Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings
Abstract
1. Introduction
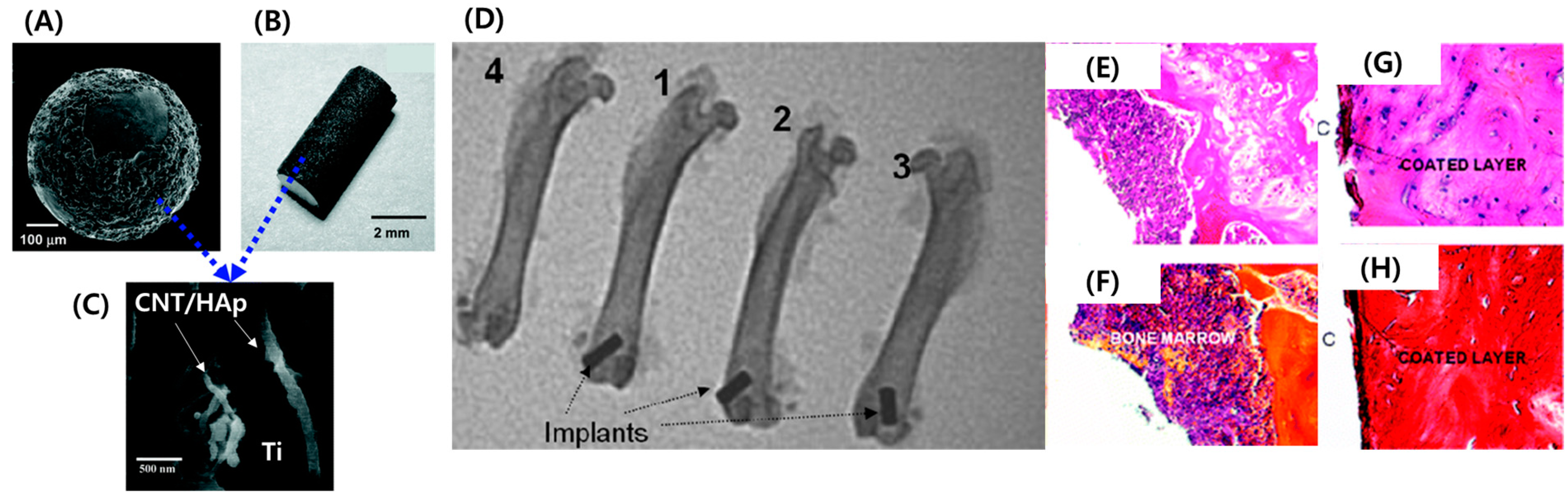
2. Physicomechanical Coating
3. Electrochemical Coating
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, J.C.; Barbosa, S.L.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.-P.; Rocha, L.A. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater. Sci. Eng. C 2015, 47, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided bone regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.; Buser, D. Guided bone regeneration for dental implants. Curr. Opin. Periodontol. 1996, 3, 168–177. [Google Scholar] [PubMed]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering? Tissue Eng. Part B Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- Silva, R.; Poon, R.; Milne, J.; Syed, A.; Zhitomirsky, I. New developments in liquid-liquid extraction, surface modification and agglomerate-free processing of inorganic particles. Adv. Colloid Interface Sci. 2018, 261, 15–27. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Liu, Z.; Tabakman, S.M.; Chen, Z.; Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009, 4, 1372–1381. [Google Scholar] [CrossRef]
- Yang, W.; Thordarson, P.; Gooding, J.J.; Ringer, S.P.; Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007, 18, 412001. [Google Scholar] [CrossRef]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Sumant, A.V. The CVD of nanodiamond materials. Chem. Vap. Depos. 2008, 14, 145–160. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond particles: Properties and perspectives for bioapplications. Crit. Rev. Solid State 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.-J.; Hyun, J.K.; Jung, T.-G.; Hong, S.W.; Han, D.-W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shin, Y.C.; Lee, J.-J.; Bae, E.-B.; Jeon, Y.-C.; Jeong, C.-M.; Yun, M.-J.; Lee, S.-H.; Han, D.-W.; Huh, J.-B. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.-W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 762–774. [Google Scholar] [CrossRef]
- Kang, S.H.; Shin, Y.C.; Hwang, E.Y.; Lee, J.H.; Kim, C.-S.; Lin, Z.; Hur, S.H.; Han, D.-W.; Hong, S.W. Engineered “coffee-rings” of reduced graphene oxide as ultrathin contact guidance to enable patterning of living cells. Mater. Horiz. 2019, 6, 1066–1079. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Han, D.-W.; Kang, S.H.; Hong, S.W.; Kim, J.M. Enhanced neurite outgrowth of PC-12 cells on graphene-monolayer-coated substrates as biomimetic cues. J. Korean Phys. Soc. 2012, 61, 1696–1699. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.H.; Kang, S.H.; Hwang, E.Y.; Hwang, Y.-S.; Lee, M.H.; Han, D.-W.; Park, J.-C. Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Facca, S.; Lahiri, D.; Fioretti, F.; Messadeq, N.; Mainard, D.; Benkirane-Jessel, N.; Agarwal, A. In vivo osseointegration of nano-designed composite coatings on titanium implants. ACS Nano 2011, 5, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, H.; Ding, C.; Zheng, X.; Li, K. Effects of graphene plates’ adoption on the microstructure, mechanical properties, and in vivo biocompatibility of calcium silicate coating. Int. J. Nanomed. 2015, 10, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z. Involvement of FAK/P38 signaling pathways in mediating the enhanced osteogenesis induced by nano-graphene oxide modification on titanium implant surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.; Tran, N.; Reineck, P.; Elbourne, A.; Mayes, E.; Sarker, A.; Dekiwadia, C.; Ivanova, E.P.; Crawford, R.J.; Ohshima, T. Engineering the interface: Nanodiamond coating on 3D-printed titanium promotes mammalian cell growth and inhibits Staphylococcus aureus colonization. ACS Appl. Mater. Interfaces 2019, 11, 24588–24597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, I.