Fire Protective Surface Coating Containing Nanoparticles for Marine Composite Laminates
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
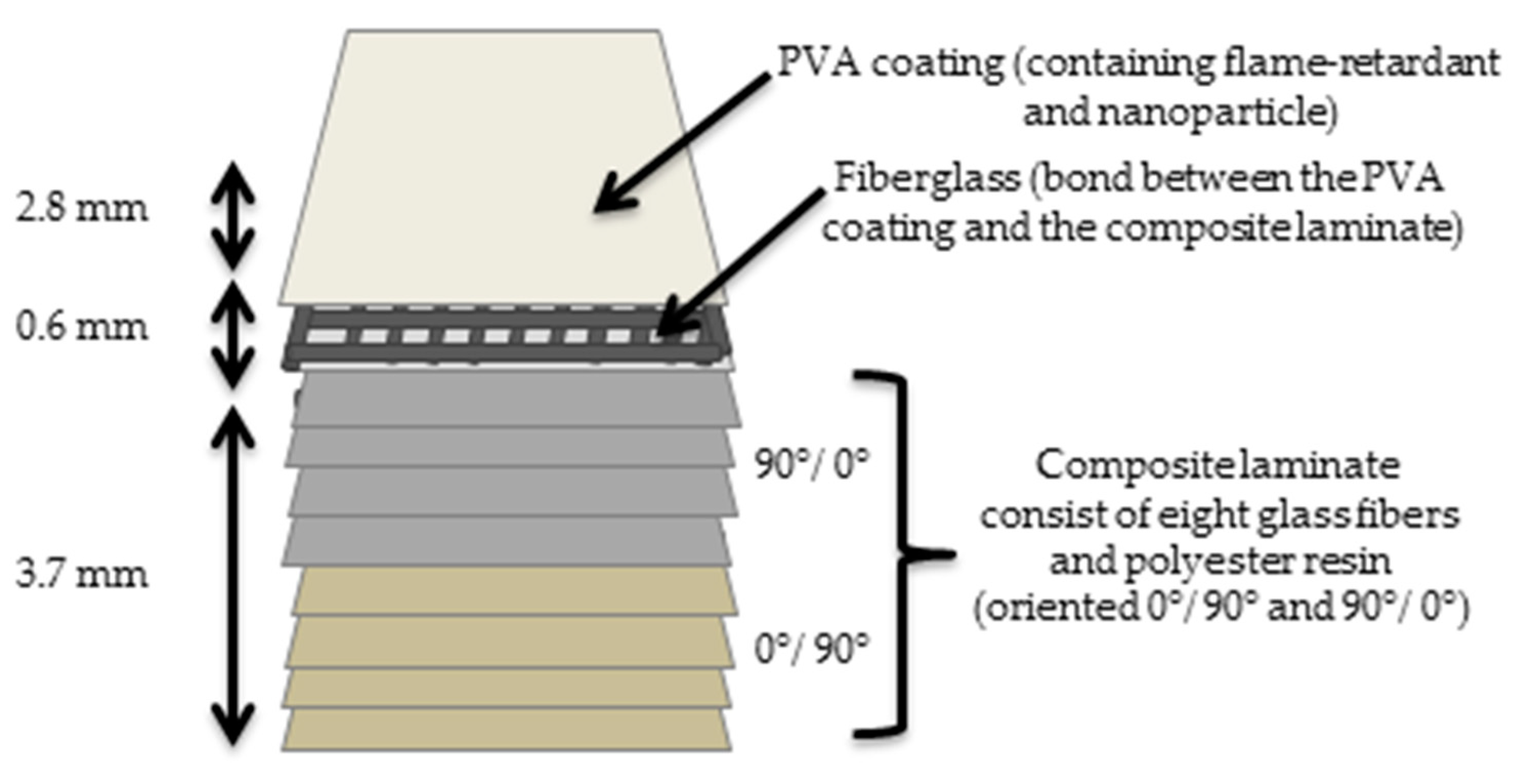
2.2. Sample Preparation
2.3. Characterization
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Cone Calorimeter
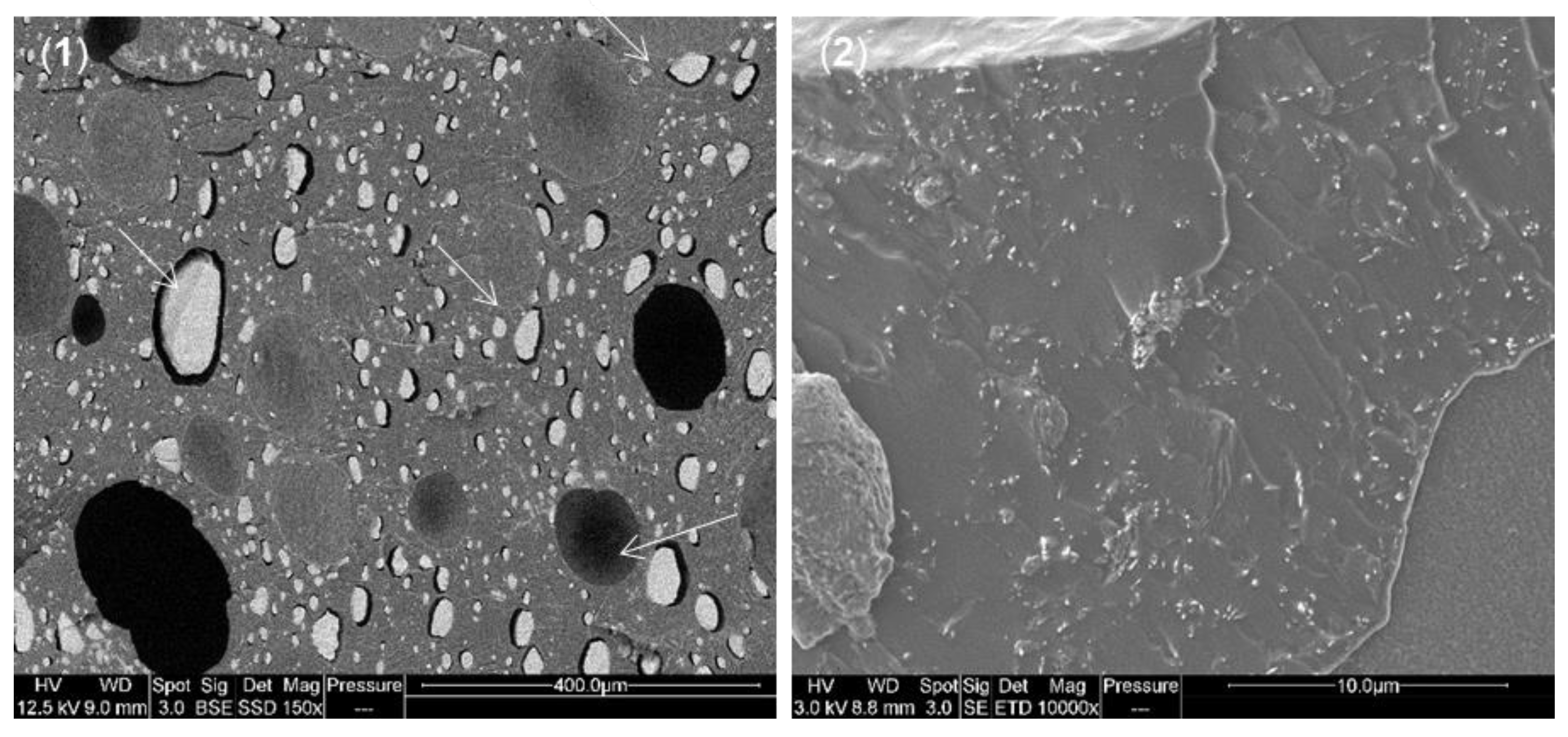
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. XRD Analysis
2.3.5. Photogrammetry
3. Results and Discussions
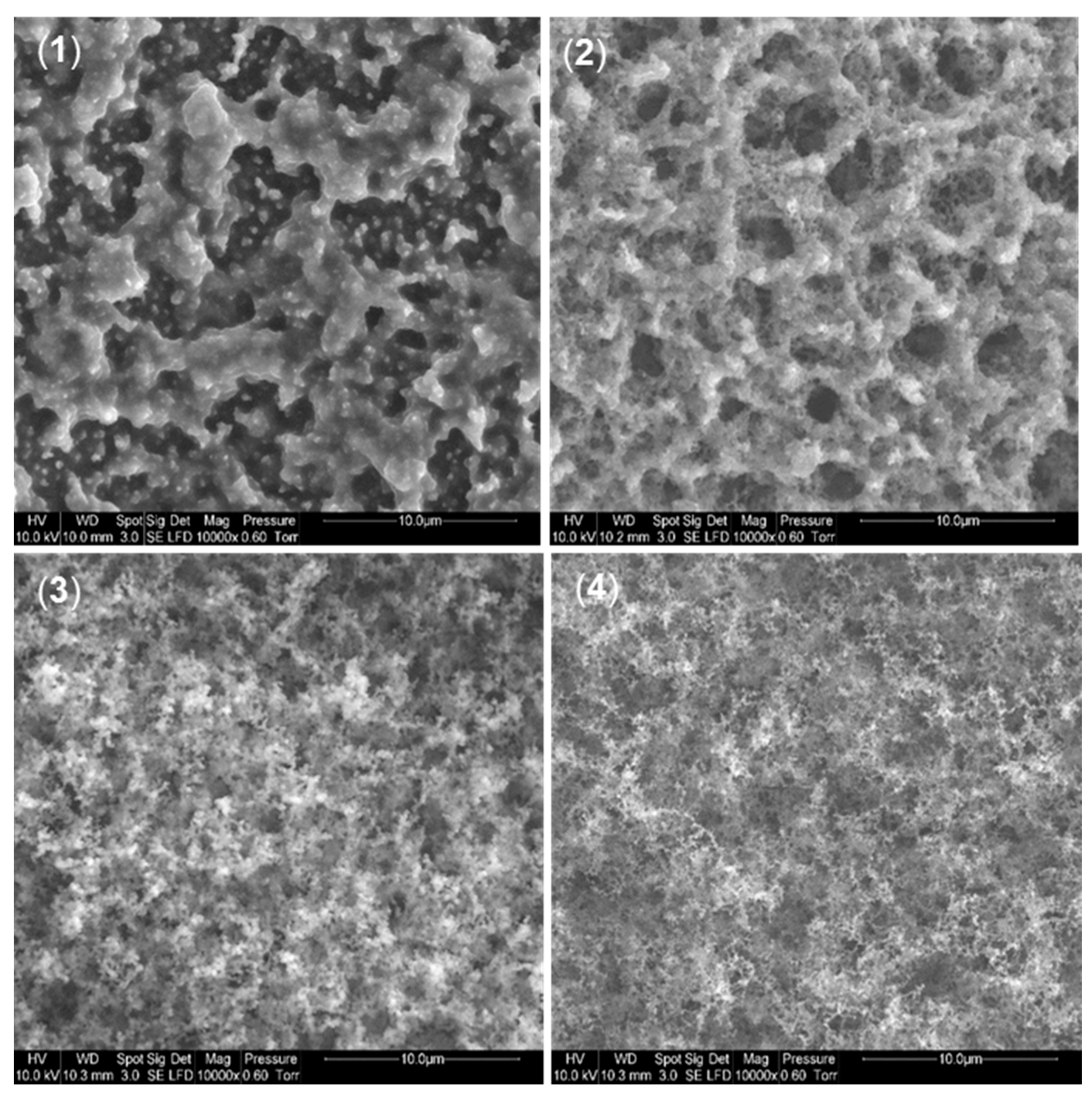
3.1. Dispersion of Particles in PVA/APP/SP Coatings
3.2. Thermal Behavior of PVA Coatings
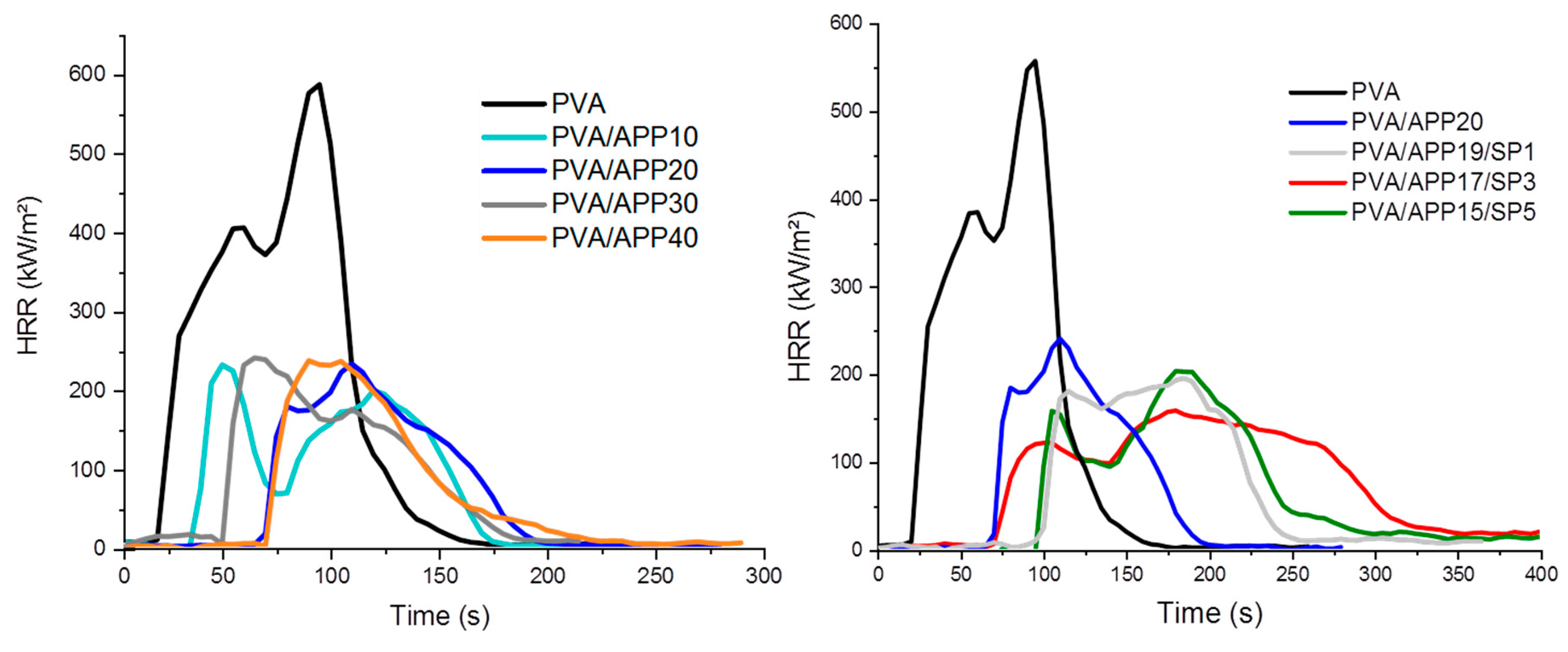
3.3. Fire Behavior of PVA/APP/SP Coatings
3.4. Characterization of Cone Calorimeter Residues
3.4.1. Composition of the Char Residue by EDX Analysis
3.4.2. XRD Analysis of Residues
3.4.3. Morphology of the Residues
3.5. Fire Resistance of GFRP Composite Laminate with PVA Coatings
3.5.1. Cone Calorimeter Tests
3.5.2. Characterization of Residues
SEM Analysis
XRD Analysis
Char Volume of Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, P.; Nguyen, Q.T.; Lau, K.T. Fire Performance of Polymer-Based Composites for Maritime Infrastructure. Compos. Part B 2018, 155, 31–48. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Gellert, E.; Burchill, P.; Challis, K. Review of Advanced Composite Structures for Naval Ships and Submarines. Compos. Struct. 2001, 53, 21–41. [Google Scholar] [CrossRef]
- Kandola, B.K.; Kandare, E. Advances in Fire Retardant Materials: Composites Having Improved Fire Resistance; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Kandola, B.K.; Ebdon, J.R.; Luangtriratana, P.; Krishnan, L. Novel Flame Retardant Thermoset Resin Blends Derived from a Free-Radically Cured Vinylbenzylated Phenolic Novolac and an Unsaturated Polyester for Marine Composites. Polym. Degrad. Stab. 2016, 127, 56–64. [Google Scholar] [CrossRef]
