interlayer as a high-performance catalyst. Nanotechnology 2019, 31, 015701. [Google Scholar] [CrossRef]
- Mestre, R.; Cadefau, N.; Hortelão, A.C.; Grzelak, J.; Gich, M.; Roig, A.; Sánchez, S. Nanorods Based on Mesoporous Silica Containing Iron Oxide Nanoparticles as Catalytic Nanomotors: Study of Motion Dynamics. ChemNanoMat 2021, 7, 134–140. [Google Scholar] [CrossRef]
- Chen, I.-H.; Lee, T.-Y.; Tseng, Y.-C.; Liou, J.-H.; Jan, J.-S. Biomineralization of mesoporous silica and metal nanoparticle/mesoporous silica nanohybrids by chemo-enzymatically prepared peptides. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610, 125753. [Google Scholar] [CrossRef]
- Elahimehr, Z.; Nemati, F.; Elhampour, A. Synthesis of a magnetic-based yolk-shell nano-reactor: A new class of monofunctional catalyst by Cu0-nanoparticles and its application as a highly effective and green catalyst for A3 coupling reaction. Arab. J. Chem. 2020, 13, 3372–3382. [Google Scholar] [CrossRef]
- Le Ferrand, H. Pressure-Less Processing of Ceramics with Deliberate Elongated Grain Orientation and Size. Light Met. 2020, 2020, 45–56. [Google Scholar]
- Yang, Y.; Veser, G. Exploiting the impact of pore diffusion in core@shell nanocatalysts. Catal. Today 2020, 2–9. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, S.; Yang, Z.-L.; Liu, B. Patchy Templated Synthesis of Macroporous Colloidal Hollow Spheres and Their Application as Catalytic Microreactors. ACS Appl. Mater. Interfaces 2019, 11, 47008–47014. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Corvo, M.C.; Pires, J.; Campos-Martin, J.M.; De Castro, B.; Balula, S.S. Mesoporous Silica vs. Organosilica Composites to Desulfurize Diesel. Front. Chem. 2019, 7, 7. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kawamoto, K.; Kobayashi, N. Effect of porous silica on the removal of tar components generated from waste biomass during catalytic reforming. Fuel Process. Technol. 2019, 194, 106104. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Chen, D.; Yu, Q.; Yang, W.; Zhou, J.; Wu, S. Facile, template-free synthesis of macroporous SiO2 as catalyst support towards highly enhanced catalytic performance for soot combustion. Chem. Eng. J. 2019, 375, 121958. [Google Scholar] [CrossRef]
- Ayakar, S.R.; Yadav, G.D. Development of novel support for penicillin acylase and its application in 6-aminopenicillanic acid production. Mol. Catal. 2019, 476, 110484. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Shao, Z.-D.; Liu, Q.; Xie, Z.; Zhao, F.; Zheng, Y.-M. Flexible and porous TiO2/SiO2/carbon composite electrospun nanofiber mat with enhanced interfacial charge separation for photocatalytic degradation of organic pollutants in water. J. Colloid Interface Sci. 2019, 553, 156–166. [Google Scholar] [CrossRef]