-S.; Neupane, M.P.; Bae, T.-S.; Lee, M.-H. Effects of a carbon nanotube-collagen coating on a titanium surface on osteoblast growth. Appl. Surf. Sci. 2014, 292, 828–836. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Choi, K.S.; Ji, M.-K.; Kim, S.; Gwon, Y.; Park, C.; Kim, J.; Lim, H.-P. Graphene–Chitosan Hybrid Dental Implants with Enhanced Antibacterial and Cell-Proliferation Properties. Appl. Sci. 2020, 10, 4888. [Google Scholar] [CrossRef]
- Jung, H.S.; Choi, Y.-j.; Jeong, J.; Lee, Y.; Hwang, B.; Jang, J.; Shim, J.-H.; Kim, Y.S.; Choi, H.S.; Oh, S.H. Nanoscale graphene coating on commercially pure titanium for accelerated bone regeneration. RSC Adv. 2016, 6, 26719–26724. [Google Scholar] [CrossRef]
- Park, K.O.; Lee, J.H.; Park, J.H.; Shin, Y.C.; Huh, J.B.; Bae, J.-H.; Kang, S.H.; Hong, S.W.; Kim, B.; Yang, D.J. Graphene oxide-coated guided bone regeneration membranes with enhanced osteogenesis: Spectroscopic analysis and animal study. Appl. Spectrosc. Rev. 2016, 51, 540–551. [Google Scholar] [CrossRef]
- Tao, B.; Chen, M.; Lin, C.; Lu, L.; Yuan, Z.; Liu, J.; Liao, Q.; Xia, Z.; Peng, Z.; Cai, K. Zn-incorporation with graphene oxide on Ti substrates surface to improve osteogenic activity and inhibit bacterial adhesion. J. Biomed. Mater. Res. 2019, 107, 2310–2326. [Google Scholar] [CrossRef]
- Mehrali, M.; Akhiani, A.R.; Talebian, S.; Mehrali, M.; Latibari, S.T.; Dolatshahi-Pirouz, A.; Metselaar, H.S.C. Electrophoretic deposition of calcium silicate–reduced graphene oxide composites on titanium substrate. J. Eur. Ceram. Soc. 2016, 36, 319–332. [Google Scholar] [CrossRef]
- Elangomannan, S.; Louis, K.; Dharmaraj, B.M.; Kandasamy, V.S.; Soundarapandian, K.; Gopi, D. Carbon nanofiber/polycaprolactone/mineralized hydroxyapatite nanofibrous scaffolds for potential orthopedic applications. ACS Appl. Mater. Interfaces 2017, 9, 6342–6355. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y. Electrophoretic deposition of graphene oxide reinforced chitosan–hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci.: Mater. 2016, 27, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Pei, X.; Zeng, Y.; He, R.; Li, Z.; Tian, L.; Wang, J.; Wan, Q.; Li, X.; Bao, H. Single-walled carbon nanotubes/hydroxyapatite coatings on titanium obtained by electrochemical deposition. Appl. Surf. Sci. 2014, 295, 71–80. [Google Scholar] [CrossRef]
- Strąkowska, P.; Beutner, R.; Gnyba, M.; Zielinski, A.; Scharnweber, D. Electrochemically assisted deposition of hydroxyapatite on Ti6Al4V substrates covered by CVD diamond films—Coating characterization and first cell biological results. Mater. Sci. Eng. C 2016, 59, 624–635. [Google Scholar] [CrossRef]
- Metzler, P.; von Wilmowsky, C.; Stadlinger, B.; Zemann, W.; Schlegel, K.A.; Rosiwal, S.; Rupprecht, S. Nano-crystalline diamond-coated titanium dental implants–A histomorphometric study in adult domestic pigs. J. Craniomaxillofac. Surg. 2013, 41, 532–538. [Google Scholar] [CrossRef]