- Kandare, E.; Chukwudolue, C.; Kandola, B.K. The Use of Fire-Retardant Intumescent Mats for Fire and Heat Protection of Glass Fibre-Reinforced Polyester Composites: Thermal Barrier Properties. Fire Mater. 2010, 34, 21–38. [Google Scholar] [CrossRef]
- Tibiletti, L.; Longuet, C.; Ferry, L.; Coutelen, P.; Mas, A.; Robin, J.J.; Lopez-Cuesta, J.M. Thermal Degradation and Fire Behaviour of Unsaturated Polyesters Filled with Metallic Oxides. Polym. Degrad. Stab. 2011, 96, 67–75. [Google Scholar] [CrossRef]
- Kandare, E.; Kandola, B.K.; Price, D.; Nazaré, S.; Horrocks, R.A. Study of the Thermal Decomposition of Flame-Retarded Unsaturated Polyester Resins by Thermogravimetric Analysis and Py-GC/MS. Polym. Degrad. Stab. 2008, 93, 1996–2006. [Google Scholar] [CrossRef]
- Tibiletti, L.; Ferry, L.; Longuet, C.; Mas, A.; Robin, J.; Lopez-Cuesta, J.-M. Thermal Degradation and Fire Behavior of Thermoset Resins Modified with Phosphorus Containing Styrene. Polym. Degrad. Stab. 2012, 97, 2602–2610. [Google Scholar] [CrossRef]
- Shekarchi, M.; Farahani, E.M.; Yekrangnia, M.; Ozbakkaloglu, T. Mechanical Strength of CFRP and GFRP Composites Filled with APP Fire Retardant Powder Exposed to Elevated Temperature. Fire Saf. J. 2020, 115, 15. [Google Scholar] [CrossRef]
- Sorathia, U.; Gracik, T.; Ness, J.; Durkin, A.; Williams, F.; Hunstad, M.; Berry, F. Evaluation of Intumescent Coatings for Shipboard Fire Protection. J. Fire Sci. 2003, 21, 423–450. [Google Scholar] [CrossRef]
- Luangtriratana, P.; Kandola, B.K.; Myler, P. Ceramic Particulate Thermal Barrier Surface Coatings for Glass Fibre-Reinforced Epoxy Composites. Mater. Des. 2014, 68, 232–244. [Google Scholar] [CrossRef]