- Kinkead, B.; Malone, R.; Smith, G.; Pandey, A.; Trifkovic, M. Bicontinuous Intraphase Jammed Emulsion Gels: A New Soft Material Enabling Direct Isolation of Co-Continuous Hierarchial Porous Materials. Chem. Mater. 2019, 31, 7601–7607. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-W.; Zheng, R.-R.; Yu, H.; Dong, X.-T. Preparation and characterization of mesoporous g-C3N4/SiO2 material with enhanced photocatalytic activity. J. Mater. Res. 2019, 34, 1785–1794. [Google Scholar] [CrossRef]
- Xu, W.-M.; Chai, K.; Jiang, Y.-W.; Mao, J.; Wang, J.; Zhang, P.; Shi, Y. 2D Single Crystal WSe2 and MoSe2 Nanomeshes with Quantifiable High Exposure of Layer Edges from 3D Mesoporous Silica Template. ACS Appl. Mater. Interfaces 2019, 11, 17670–17677. [Google Scholar] [CrossRef]
- Mahy, J.G.; Tasseroul, L.; Tromme, O.; Lavigne, B.; Lambert, S.D. Hydrodechlorination and complete degradation of chlorinated compounds with the coupled action of Pd/SiO2 and Fe/SiO2 catalysts: Towards industrial catalyst synthesis conditions. J. Environ. Chem. Eng. 2019, 7, 103014. [Google Scholar] [CrossRef]
- Song, L.; Lin, Z.; Zhao, Y.; Hu, X.; Hong, Y.; Su, Y.; Wang, H.; Li, J. A CO2-expanded gelation approach to prepare bimodal porous silica materials and their catalytic applications. J. Supercrit. Fluids 2019, 144, 91–97. [Google Scholar] [CrossRef]
- Weinberger, C.; Heckel, T.; Schnippering, P.; Schmitz, M.; Guo, A.; Keil, W.; Marsmann, H.C.; Schmidt, C.; Tiemann, M.; Wilhelm, R. Straightforward Immobilization of Phosphonic Acids and Phosphoric Acid Esters on Mesoporous Silica and Their Application in an Asymmetric Aldol Reaction. Nanomater. 2019, 9, 249. [Google Scholar] [CrossRef]
- Fathali, Z.; Rezaei, S.; Faramarzi, M.A.; Habibi-Rezaei, M. Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int. J. Biol. Macromol. 2019, 122, 359–366. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Fang, R.; Li, Y.; Chen, Z. Hollow-Co3 O4 @Co3 O4 @SiO2 Multi-Yolk-Double-Shell Nanoreactors for Highly Efficient CO Oxidation. ChemCatChem 2019, 11, 772–779. [Google Scholar] [CrossRef]
- Bolshakov, A.; Van Diepen, M.; Van Hoof, A.J.F.; Hidalgo, D.E.R.; Kosinov, N.; Hensen, E.J.M. Hierarchically Porous (Alumino)Silicates Prepared by an Imidazole-Based Surfactant and Their Application in Acid-Catalyzed Reactions. ACS Appl. Mater. Interfaces 2019, 11, 40151–40162. [Google Scholar] [CrossRef] [PubMed]