- Patel, S.C.; Lalwani, G.; Grover, K.; Qin, Y.-X.; Sitharaman, B. Fabrication and cytocompatibility of in situ crosslinked carbon nanomaterial films. Sci. Rep. 2015, 5, 10261–10273. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K. Enhanced antibacterial and corrosion resistance properties of Ag substituted hydroxyapatite/functionalized multiwall carbon nanotube nanocomposite coating on 316L stainless steel for biomedical application. Ultrason. Sonochem. 2019, 59, 104730–104740. [Google Scholar] [CrossRef]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T. Enhanced osseointegration of titanium alloy implants with laser microgrooved surfaces and graphene oxide coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef] [PubMed]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Electrically polarized HAp-coated Ti: In vitro bone cell–material interactions. Acta Biomater. 2010, 6, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Uenoyama, K.; Matsuguchi, N.; Sugioka, Y. Quantitative analysis of in vivo tissue responses to titanium-oxide-and hydroxyapatite-coated titanium alloy. J. Biomed. Mater. Res. 1991, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shaw, L.L. Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials 2009, 30, 6565–6572. [Google Scholar] [CrossRef]
- Thomsen, M.G.; Latifi, R.; Kallemose, T.; Husted, H.; Troelsen, A. Does knee awareness differ between different knee arthroplasty prostheses? A matched, case-control, cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 141–147. [Google Scholar] [CrossRef]
- Vedantam, R.; Ruddlesdin, C. The fully hydroxyapatite-coated total hip implant: Clinical and roentgenographic results. J. Arthroplast. 1996, 11, 534–542. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Jones, M.D.; Hunter, T.B.; Benjamin, J.B.; Ruth, J.T.; Brown, A.W.; Sheppard, J.E. Joint arthroplasties and prostheses. Radiographics 2003, 23, 1295–1314. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite–carbon nanotube composites for biomedical applications: A review. Int. J. Appl. Ceram. Technol. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Campbell, A.A. Bioceramics for implant coatings. Mater. Today 2003, 6, 26–30. [Google Scholar] [CrossRef]
- Unabia, R.; Candidato, R.; Pawłowski, L. Current progress in solution precursor plasma spraying of cermets: A review. Metals 2018, 8, 420. [Google Scholar] [CrossRef]
- Lahiri, D.; Benaduce, A.P.; Rouzaud, F.; Solomon, J.; Keshri, A.K.; Kos, L.; Agarwal, A. Wear behavior and in vitro cytotoxicity of wear debris generated from hydroxyapatite–carbon nanotube composite coating. J. Biomed. Mater. Res. A 2011, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Kissel, T. Ultrasonic atomization for spray drying: A versatile technique for the preparation of protein loaded biodegradable microspheres. J. Microencapsul. 1999, 16, 325–341. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, A.A.; Hinks, J.A.; Marks, N.A.; Greaves, G.; Valencia, F.J.; Donnelly, S.E.; González, R.I.; Kiwi, M.; Trigub, A.L.; Bringa, E.M. Ion implantation in nanodiamonds: Size effect and energy dependence. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Grausova, L.; Bacakova, L.; Kromka, A.; Potocky, S.; Vanecek, M.; Nesladek, M.; Lisa, V. Nanodiamond as promising material for bone tissue engineering. J. Nanosci. Nanotechnol. 2009, 9, 3524–3534. [Google Scholar] [CrossRef]