- Luangtriratana, P.; Kandola, B.K.; Duquesne, S.; Bourbigot, S. Quantification of Thermal Barrier Efficiency of Intumescent Coatings on Glass Fibre-Reinforced Epoxy Composites. Coatings 2018, 8, 347. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Alkan, M.; Benlikaya, R. Poly(Vinyl Alcohol) Nanocomposites with Sepiolite and Heat-Treated Sepiolites. J. Appl. Polym. Sci. 2009, 112, 3764–3774. [Google Scholar] [CrossRef]
- Lin, J.S.; Liu, Y.; Wang, D.Y.; Qin, Q.; Wang, Y.Z. Poly(Vinyl Alcohol)/Ammonium Polyphosphate Systems Improved Simultaneously Both Fire Retardancy and Mechanical Properties by Montmorillonite. Ind. Eng. Chem. Res. 2011, 9998–10005. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Wang, D.; Wang, D.; Wang, Y. Synergistic Effect of Ammonium Polyphosphate and Layered Double Hydroxide on Flame Retardant Properties of Poly (Vinyl Alcohol). Polym. Degrad. Stab. 2008, 93, 1323–1331. [Google Scholar] [CrossRef]
- Quach, Y.; Ferry, L.; Sonnier, R. Efficiency of Wollastonite and Ammonium Polyphosphate Combinations on Flame Retardancy of Polystyrene. Polym. Adv. Technol. 2012, 10, 104–113. [Google Scholar] [CrossRef]
- Rimez, B.; Rahier, H.; Biesemans, M.; Bourbigot, S.; Van Mele, B. Flame Retardancy and Degradation Mechanism of Poly(Vinyl Acetate) in Combination with Intumescent Flame Retardants: I. Ammonium Poly(Phosphate). Polym. Degrad. Stab. 2015, 121, 321–330. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New Prospects in Flame Retardant Polymer Materials: From Fundamentals to Nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T. Composites: Part A Multiwalled Carbon Nanotubes and Sepiolite Nanoclays as Flame Retardants for Polylactide and Its Natural Fibre Reinforced Composites. Compos. Part A 2010, 41, 954–963. [Google Scholar] [CrossRef]
