Measuring the Electrical and Photonic Properties of Cobalt Oxide-Containing Composite Carbon Fibers
Abstract
1. Introduction
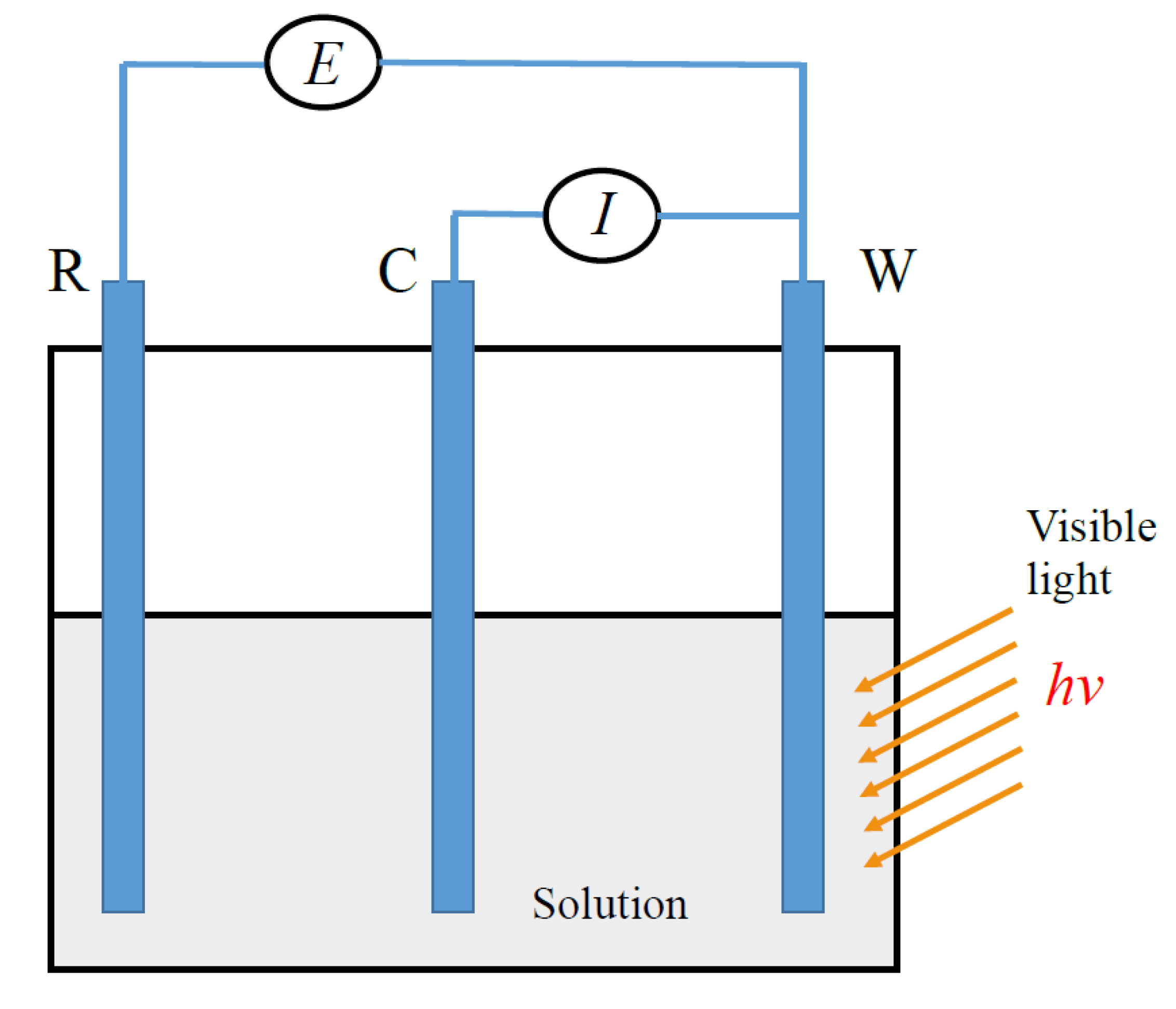
2. Materials and Experimental
2.1. Materials and Instruments
2.2. FiberPrrocessing
2.3. Characterization
3. Results and Discussion
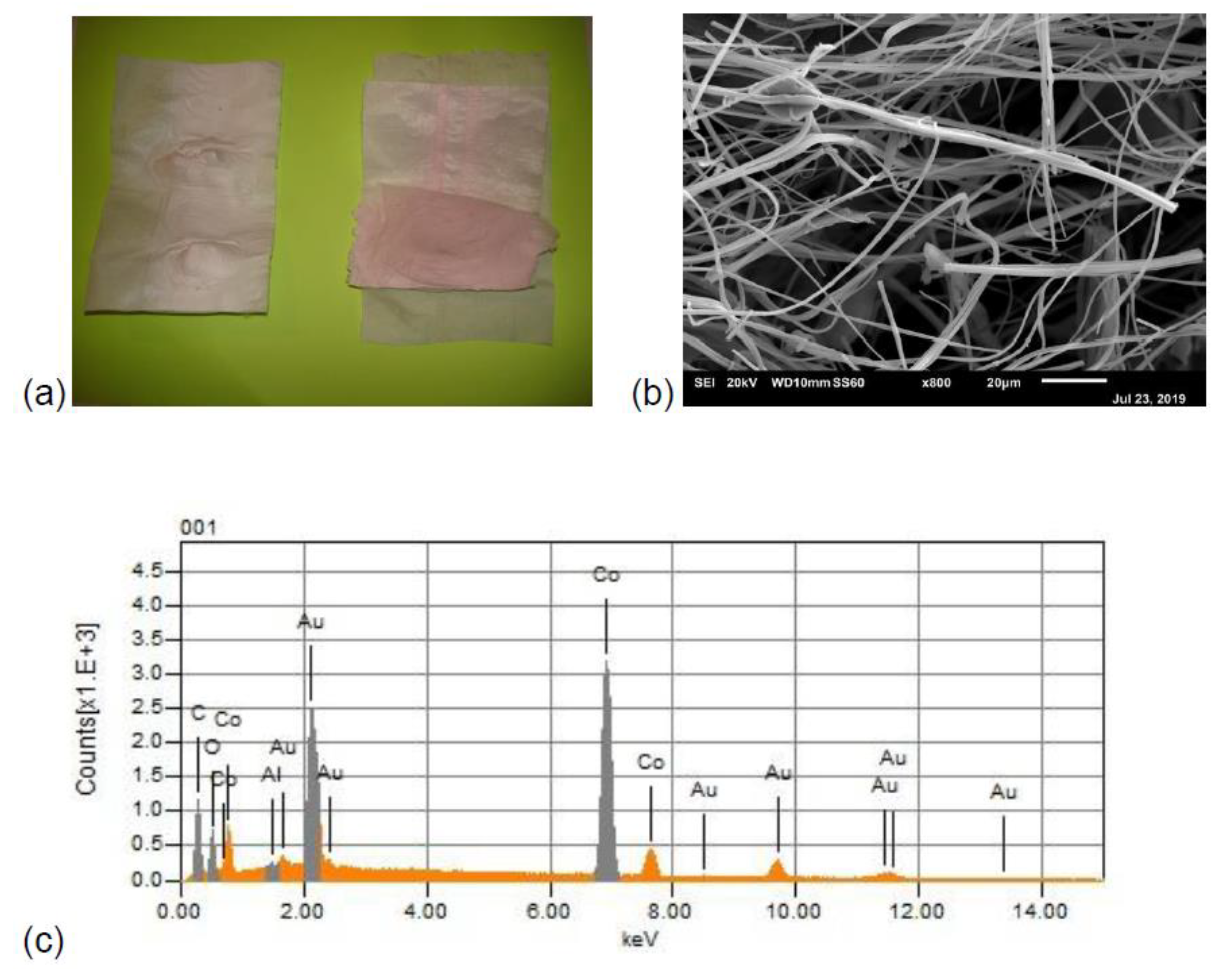
3.1. Morphology and Composition
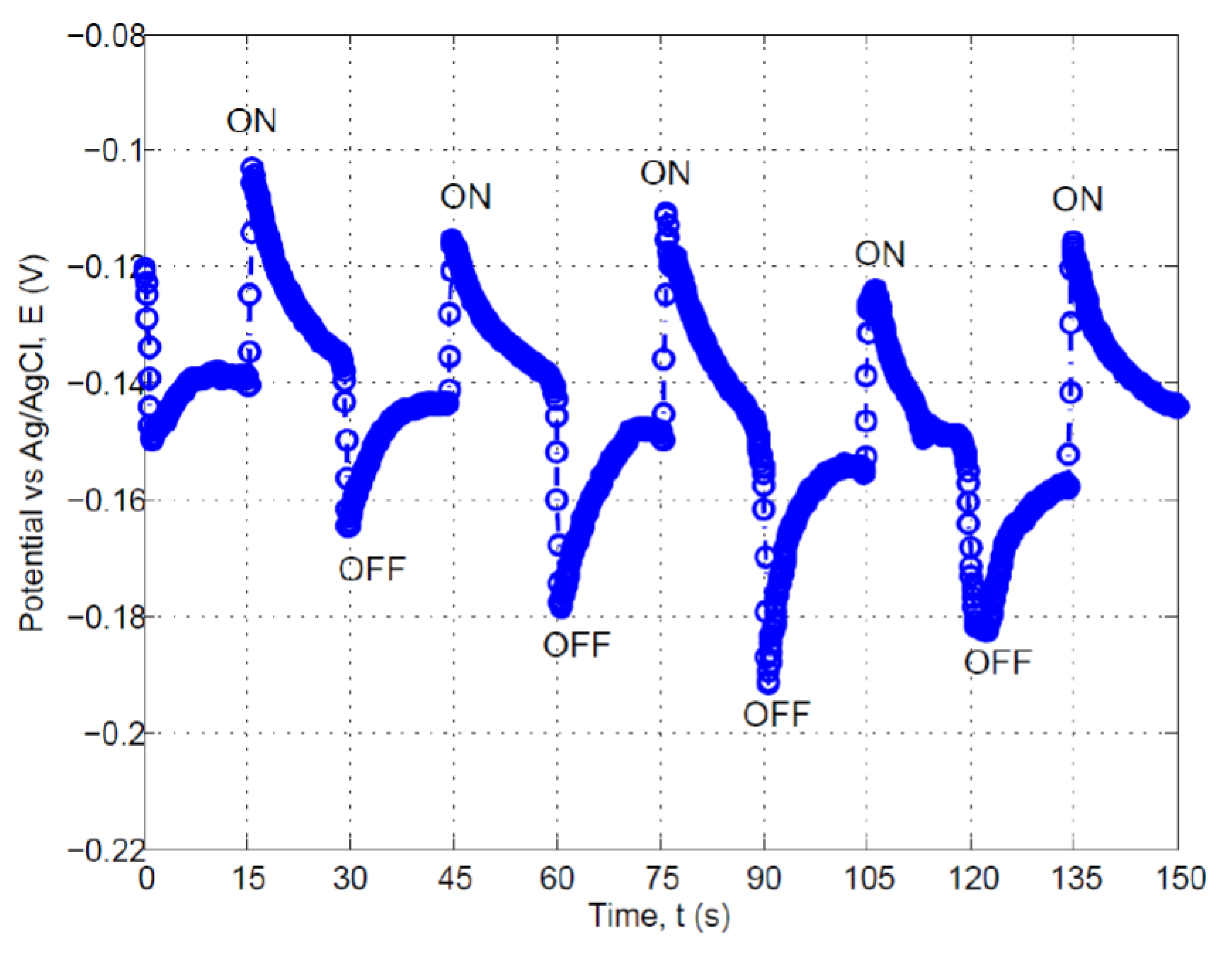
3.2. Photosensitivity of the Composite Carbon Fiber
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Lv, P.; Duan, F.; Sheng, J.L.; Lu, S.L.; Zhu, H.; Du, M.L.; Chen, M.Q. Direct Z-scheme Bi2S3/BiFeO3 heterojunction nanofibers with enhanced photocatalytic activity. J. Alloys Compd. 2020, 834, 155158. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.R.; Ayub, I.; Wang, S.L.; Yan, W. In situ decoration of g-C3N4 quantum dots on 1D branched TiO2 loaded with plasmonic Au nanoparticles and improved the photocatalytic hydrogen evolution activity. Appl. Surf. Sci. 2020, 519, 146208. [Google Scholar] [CrossRef]
- Gadisa, B.T.; Kassahun, S.K.; Appiah-Ntiamoah, R.; Kim, H. Tuning the charge carrier density and exciton pair separation in electrospun 1D ZnO-C composite nanofibers and its effect on photodegradation of emerging contaminants. J. Colloid Interface Sci. 2020, 570, 251–263. [Google Scholar] [CrossRef] [PubMed]
- El-Fawal, E.M.; Younis, S.A.; Moustafa, Y.M.; Serp, P. Preparation of solar-enhanced AlZnO@carbon nano-substrates for remediation of textile wastewaters. J. Environ. Sci. 2020, 92, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Cinar, B.; Kerimoglu, I.; Tonbul, B.; Demirbuken, A.; Dursun, S.; Kaya, I.C.; Kalem, V.; Akyildiz, H. Hydrothermal/electrospinning synthesis of CuO plate-like particles/TiO2 fibers heterostructures for high-efficiency photocatalytic degradation of organic dyes and phenolic pollutants. Mater. Sci. Semiconduc. Proc. 2020, 109, 104919. [Google Scholar] [CrossRef]