- Gößl, D.; Singer, H.; Chiu, H.-Y.; Schmidt, A.; Lichtnecker, M.; Engelke, H.; Bein, T. Highly active enzymes immobilized in large pore colloidal mesoporous silica nanoparticles. New J. Chem. 2018, 43, 1671–1680. [Google Scholar] [CrossRef]
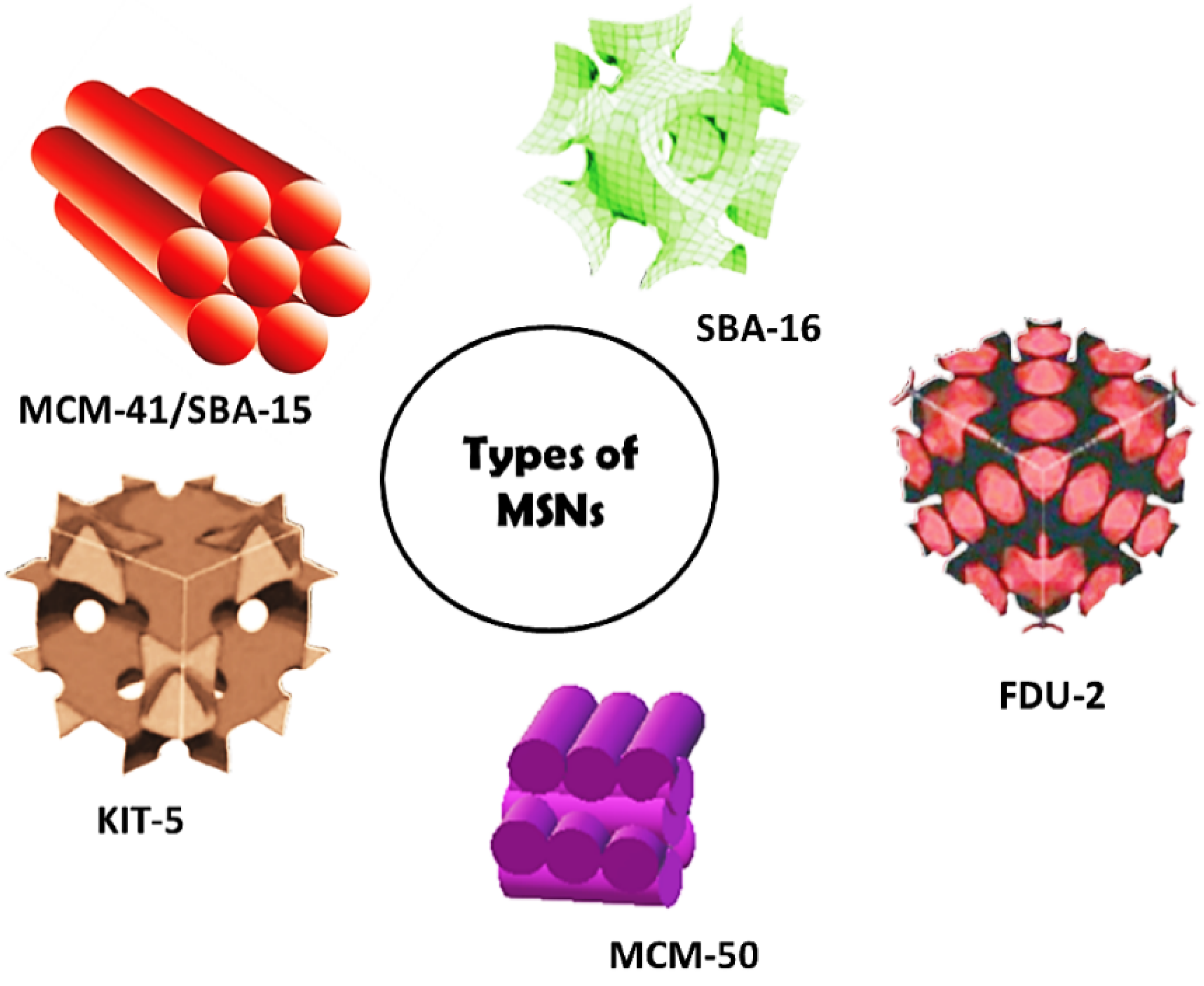

| MSN Family | MSN Type | Pore Symmetry | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| M41S | MCM-41 | 2D hexagonal | 1.5–8 | >1.0 |
| MCM-48 | 3D cubic | 2–5 | >1.0 | |
| MCM-50 | Lamellar | 2–5 | >1.0 | |
| SBA | SBA-11 | 3D cubic | 2.1–3.6 | 0.68 |
| SBA-12 | 3D hexagonal | 3.1 | 0.83 | |
| SBA-15 | 2D hexagonal | 6–0 | 1.17 | |
| SBA-16 | Cubic | 5–15 | 0.91 | |
| KIT | KIT-5 | Cubic | 9.3 | 0.45 |
| COK | COK-12 | Hexagonal | 5.8 | 0.45 |
| FDU | FDU-12 | 3D Cubic | 10–26 | 0.66 |
| Full Name | Santa Barbara Amorphous Type 15 | Santa Barbara Amorphous Type 16 | Mobil Composition of Matter No. 41 | Mobil Composition of Matter No. 48 | Hexagonal Mesoporous Silica |
|---|---|---|---|---|---|
| Short name | SBA-15 | SBA-16 | MCM-41 | MCM-48 | HMS |
| Structure directing agent | Pluronic 123 (non-ionic) | Pluronic F127 (non-ionic) | CTAB (cationic) | CTAB (cationic) | Amines (non-ionic) |
| pH at synthesis | Acidic (pH ~ 1) | Acidic (pH ~ 1) | Basic (pH ~ 11–13) | Basic (pH ~ 11–13) | Basic (pH ~ 9) |
| Features | Hexagonal pores, 2D array, p6mm symmetry, channels interconnected by small micropores | 3D cubic arrangement connected by spherical cavities, Im3m space symmetry | 1D mesopores, p6mm hexagonal, absence of interconnected pores | Ia3d 3D cubic continuous pore arrangement | Sponge-like particles, warm-hole mesostructured framework |
| Pore diameter | Uniform and larger pore diameter (4–30 nm) facilitating easy diffusion | Similar pore diameter values but nonuniform mesopores | Smaller pore diameter (1.5–10 nm) hindering the diffusion of substrates | Smaller pore diameter (2–3 nm) hindering the diffusion of substrates | Smaller pore diameter than SBA-15 (2–10) |
| Range of surface area | Higher surface area (~1000 m2/g), high surface area to volume ratio | Comparable surface area values to SBA-15 | Lower surface area (~800 m2/g) | Higher surface area (~1100 m2/g) | Surface area (800–1000 m2/g) |
| Stability | Thick walls (up to 9 nm) and hence more thermally stable | Thick walls comparable to SBA-15 | Thin walls (0.5 nm) and thus poor hydrothermal stability | Thin walls and hence comparatively less thermally stable | Less ordered structure but comparable stability |