- Bodzay, B.; Bocz, K.; Bárkai, Z.; Marosi, G. Influence of Rheological Additives on Char Formation and Fire Resistance of Intumescent Coatings. Polym. Degrad. Stab. 2011, 96, 355–362. [Google Scholar] [CrossRef]
- Tian, G.; Han, G.; Wang, F.; Liang, J. Sepiolite Nanomaterials: Structure, Properties and Functional Applications, Nanomateri. In Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Huang, N.H.; Chen, Z.J.; Wang, J.Q.; Wei, P. Synergistic Effects of Sepiolite on Intumescent Flame Retardant Polypropylene. Express Polym. Lett. 2010, 4, 743–752. [Google Scholar] [CrossRef]
- Vahabi, H.; Lin, Q.; Vagner, C.; Cochez, M.; Ferriol, M.; Laheurte, P. Investigation of Thermal Stability and Flammability of Poly(Methyl Methacrylate) Composites by Combination of APP with ZrO2, Sepiolite or MMT. Polym. Degrad. Stab. 2016, 124, 60–67. [Google Scholar] [CrossRef]
- Pappalardo, S.; Russo, P.; Acierno, D.; Rabe, S.; Schartel, B. The Synergistic Effect of Organically Modified Sepiolite in Intumescent Flame Retardant Polypropylene. Eur. Polym. J. 2016, 76, 196–207. [Google Scholar] [CrossRef]
- Carretier, V.; Delcroix, J.; Pucci, M.F.; Rublon, P. Influence of Sepiolite and Lignin as Potential Synergists on Flame Retardant Systems in Polylactide (PLA) and Polyurethane Elastomer (PUE). Materials 2020, 13, 2450. [Google Scholar] [CrossRef]
- García-romero, E.; Suárez, M. Sepiolite–Palygorskite: Textural Study and Genetic Considerations. Appl. Clay Sci. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, M.; Liu, F.; Zhang, C. Flame-Retardant Polyvinyl Alcohol Membrane with High Transparency Based on a Reactive Phosphorus- Containing Compound. R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Jia, D.-M.; Cui, Y.-F.; Xie, D. Kinetics Analysis of Thermal Degradation Reaction of PVA and PVA/Starch Blends. Reinf. Plast. Compos. 2009, 28, 2771–2780. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L.; Costanzi, F.; Pagliari, A. Study of the Mechanism of Intumescence in Fire Retardant Polymers: Part VI-Mechanism of Ester Formation in Ammonium Polyphosphate-Pentaerythritol Mixtures. Polym. Degrad. Stab. 1985, 12, 213–228. [Google Scholar] [CrossRef]
- Camino, G.; Luda, M.P. Mechanistic Study of Intumescence. In FRPM 97: 6th European Meeting on Fire Retardancy of Polymeric Materials; Royal Society of Chemistry: London, UK, 1998. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wang, F.; Liang, J.; Ran, S. Phase Transformation and Morphology Evolution of Sepiolite Fibers during Thermal Treatment. Appl. Clay Sci. 2017, 143, 205–211. [Google Scholar] [CrossRef]
- Winans, R.E.; Seifert, S.; Carrado, K.A. In Situ SAXS Studies of the Structural Changes of Sepiolite Clay and Sepiolite-Carbon Composites with Temperature. Chem. Mater. 2002, 14, 739–742. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of Fire-Retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Dumazert, L.; Rasselet, D.; Lopez-Cuesta, J.-M.; Pang, B.; Gallard, B.; Kennouche, S. Thermal Stability and Fire Reaction of Poly (Butylene Succinate) Nanocomposites Using Natural Clays and FR Additives. Polym. Adv. Technol. 2018, 29, 69–83. [Google Scholar] [CrossRef]
- Duquesne, S.; Samyn, F.; Bourbigot, S.; Amigouet, P.; Jouffret, F.; Shen, K. Influence of Talc on the Fire Retardant Properties of Highly Filled Intumescent Polypropylene Composites. Polym. Adv. Technol. 2008, 19, 620–627. [Google Scholar] [CrossRef]
- Pötzsch, S.; Krüger, S.; Sklorz, C.; Borch, J.; Hilse, T.; Otremba, F. The Fire Resistance of Lightweight Composite Tanks Depending on Fire Protection Systems. Fire Saf. J. 2018, 100, 118–127. [Google Scholar] [CrossRef]