- Shidid, D.; Leary, M.; Choong, P.; Brandt, M. Just-in-time design and additive manufacture of patient-specific medical implants. Phys. Procedia 2016, 83, 4–14. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.; Sarker, A.; Ivanova, E.P.; Crawford, R.J. Polycrystalline diamond coating of additively manufactured titanium for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Chłopek, J.; Czajkowska, B.; Szaraniec, B.; Frackowiak, E.; Szostak, K.; Beguin, F. In vitro studies of carbon nanotubes biocompatibility. Carbon 2006, 44, 1106–1111. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Sun, W.; Sun, W.; Lin, H.; Sun, W.; Lin, H.; Xie, H.; Chen, B.; Zhao, W.; Han, Q.; Zhao, Y. Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors 2007, 25, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental understanding and modeling of spin coating process: A review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef]
- Scriven, L. Physics and applications of dip coating and spin coating. MRS Online Proc. Lib. Arch. 1988, 121, 717. [Google Scholar] [CrossRef]
- Lawrence, C. The mechanics of spin coating of polymer films. Phys. Fluids 1988, 31, 2786–2795. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, N.H.; Lee, N.R.; Chang, S.T. Meniscus-dragging deposition of single-walled carbon nanotubes for highly uniform, large-area, transparent conductors. Carbon 2014, 77, 964–972. [Google Scholar] [CrossRef]
- Ko, Y.U.; Cho, S.-r.; Choi, K.S.; Park, Y.; Kim, S.T.; Kim, N.H.; Kim, S.Y.; Chang, S.T. Microlitre scale solution processing for controlled, rapid fabrication of chemically derived graphene thin films. J. Mater. Chem. 2012, 22, 3606–3613. [Google Scholar] [CrossRef]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic deposition (EPD): Fundamentals and applications from nano-to micro-scale structures. In Handbook of Nanoelectrochemistry; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–27. [Google Scholar]
- Liu, X.; Ding, C.; Wang, Z. Apatite formed on the surface of plasma-sprayed wollastonite coating immersed in simulated body fluid. Biomaterials 2001, 22, 2007–2012. [Google Scholar] [CrossRef]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, M.; Zhai, W.; Qu, H.; Chang, J. Fabrication and characterization of hydroxyapatite/wollastonite composite bioceramics with controllable properties for hard tissue repair. J. Am. Ceram. Soc. 2011, 94, 99–105. [Google Scholar] [CrossRef]
- Liu, X.; Ding, C. Phase compositions and microstructure of plasma sprayed wollastonite coating. Surf. Coat. Technol. 2001, 141, 269–274. [Google Scholar] [CrossRef]
- Rodriguez, H.; Vargas, G.; Cortés, D. Electrophoretic deposition of bioactive wollastonite and porcelain–wollastonite coatings on 316L stainless steel. Ceram. Int. 2008, 34, 1303–1307. [Google Scholar] [CrossRef]
- Sharma, S.; Soni, V.P.; Bellare, J.R. Chitosan reinforced apatite–wollastonite coating by electrophoretic deposition on titanium implants. J. Mater. Sci. Mater. Med. 2009, 20, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Blazewicz, M. Carbon materials in the treatment of soft and hard tissue injuries. Eur. Cells Mater. 2001, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Naskar, D.; Bhattacharjee, P.; Ghosh, A.K.; Mandal, M.; Kundu, S.C. Carbon nanofiber reinforced nonmulberry silk protein fibroin nanobiocomposite for tissue engineering applications. ACS Appl. Mater. Interfaces 2017, 9, 19356–19370. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.; Zhou, O.; Zhu, W.; Werder, D.; Jin, S. Nucleation and growth of carbon nanotubes by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 2767–2769. [Google Scholar] [CrossRef]