| Sr. No. | Material | Type of Synthesis | Catalyst | Chemical Reaction | Ref. |
|---|---|---|---|---|---|
| 1. | Core-shell structured magnetic silica. | Sol-gel | Bronsted acid | Transesterification of soybean oils, low-quality oils to biodiesel | [101] |
| 2. | Chondroitinase ABC (I) on red porous silicon nanoparticles | Electrochemical etching | chrondroitinase | Biological enzyme catalysis reaction | [102] |
| 3. | Mesoporous-silica-supported metal nanocatalysts | Sol–gel | Metal nanocatalyst (Ag, Pd, amines) | Dehydrogenation of formic acid for Hydrogen generation (HCOOH -> H2 + CO2) | [103] |
| 4. | Mesoporous silica (SBA-15, MCM-41) | Sol–gel | Palladium and platinum nanoparticles | Organic synthesis alcohols, carboxylic acids, and esters | [104] |
| 5. | Mesoporous silica spheres and nanocapsules | Soft and hard dual template | Sulfonic acid | Biomass valorization catalysis, conversion of cellobiose into glucose | [105] |
| 6. | Silica nanoshell (Pd/Fe3O4@h-SiO2) | Sol–gel | Pd nanocrystals | Biorthogonal Organic Synthesis, carbocyclization reactions, converting a range of non-fluorescent substrates to fluorescent products | [106] |
| 7. | Mesoporous fumed silica | Sol–gel | Palladium, Cobalt, Nickel, and Copper | Suzuki cross-coupling (SCC) reactions (C-C bond forming reaction) Cross-coupling reaction between bromobenzene with benzeneboronic acid give biphenyl | [107] |
| 8. | Mesoporous MoO3/SiO2 nanosphere networks | Self-assembly | MoO3 | oxidative desulfurization of Dibenzothiophene (DBT) | [108] |
| 9. | Silica | Sol–gel | IrO2 | Photodegradation of methylene blue | [109] |
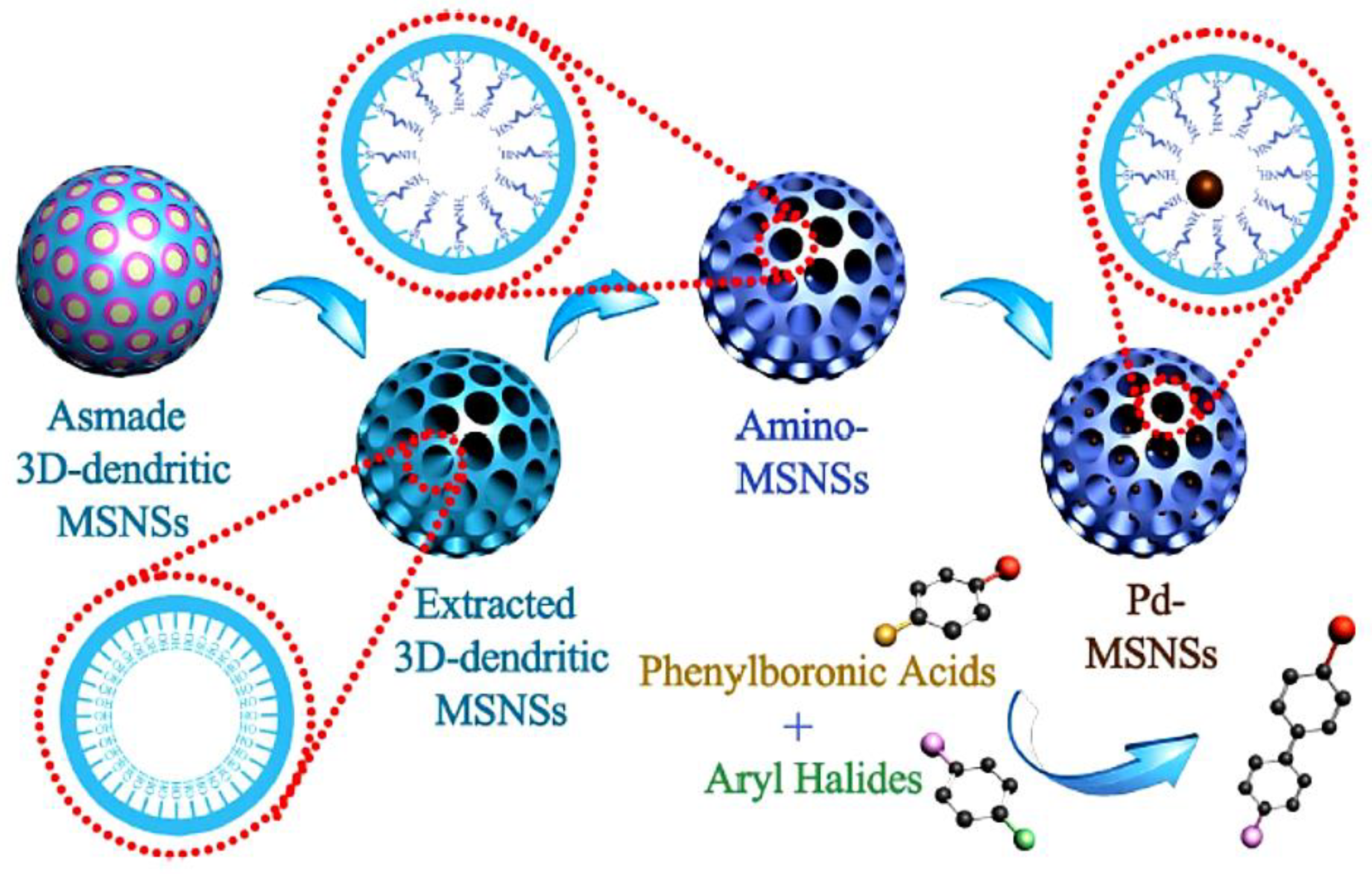
| 10. | Dendrimer-like Porous Silica Nanoparticles (DPSNs) | Template-mediated self-assembly | Cu-BTC MOFs | Catalytic aerobic epoxidation of olefins, cyclooctene to cyclooctane oxide | [110] |
| 11. | Mesoporous Silica (Fe@silica) | Sol-gel and Hydrothermal | Iron | Oxidation | [82] |
| 12. | Silica (CuO@SiO2) | Sol-gel | Cu nanoparticles (NPs) | Cu-catalyzed organic transformations, C-C bond formation, reduction of organic dye | [111] |
| 13. | Mesoporous silica matrix (MMS) | Direct growth technique | Hongkong University of Science and Technology (HKUST-1) (Cu3(BTC)2, BTC ¼ benzene-1,3,5-tricarboxylate) nanoparticles | Condensation reaction, Friedlander reaction between 2-amino-5-chlorobenzophenone and acetylacetone and Henry reaction nitroaldol condensation between nitromethane and 4- nitrobenzaldehyde | [112] |