- Geoffroy, L.; Samyn, F.; Jimenez, M.; Bourbigot, S. Intumescent Polymer Metal Laminates for Fire Protection. Polymers 2018, 10, 995. [Google Scholar] [CrossRef]
- Wu, G.M.; Schartel, B.; Bahr, H.; Kleemeier, M.; Yu, D.; Hartwig, A. Experimental and Quantitative Assessment of Flame Retardancy by the Shielding Effect in Layered Silicate Epoxy Nanocomposites. Combust. Flame 2012, 159, 3616–3623. [Google Scholar] [CrossRef]



 ): SiP2O7, (○): NH4Mg(PO3)3, (△): SixPyOz phase).
): SiP2O7, (○): NH4Mg(PO3)3, (△): SixPyOz phase).
 ): SiP2O7, (○): NH4Mg(PO3)3, (△): SixPyOz phase).
): SiP2O7, (○): NH4Mg(PO3)3, (△): SixPyOz phase).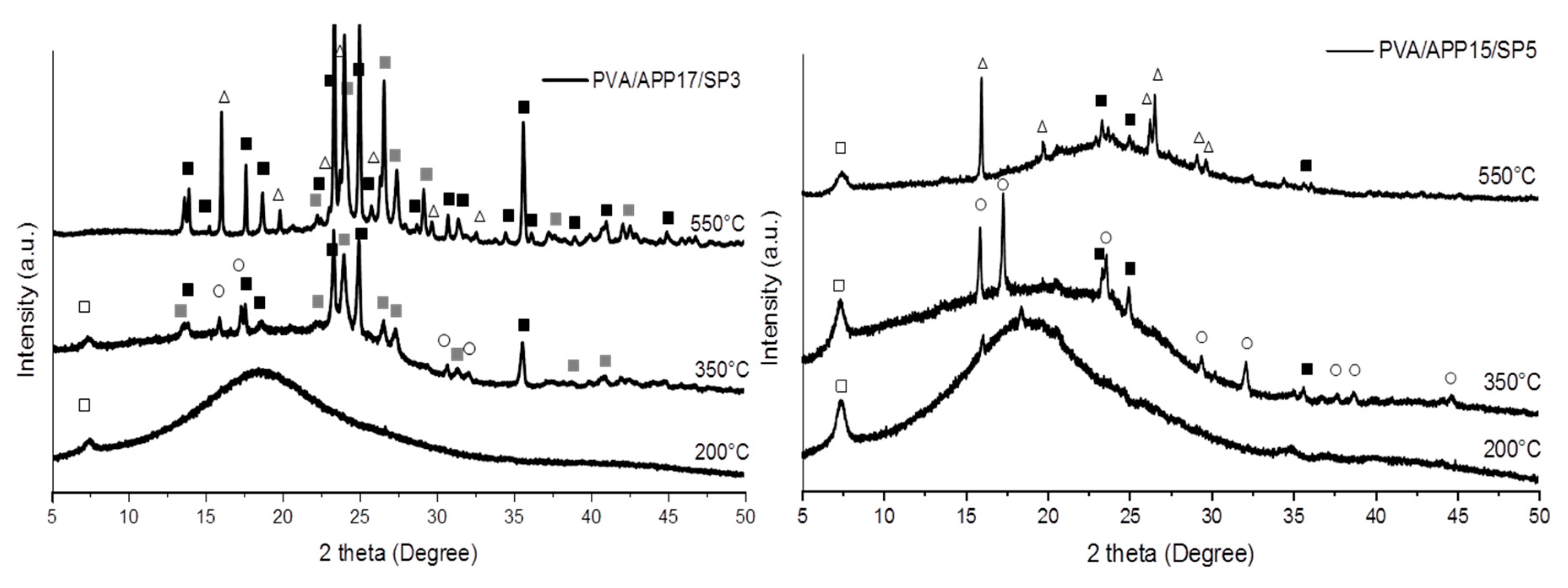
 ): SiP2O7, (●): (NH4)2MgH4(P2O7),2H2O, (⬚): Mg2P4O12, (○): NH4Mg(PO3)3, (
): SiP2O7, (●): (NH4)2MgH4(P2O7),2H2O, (⬚): Mg2P4O12, (○): NH4Mg(PO3)3, ( ): NH4(PO3), (△): SixPyOz phase).
): NH4(PO3), (△): SixPyOz phase).
 ): SiP2O7, (●): (NH4)2MgH4(P2O7),2H2O, (⬚): Mg2P4O12, (○): NH4Mg(PO3)3, (
): SiP2O7, (●): (NH4)2MgH4(P2O7),2H2O, (⬚): Mg2P4O12, (○): NH4Mg(PO3)3, ( ): NH4(PO3), (△): SixPyOz phase).
): NH4(PO3), (△): SixPyOz phase).


 ): SiP2O7, (
): SiP2O7, ( ): (NH4)H2PO4, (●): (NH4)2MgH4(P2O7),2H2O, (△): SixPyOz).
): (NH4)H2PO4, (●): (NH4)2MgH4(P2O7),2H2O, (△): SixPyOz).
 ): SiP2O7, (
): SiP2O7, ( ): (NH4)H2PO4, (●): (NH4)2MgH4(P2O7),2H2O, (△): SixPyOz).
): (NH4)H2PO4, (●): (NH4)2MgH4(P2O7),2H2O, (△): SixPyOz).


| Nomenclature | Component (wt%) | ||
|---|---|---|---|
| PVA | APP | Sepiolite | |
| PVA | 100 | 0 | 0 |
| PVA/APP10 | 90 | 10 | 0 |
| PVA/APP20 | 80 | 20 | 0 |
| PVA/APP30 | 70 | 30 | 0 |
| PVA/APP40 | 60 | 40 | 0 |
| PVA/APP19/SP1 | 80 | 19 | 1 |
| PVA/APP17/SP3 | 80 | 17 | 3 |
| PVA/APP15/SP5 | 80 | 15 | 5 |
| Residue (%) | ||||
|---|---|---|---|---|
| Nomenclature | Tonset (°C) | 700 °C (Expres) | Thres | Expres–Theres |
| PVA | 291 | 3.47 | 2.34 | - |