- Zendehzaban, M.; Ashjari, M.; Sharifnia, S. Preparation of floatable TiO2/poly(vinyl alcohol)-alginate composite for the photodegradation of ammonia wastewater. Int. J. Energy Res. 2020, 44, 2150–2163. [Google Scholar] [CrossRef]
- Baylan, E.; Yildirim, O.A. Highly efficient photocatalytic activity of stable manganese-doped zinc oxide (Mn:ZnO) nanofibers via electrospinning method. Mater. Sci. Semicond. Proc. 2019, 103, 104621. [Google Scholar] [CrossRef]
- Xiao, Y.; He, Z.Q.; Wang, R.S.; Tao, X.Q.; Li, B.X. Synthesis of WO3 nanofibers decorated with BiOCl nanosheets for photocatalytic degradation of organic pollutants under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123752. [Google Scholar] [CrossRef]
- Li, Y.J.; Cao, T.P.; Zhao, Y.H.; Sun, D.W.; Wang, X. Preparation of Bi@Bi2Sn2O7/TiO2 plasmonic composite fibers with enhanced photocatalytic hydrogen generation activity. Chin. J. Inorg. Chem. 2019, 35, 1371–1378. [Google Scholar] [CrossRef]
- Ramos, P.G.; Flores, E.; Luyo, C.; Sanchez, L.A.; Rodriguez, J. Fabrication of ZnO-RGO nanorods by electrospinning assisted hydrothermal method with enhanced photocatalytic activity. Mater. Today Commun. 2019, 19, 407–412. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, M.; Li, J.; Yang, H.C.; Gao, J.; He, G.; Sun, Z.Q. Construction of Ag@AgCl decorated TiO2 nanorod array film with optimized photoelectrochemical and photocatalytic performance. Appl. Surf. Sci. 2019, 476, 84–93. [Google Scholar] [CrossRef]