| 14. | Functionalized mesoporous SBA-15, SBA-16, MCM-41, MCM-48 | Sol-gel | Metal nanoparticles (Ti, V, Cr, and Mo) | Catalytic transformation, Dry Reforming of Methane (DRM) reaction as CH4 + CO2 -> 2H2+ 2CO | [33] |
| 15. | Porous SiO2 (Pt@HS-SiO2 PL) | So-gel | Platinum Nanoparticles | Hydrogenation of alkenes (decene to decane) and nitroarenes to amino phenol | [113] |
| 16. | Silica (SiO2) powder | Sol-gel | Diethylenetriamine | Knoevenagel reaction (carbon-carbon (C-C) coupling). | [114] |
| 17. | Biosilica Microparticles | Diatom (Thalassiosira pseudonana) | Horseradish peroxidase (HRP), glucose oxidase, Gold nanoparticles | Biological enzyme catalysis reaction of T. pseudonana, Oxidation of glucose. | [115] |
| 18. | Folic acid-functionalized dendritic fibrous nano-silica (FA-KCC-1-NH2) (FA = Folic acid, KCC-I = fibrous nano silica) | Hydrothermal | KCC-1-NH-FA nanoparticles | Amidation of carboxylic acids with amines | [116] |
| 19. | Monodisperse mesoporous silica microspheres (M-MSMs) | Sol–gel | Au Nanoparticles | Reduction of 4-nitrophenol (4-NP) to 4-amino phenol | [117] |
| 20. | CaSiO3-SiO2 powder | Co-precipitation method | CaSiO3 (Na2O·nSiO2) | Decomposition of isopropyl alcohol, dehydrogenation of the alcohol producing acetone | [118] |
| 21. | ReO3/SiO2, silica matrix | Sol-gel | ReO3 nanoparticles | Photodegradation of Blue Methylene | [119] |
| 22. | Porous silicon dioxide (SiO2) and carboxyl-functionalized carbon nanotube (PtIrNi/SiO2-CNT-COOH) | Sol-gel | PtIrNi alloy nanoparticles | Electrochemical Ammonia Oxidation reaction (AOR) | [120] |
| 23. | Expanded mesoporous silica (EMSN)-encapsulated Pt nanoclusters. | Sol-gel | Pt nanoclusters | Artificial enzymes for tracking hydrogen peroxide secretion from live cells | [121] |
| 24. | Microporous silica microcapsules | Gas-in-water-in oil emulsions (g/w/o) | Microporous silica microcapsules | Ostwald ripening, generation of gas-in-water-in-oil emulsions | [122] |
| 25. | Pt-loaded ZSM-22/MCM-4 (Pt-MES) | Sol-gel | Bronsted acid, Pt nanoparticles | N-alkane isomerization for refinery process by converting the petroleum into the gasoline with high quality and the diesel | [90] |
| 26. | Organo-amine-functionalized castor oil templated mesoporous silicas | Valorization of rice husk | Amine groups | Biodiesel synthesis, transesterification of model C4-C12 triglycerides (TAG) to fatty acid methyl esters | [123] |
| 27. | TEMPO-functionalized mesoporous silica particles, MCM-41 and SBA- | Co-condensation | (2,2,6,6 tetramethylpiperidin-1-yl) oxyl (TEMPO) | Heterogeneous oxidation (oxidation of alcohols to aldehydes), Knoevenagel condensation (C-C bond formation) | [124] |