- Kobashi, K.; Nishimura, K.; Kawate, Y.; Horiuchi, T. Synthesis of diamonds by use of microwave plasma chemical-vapor deposition: Morphology and growth of diamond films. Phys. Rev. B 1988, 38, 4067–4084. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ishihara, M.; Hasegawa, M. Large area coating of graphene at low temperature using a roll-to-roll microwave plasma chemical vapor deposition. Thin Solid Films 2013, 532, 89–93. [Google Scholar] [CrossRef]
- Elliott, M.; May, P.; Petherbridge, J.; Leeds, S.; Ashfold, M.; Wang, W. Optical emission spectroscopic studies of microwave enhanced diamond CVD using CH4/CO2 plasmas. Diam. Relat. Mater. 2000, 9, 311–316. [Google Scholar] [CrossRef]
- Krishnia, L.; Tyagi, P.K. Growth and characterization of polycrystalline diamond films on silicon using sugarcane bagasse as carbon precursor at atmospheric pressure by thermal chemical vapor deposition. Diam. Relat. Mater. 2018, 87, 18–26. [Google Scholar] [CrossRef]
- Azboy, I.; Groff, H.; Goswami, K.; Vahedian, M.; Parvizi, J. Low-dose aspirin is adequate for venous thromboembolism prevention following total joint arthroplasty: A systematic review. J. Arthroplast. 2020, 35, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, J.; Wu, T.; Tang, Z.; Ding, G.; Zhang, C.; Wang, S.; Liu, Y. Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d-PGJ2/PPARγ/TGF-β1 pathway. Stem Cells Dev. 2014, 23, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Liu, Y.; Zhang, X.; Zhou, Y. Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin. Stem Cell Res. Ther. 2015, 6, 200. [Google Scholar] [CrossRef]
- Shin, Y.C.; Song, S.-J.; Jeong, S.J.; Kim, B.; Kwon, I.K.; Hong, S.W.; Oh, J.-W.; Han, D.-W. Graphene-based nanocomposites as promising options for hard tissue regeneration. Adv. Exp. Med. Biol. 2018, 1078, 103–117. [Google Scholar] [PubMed]
- Shin, Y.C.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Hwang, Y.-S.; Choi, Y.S.; Han, D.-W. Graphene-functionalized biomimetic scaffolds for tissue regeneration. Adv. Exp. Med. Biol. 2018, 164, 73–89. [Google Scholar]


| Clarification | Coating Method | CNM | Conjugation | Coating Quality (Features and Process Rate) | Biological Evaluation | Osteogenic and Antibacterial Activities | Ref. |
|---|---|---|---|---|---|---|---|
| Physicomechanical Method | Plasma spraying | CNT | HAp | FDA-approved method and commonly used | In vivo (rat and mouse) | Newly grown bone, no periosteal reactions, and restoration of healthy osteoblast and osteocyte | [21] |
| Graphene | CS | In vivo (rabbit) | Newly grown bone cover pores in interface | [22] | |||
| Ultrasonic atomization spraying | GO | - | Retains original particle structure; thin and uniform layer | In vitro (BM-MSC) and in vivo (rat) | Increased cell adhesion, proliferation, and osteogenic markers; in vivo osseointegration | [23] | |
| Dip coating | ND | - | Simple, fast, and cost-effective | In vitro (NHDF and calvariae primary osteoblast) | Enhanced cell growth; inhibition of Staphylococcus aureus colonization | [24] | |
| MWCNT | Collagen | In vitro (MSC) | Increased proliferation and ALP activity | [25] | |||
| Spin coating | GO | Chitosan | Fast process rate and simple process | In vitro (MC3T3-E1) and in vivo (rat) | Antibacterial effect on Streptococcus mutans; enhanced cell proliferation | [26] | |
| rGO | Dex, AA | In vitro (MC3T3-E1) and in vivo (rat) | Enhanced cell viability and adhesion; formation of collagen type I and new bone | [27] | |||
| MDD | GO | - | Transparent coating by precise control in nanometer scale | In vitro (MC3T3-E1) and in vivo (rat) | Enhanced proliferation and ALP activity; new bone formation | [28] | |