- Li, Y.J.; Cao, T.P.; Mei, Z.M.; Li, X.P.; Sun, D.W.; Yang, D.K. Preparation and photocatalytic activity of Gd-N co-doped SrTiO3/TiO2 composite nanofibers. Chin. J. Inorg. Chem. 2019, 35, 82–88. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, X.Z.; Zhang, M.; Fang, L.L.; Jin, L.P.; Gao, J.; Zhang, Y.C.; Yang, B.; He, G.; Sun, Z.Q. TiO2 nanorod array film decorated with rGO nanosheets for enhancing photocatalytic and photoelectrochemical properties. J. Alloy. Compd. 2019, 770, 243–251. [Google Scholar] [CrossRef]
- Roy, S.; Payra, S.; Challagulla, S.; Arora, R.; Roy, S.; Chakraborty, C. Enhanced photoinduced electrocatalytic oxidation of methanol using Pt nanoparticle-decorated TiO2-polyaniline ternary nanofibers. ACS Omega 2018, 3, 17778–17788. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.G.; Wang, X.X.; Nie, G.D.; Zhang, J.; Zhang, S.X.; Cao, N.; Yan, S.Y.; Long, Y.Z. Highly flexible Fe2O3/TiO2 composite nanofibers for photocatalysis and ultraviolet detection. J. Phys. Chem. Solid. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Uyar, T. Conscientious design of Zn-S/Ti-N layer by transformation of ZnTiO3 on electrospun ZnTiO3@TiO2 nanofibers: Stability and reusable photocatalytic performance under visible irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12980–12992. [Google Scholar] [CrossRef]
- Ma, G.; Lu, J.L.; Meng, Q.G.; Lv, H.Q.; Shui, L.L.; Zhang, Y.G.; Jin, M.L.; Chen, Z.H.; Yuan, M.Z.; Notzel, R.; et al. Synergistic effect of Cu-ion and WO3 nanofibers on the enhanced photocatalytic degradation of Rhodamine B and aniline solution. Appl. Surf. Sci. 2018, 451, 306–314. [Google Scholar] [CrossRef]