| 28. | Silica-encapsulated core–shell Co@SiO2 | Hydrothermal | Cobalt | Fischer-Tropsch synthesis (FTS) | [125] |
| 29. | Porous silica | Sol-gel | Gold nanoparticles | Biomedical, catalytic, and optical properties | [14] |
| 30. | Palladium Nanocatalysts Encapsulated on Porous Silica @ Magnetic Carbon-Coated Cobalt Nanoparticles | Sol-gel | Palladium nanoparticles | Sustainable hydrogenation of nitroarenes to aniline, alkenes and alkynes | [126] |
| 31. | SBA-15-based composites (X@SBA-15) | Impregnation and hydrothermal methods | Transition metals/metal oxides and nanocarbons | Water decontamination by advanced oxidation processes | [25] |
| 32. | Mesoporous silica | Sol-gel | Ni-Co bimetallic hydroxide particles | Urea oxidation reaction | [127] |
| 33. | Porous silica nanotubes loaded Au nanoparticles (SiO2@Au@SiO2 NTs) | Sol-gel | Gold nanoparticles | Catalytic reduction of 4-Nitrophenol to 4-amino phenol | [128] |
| 34. | Self-propelled mesoporous silica nanorods (MSNRs) | Sol-gel | Iron oxide (Fe2O3) nanoparticles | Catalytic decomposition of hydrogen peroxide by a sputtered Pt layer | [129] |
| 35. | Porous silica | Self-assembly | Metal and alloy nanoparticles (Au, Ag, pd, Ag/Pd) | Biomineralization, reduction of 4-nitrophenol to 4-amino phenol | [130] |
| 36. | A novel and yolk/shell nanoreactor catalyst (H-Fe3O4@h-Cu0@m-SiO2) | Hydrothermal | CuO-nanoparticles | A3 coupling reaction of alkynes, aldehydes, and amines | [131] |
| 37. | Micron-sized, spherical SiO2 | Mechanochemical | Spherical silica | Polyolefin catalyst production | [132] |
| 38. | Ni@SiO2 core–shell nanocatalysts | Sol-gel | Ni particles | Catalytic oxidation of CH4 to CO2 | [133] |
| 39. | Hollow SiO2 spheres | Template synthesis | Au nanoparticles | Catalytic Microreactors, reduction of 4-nitrophenol to 4-aminophenol | [134] |
| 40. | Monolacunary Keggin-type [PW11O39] 7-(PW11) heteropolyanion SBA-15 (PW11@TMA-SBA-15) | Sol-gel | N-trimethylammonium (TMA) | Oxidative desulfurization of organosilica composite | [135] |
| 41. | Porous silica | Sol-gel | Ni nanocatalyst | Thermal gasification of waste biomass | [136] |
| 42. | Macroporous SiO2 | Sol-gel | Ag2O, Na2O or K2O | Soot combustion reactions, gas-solid-solid reactions | [137] |
| 43. | Aminopropyl functionalized mesocellular foam silica (MCF) | Sol-gel | Penicillin acylase | 6-aminopenicillanic acid production, biocatalytic transformation | [138] |