| Electrochemical Method | EPD | GOMA | PBA functionalization GelMA-PBA | High versatility and cost-effectiveness; uniform coating on a porous and complex-shaped substrate with easy accessibility and low cost of equipment | In vitro (osteoblast from rat calvaria) | Enhanced cell viability, proliferation, mineralization, collagen secretion, ALP activity, and osteogenic-relative gene expression; antibacterial effect on Pseudomonas aeruginosa and S. aureus | [29] |
| rGO | CS | In vitro (hFOB) | Increased cell viability | [30] | |||
| CNF | HAp, PCL | In vitro (MG63) and in vivo (rat) | Antibacterial effect on S. aureus and Escherichia coli; enhanced proliferation and ALP activity | [31] | |||
| GO | Chitosan, HAp | In vitro (MG63) | Antibacterial effect on S. aureus; enhanced proliferation and ALP activity | [32] | |||
| GO | Chitosan, HAp | In vitro (BM-MSC) and in vivo (rat) | Improved proliferation and differentiation; improved in vivo osseointegration | [33] | |||
| ECD | GO | HAp | Low process temperature; coating on geometrically complex surface; controllable coating properties; low cost of equipment | In vitro (MG63) | Enhanced proliferation and ALP activity | [34] | |
| SWCNT | HAp | In vitro (human osteoblast) | Enhanced proliferation and ALP activity | [35] | |||
| MW-PACVD | ND | HAp | Dense and homogeneous coating; varying crystalline structure; | In vitro (hMSC) | Enhanced proliferation and ALP activity | [36] | |
| ND | - | ultrahardness with a very low friction coefficient, chemical inertness, impermeability of the carbon coating, and highly resistant corrosion and erosion processes | In vivo (pig) | Enhanced bone-to-implant contact (BIC) | [37] | ||
| Spraying and in situ crosslinking | MWCNT | - | Facile, cheap, and scalable | In vitro (ADSC) | - | [38] | |
| Chemical spray pyrolysis | MWCNT | Silver, HAp | Uniform deposition rate at low temperature; pure and reproducible; mass productivity | In vivo (human osteoblast) | Antibacterial property on E. coli, Shigella flexeri, S. aureus, and Bacillus subtilis | [39] | |
| Alkali hydrothermal reaction and silane coupling; APTES conjugation | GO | Aspirin | Stable bonding; the feasibility of functionalization | In vitro (MC3T3-E1) | Enhanced proliferation and ALP activity | [40] | |
| Chemical assembly | GO | Dopamine | Uniform coating on any shape or structure | In vitro (BM-MSC) and in vivo (rabbit) | Improved cell viability, ALP activity, and mineralization; improved in vivo osseointegration | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Lee, J.H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings. J. Compos. Sci. 2021, 5, 23. https://doi.org/10.3390/jcs5010023
Kang MS, Lee JH, Hong SW, Lee JH, Han D-W. Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings. Journal of Composites Science. 2021; 5(1):23. https://doi.org/10.3390/jcs5010023
Chicago/Turabian StyleKang, Moon Sung, Jong Ho Lee, Suck Won Hong, Jong Hun Lee, and Dong-Wook Han. 2021. "Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings" Journal of Composites Science 5, no. 1: 23. https://doi.org/10.3390/jcs5010023
APA StyleKang, M. S., Lee, J. H., Hong, S. W., Lee, J. H., & Han, D.-W. (2021). Nanocomposites for Enhanced Osseointegration of Dental and Orthopedic Implants Revisited: Surface Functionalization by Carbon Nanomaterial Coatings. Journal of Composites Science, 5(1), 23. https://doi.org/10.3390/jcs5010023