- Tahir, M.B. Construction of MoS2/CND-WO3 ternary composite for photocatalytic hydrogen evolution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2160–2168. [Google Scholar] [CrossRef]
- Panthi, G.; Hassan, M.M.; Kuk, Y.S.; Kim, J.Y.; Chung, H.J.; Hong, S.T.; Park, M. Enhanced antibacterial property of sulfate-doped Ag3PO4 nanoparticles supported on PAN electrospun nanofibers. Molecules 2020, 25, 1411. [Google Scholar] [CrossRef]
- Chueachot, R.; Nakhowong, R. Synthesis and optical properties of blue pigment CoAl2O4 nanofibers by electrospinning. Mater. Lett. 2020, 259, 126904. [Google Scholar] [CrossRef]
- Jiang, D.H.; Kobayashi, S.; Jao, C.C.; Mato, Y.; Isono, T.; Fang, Y.H.; Lin, C.C.; Satoh, T.; Tung, S.H.; Kuo, C.C. Light down-converter based on luminescent nanofibers from the blending of conjugated rod-coil block copolymers and perovskite through electrospinning. Polymers 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.Y.; Liu, Y.; Hou, Y.J.; Wang, S.H. Electrospinning preparation and luminescence properties of Eu2(PBT)(3)(NO3)(3)/PMMA composite nanofibers. Mater. Chem. Phys. 2018, 217, 486–492. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, P.J.; Pun, E.Y.B.; Lin, H.; Yuan, J.L.; Zhao, X. Hybrid excitation mechanism of upconversion fluorescence in hollow La2Ti2O7: Tm3+/Yb3+ submicron fibers. J. Mater. Sci. 2020, 55, 4633–4645. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, S.; Zhang, J.C.; Yan, X.; Yu, G.F.; Yang, D.P.; Long, Y.Z. Ultrasensitive fluorescence lifetime tuning in patterned polymer composite nanofibers with plasmonic nanostructures for multiplexing. Macromol. Rap. Comm. 2019, 40, 1800022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.H.; Tsai, Y.H.; Veeramuthu, L.; Liang, F.C.; Chen, L.C.; Lin, C.C.; Satoh, T.; Tung, S.H.; Kuo, C.C. Novel ultra-stable and highly luminescent white light-emitting diodes from perovskite quantum dots-Polymer nanofibers through biaxial electrospinning. APL Mater. 2019, 7, 111105. [Google Scholar] [CrossRef]