| 44. | TiO2/SiO2/C nanofiber mat, SiO2 nanoparticles | Calcination | TiO2/SiO2/C | Photocatalytic degradation of organic pollutants (rhodamine B and 4-nitrophenol) in water | [139] |
| 45. | Alumina-coated silica nanoparticles (AlO-SiO NPs) | Sol-gel | Alumina | Surface reactions | [140] |
| 46. | mesoporous g-C3N4/SiO2 material | Sol-gel | Carbon nitride (g-C3N4) | Photodegradation of rhodamine B (RhB) under visible light | [141] |
| 47. | Mesoporous silica material KIT-6 | Template synthesis | Transition metals | Electrocatalytic hydrogen evolution reaction | [142] |
| 48. | Pd/SiO2 and Fe/SiO2 | Sol-gel | Metallic (Pd catalysts) or metallic oxide (Fe catalysts) nanoparticles | Pd/SiO2 Hydrodechlorination of 2,4,6-trichlorophenol (TCP) in water, Fe/SiO2 materials degrade phenol | [143] |
| 49. | Bimodal porous silica | Sol-gel | NiO | Phenol to cyclohexanol | [144] |
| 50. | Mesoporous silica materials (SBA-15 and MCM-41) | Sol-gel | Phosphonic and phosphoric acid esters | Asymmetric aldol reaction (C-C bond formation) | [145] |
| 51. | Mesoporous silica (SBA-15) | Sol-gel | Laccase | Enzyme aggregate (E-CLEA) potential in phenol removal | [146] |
| 52. | Novel hollow-Co3O4 @Co3O4@SiO2 multi-yolk-double-shell nanoreactors | Sol-gel | Metals (Pd, Pt, Ru, Rh, and Au) and metal oxides. | CO Oxidation | [147] |
| 53. | Ordered mesoporous silicas (MCM-41) | Sol-gel | Aluminum | Hydro isomerization and Friedel-Crafts alkylation of benzene with benzyl alcohol | [148] |
| 54. | Colloidal mesoporous silica nanoparticles (LP-MSNs) | Co-condensation | Alkyne-functionalized | Colorimetric reaction of guaiacol (2-methoxyphenol), hydrolysis of 4-nitrophenyl acetate (NPA) by LP-MSN-CA | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. J. Compos. Sci. 2021, 5, 75. https://doi.org/10.3390/jcs5030075
Shinde PS, Suryawanshi PS, Patil KK, Belekar VM, Sankpal SA, Delekar SD, Jadhav SA. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. Journal of Composites Science. 2021; 5(3):75. https://doi.org/10.3390/jcs5030075
Chicago/Turabian StyleShinde, Preeti S., Pradnya S. Suryawanshi, Kanchan K. Patil, Vedika M. Belekar, Sandeep A. Sankpal, Sagar D. Delekar, and Sushilkumar A. Jadhav. 2021. "A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports" Journal of Composites Science 5, no. 3: 75. https://doi.org/10.3390/jcs5030075
APA StyleShinde, P. S., Suryawanshi, P. S., Patil, K. K., Belekar, V. M., Sankpal, S. A., Delekar, S. D., & Jadhav, S. A. (2021). A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. Journal of Composites Science, 5(3), 75. https://doi.org/10.3390/jcs5030075