| PVA/APP10 | 249 | 22.60 | 8.22 | 14.37 |
| PVA/APP20 | 248 | 27.64 | 13.27 | 14.36 |
| PVA/APP30 | 247 | 23.00 | 18.31 | 4.68 |
| PVA/APP40 | 247 | 24.47 | 23.36 | 1.11 |
| PVA/APP19/SP1 | 248 | 27.27 | 14.90 | 12.37 |
| PVA/APP17/SP3 | 243 | 28.00 | 14.25 | 13.75 |
| PVA/APP15/SP5 | 238 | 26.71 | 13.59 | 13.11 |
| Formulation | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | TSR (m2/m2) | Residue (%) |
|---|---|---|---|---|---|
| PVA | 20 ± 2 | 558 ± 2 | 35 ± 1 | 334 ± 50 | 0 |
| PVA/APP10 | 27 ± 14 | 242 ± 28 | 22 ± 1 | 757 ± 68 | 11.5 ± 2.0 |
| PVA/APP20 | 81 ± 22 | 205 ± 39 | 19 ± 3 | 930 ± 89 | 17.6 ± 1.2 |
| PVA/APP30 | 54 ± 8 | 254 ± 10 | 18 ± 1 | 890 ± 66 | 14.3 ± 0.2 |
| PVA/APP40 | 72 ± 12 | 251 ± 36 | 14 ± 3 | 641 ± 70 | 22.9 ± 2.7 |
| PVA/APP19/SP1 | 90 ± 13 | 193 ± 10 | 22 ± 2 | 1105 ± 24 | 15.6 ± 4.7 |
| PVA/APP17/SP3 | 65 ± 25 | 158 ± 18 | 22 ± 6 | 1058 ± 109 | 16.5 ± 1.2 |
| PVA/APP15/SP5 | 73 ± 28 | 208 ± 62 | 21 ± 6 | 1103 ± 81 | 15.2 ± 2.0 |
| Composition | %C | %O | %P | %Si | %Mg | %Na | % N | Residue after Cone Test (%) | %Pini (%) | %Pres (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PVA/APP20 | 23.9 | 51.1 | 23.1 | - | - | 1.7 | Not detected | 17.6 | 6.3 | 4.0 |
| PVA/APP19/SP1 | 30.1 | 47.8 | 19.2 | 0.6 | 0.5 | 1.4 | Not detected | 15.6 | 5.9 | 2.9 |
| PVA/APP17/SP3 | 44.5 | 33.8 | 17.3 | 2.0 | 1.1 | 1.0 | Not detected | 16.5 | 5.3 | 2.8 |
| PVA/APP15/SP5 | 41.4 | 37.8 | 14.0 | 3.6 | 1.8 | 0.9 | Not detected | 15.2 | 4.7 | 2.1 |
| Coated Composite Laminate (CP) | 20 kW/m2 flux | 50 kW/m2 flux | ||||
|---|---|---|---|---|---|---|
| TMAX (°C) | TEND (°C) | TMAX (°C) | TEND (°C) | TTI (s) | TTF (s) | |
| CP | 263 | 263 | 353 | 301 | - | - |
| CP/PVA | 175 | 175 | 560 | 256 | 150 | 550 |
| CP/PVA/APP20 | 168 | 168 | 329 | 298 | 1610 | 2050 |