- Chen, C.; He, G.Q.; Zhang, Z.Y.; Yan, J.F.; Kou, L.; Hauffman, T.; Pletincx, S.; Zhao, W.; Yun, J.N.; De Tandt, C.; et al. Growth mechanism of novel scaly CNFs@ZnO nanofibers structure and its photoluminescence property. Appl. Surf. Sci. 2019, 491, 75–82. [Google Scholar] [CrossRef]
- Ge, W.Y.; Shi, J.D.; Xu, M.M.; Chen, X.L.; Zhu, J.F. Red upconversion luminescence (UCL) properties in one-dimensional Yb2Ti2O7: Er nanofibers via an electrospinning route. J. Alloy. Compd. 2019, 788, 993–999. [Google Scholar] [CrossRef]
- Matysiak, W.; Tanski, T.; Smok, W. Study of optical and dielectric constants of hybrid SnO2 electrospun nanostructures. Appl. Phys. A Mater. Sci. Proc. 2020, 126, 115. [Google Scholar] [CrossRef]
- Akhlaq, M.; Khan, Z.S. Synthesis and characterization of electro-spun TiO2 and TiO2-SnO2 composite nano-fibers for application in advance generation solar cells. Mater. Res. Express 2020, 7, 015523. [Google Scholar] [CrossRef]
- Bakr, Z.H.; Wali, Q.; Yang, S.Y.; Yousefsadeh, M.; Padmasree, K.P.; Ismail, J.; Ab Rahim, M.H.; Yusoff, M.M.; Jose, R. Characteristics of ZnO-SnO2 Composite nanofibers as a photoanode in dye-sensitized solar cells. Ind. Eng. Chem. Res. 2019, 58, 643–653. [Google Scholar] [CrossRef]
- Nasirian, S. Enhanced carbon dioxide sensing performance of polyaniline/tin dioxide nanocomposite by ultraviolet light illumination. Appl. Surf. Sci. 2020, 502, 144302. [Google Scholar] [CrossRef]
- Tharsika, T.; Thanihaichelvan, M.; Haseeb, A.S.M.A.; Akbar, S.A. Highly sensitive and selective ethanol sensor based on ZnO nanorod on SnO2 thin film fabricated by spray pyrolysis. Front. Mater. 2019, 6, 122. [Google Scholar] [CrossRef]
- Li, X.Q.; Gu, J.P.; Zhou, Z.; Ma, L.F.; Tang, Y.P.; Gao, J.W.; Wang, Q.M. New lanthanide ternary complex system in electrospun nanofibers: Assembly, physico-chemical property and sensor application. Chem. Eng. J. 2019, 358, 67–73. [Google Scholar] [CrossRef]
- Ashokkumar, S.P.; Vijeth, H.; Yesappa, L.; Niranjana, M.; Vandana, M.; Devendrappa, H. Electrochemically synthesized polyaniline/copper oxide nano composites: To study optical band gap and electrochemical performance for energy storage devices. Inorg. Chem. Commun. 2020, 115, 107865. [Google Scholar] [CrossRef]
- Zahan, M.; Podder, J. Surface morphology, optical properties and Urbach tail of spray deposited Co3O4 thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 4259–4269. [Google Scholar] [CrossRef]
- Patel, S.; Kansara, S.; Gan, Y.X.; Zhao, Y.T.; Gan, J.B. Hydrothermally coated oxide nanoparticle-containing composite fibers. In Proceedings of the 4th Thermal and Fluids Engineering Conference (TFEC2019), Las Vegas, NV, USA, 14–17 April 2019. Paper No. TFEC-2019-28031. [Google Scholar]
- Xu, L.Q.Y.; Zhang, L.Y.; Cheng, B.; Yu, J.G. Rationally designed hierarchical NiCo2O4-C@Ni(OH)2 core-shell nanofibers for high performance supercapacitors. Carbon 2019, 152, 652–660. [Google Scholar] [CrossRef]
- Ling, S.J.; Sanny, J.; Moebs, W. University Physics; OpenStax Rice University: Houston TX, USA, 2018; Volume 2, pp. 479–481. Available online: http://cnx.org/content/col12074/1.9 (accessed on 6 September 2020).