| CP/PVA/APP17/SP3 | 196 | 196 | 333 | 330 | - | - |
| CP/PVA/APP15/SP5 | 169 | 169 | 345 | 330 | - | - |
| (a) | |||||||
| Composition | %C | %O | %P | %Si | %Mg | %Na | %N |
| CP/PVA/APP20 | 14.7 | 53.1 | 29 | - | - | 2.7 | Not detected |
| CP/PVA/APP17/SP3 | 39.2 | 35.9 | 14.7 | 2.6 | 1.4 | 1.1 | 5 |
| CP/PVA/APP15/SP5 | 24.4 | 42.6 | 16.6 | 9.1 | 2.6 | 1.4 | 3 |
| (b) | |||||||
| Composition | %C | %O | %P | %Si | %Mg | %Na | %N |
| CP/PVA/APP20 | 31.2 | 44.6 | 21.4 | - | - | 1.43 | Not detected |
| CP/PVA/APP17/SP3 | 39 | 36.9 | 16.7 | 3.2 | 0.93 | 0.83 | 2 |
| CP/PVA/APP15/SP5 | 31.8 | 43.6 | 16.4 | 4.2 | 2.33 | 1.35 | Not detected |
| Coating Formulation | Volume (cm3) |
|---|---|
| CP/PVA | 117 |
| CP/PVA/APP20 | 100 ± 7 |
| CP/PVA/APP17/SP3 | 55 ± 4 |
| CP/PVA/APP15/SP5 | 30 ± 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floch, L.; Da Cruz Chiochetta, B.; Ferry, L.; Perrin, D.; Ienny, P. Fire Protective Surface Coating Containing Nanoparticles for Marine Composite Laminates. J. Compos. Sci. 2021, 5, 6. https://doi.org/10.3390/jcs5010006
Floch L, Da Cruz Chiochetta B, Ferry L, Perrin D, Ienny P. Fire Protective Surface Coating Containing Nanoparticles for Marine Composite Laminates. Journal of Composites Science. 2021; 5(1):6. https://doi.org/10.3390/jcs5010006
Chicago/Turabian StyleFloch, Léa, Bianca Da Cruz Chiochetta, Laurent Ferry, Didier Perrin, and Patrick Ienny. 2021. "Fire Protective Surface Coating Containing Nanoparticles for Marine Composite Laminates" Journal of Composites Science 5, no. 1: 6. https://doi.org/10.3390/jcs5010006
APA StyleFloch, L., Da Cruz Chiochetta, B., Ferry, L., Perrin, D., & Ienny, P. (2021). Fire Protective Surface Coating Containing Nanoparticles for Marine Composite Laminates. Journal of Composites Science, 5(1), 6. https://doi.org/10.3390/jcs5010006