- Gan, Y.X.; Gan, B.J.; Su, L.S. Biophotofuel cell anode containing self-organized titanium dioxide nanotube array. Mater. Sci. Eng. B Avd. Fun. Solid State Mater. 2011, 176, 1197–1206. [Google Scholar] [CrossRef]
- Gan, Y.X.; Gan, B.J.; Clark, E.; Su, L.S.; Zhang, L.H. Converting environmentally hazardous materials into clean energy using a novel nanostructured photoelectrochemical fuel cell. Mater. Res. Bull. 2012, 47, 2380–2388. [Google Scholar] [CrossRef]
- Gan, Y.X. Structural assessment of nanocomposites. Micron 2012, 43, 782–817. [Google Scholar] [CrossRef]


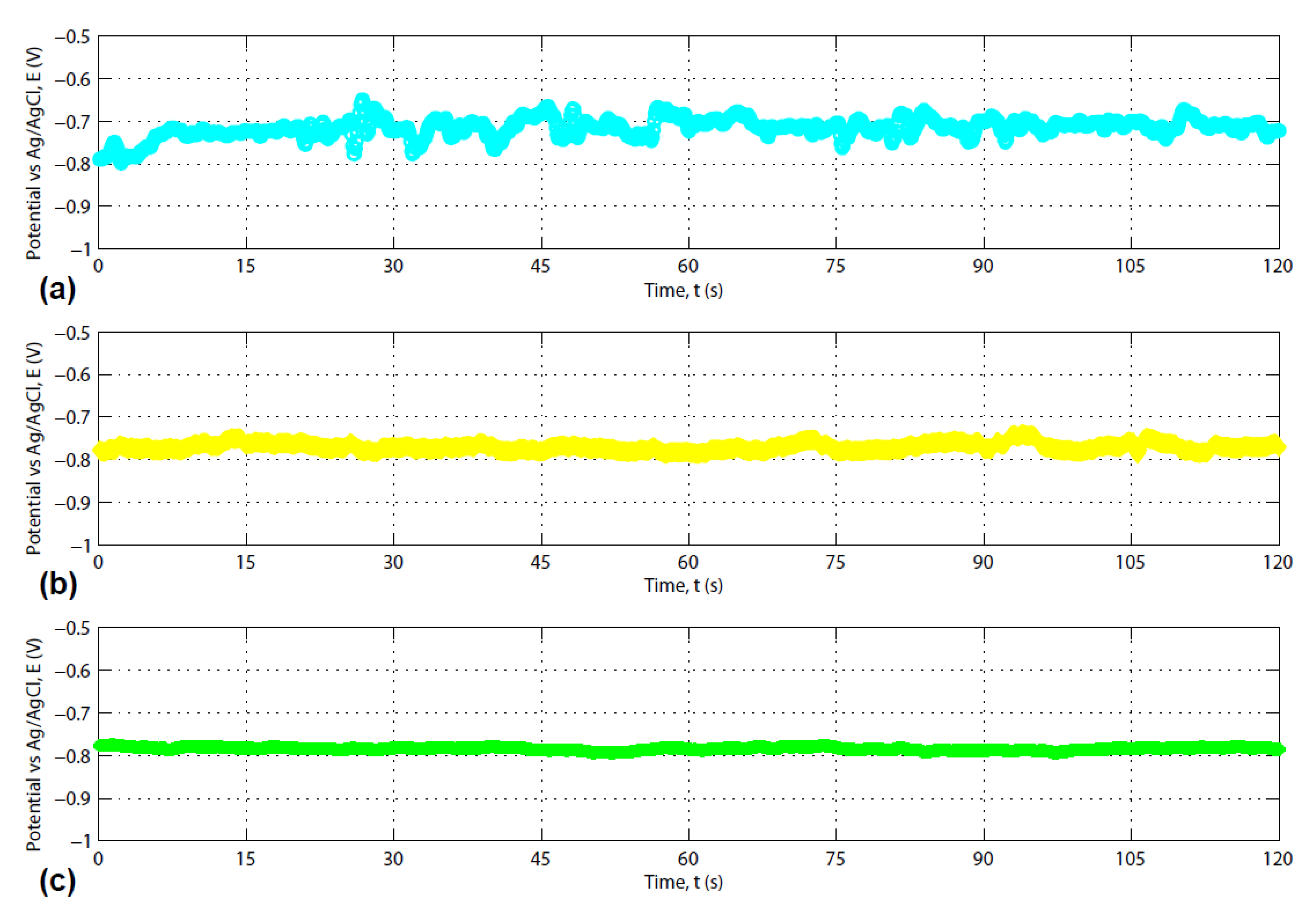

| Isopropyl Concentration (%) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 35.0 | 70.0 |
|---|---|---|---|---|---|---|---|
| Equilibrium potential (V) | −0.7846 | −0.7613 | −0.7325 | −0.7297 | −0.7259 | −0.7129 | −0.7101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X.; Gan, J.B. Measuring the Electrical and Photonic Properties of Cobalt Oxide-Containing Composite Carbon Fibers. J. Compos. Sci. 2020, 4, 156. https://doi.org/10.3390/jcs4040156
Gan YX, Gan JB. Measuring the Electrical and Photonic Properties of Cobalt Oxide-Containing Composite Carbon Fibers. Journal of Composites Science. 2020; 4(4):156. https://doi.org/10.3390/jcs4040156
Chicago/Turabian StyleGan, Yong X., and Jeremy B. Gan. 2020. "Measuring the Electrical and Photonic Properties of Cobalt Oxide-Containing Composite Carbon Fibers" Journal of Composites Science 4, no. 4: 156. https://doi.org/10.3390/jcs4040156
APA StyleGan, Y. X., & Gan, J. B. (2020). Measuring the Electrical and Photonic Properties of Cobalt Oxide-Containing Composite Carbon Fibers. Journal of Composites Science, 4(4), 156. https://doi.org/10.3390/jcs4040156





