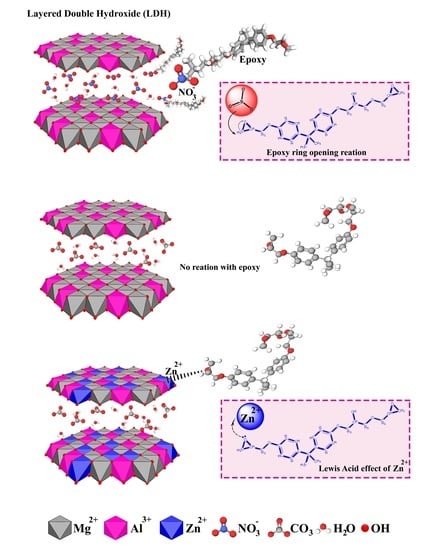
A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cure Behavior Analysis
3.2. Cure Kinetics Analysis
3.2.1. Determining the Reaction Model and the Order of Reaction
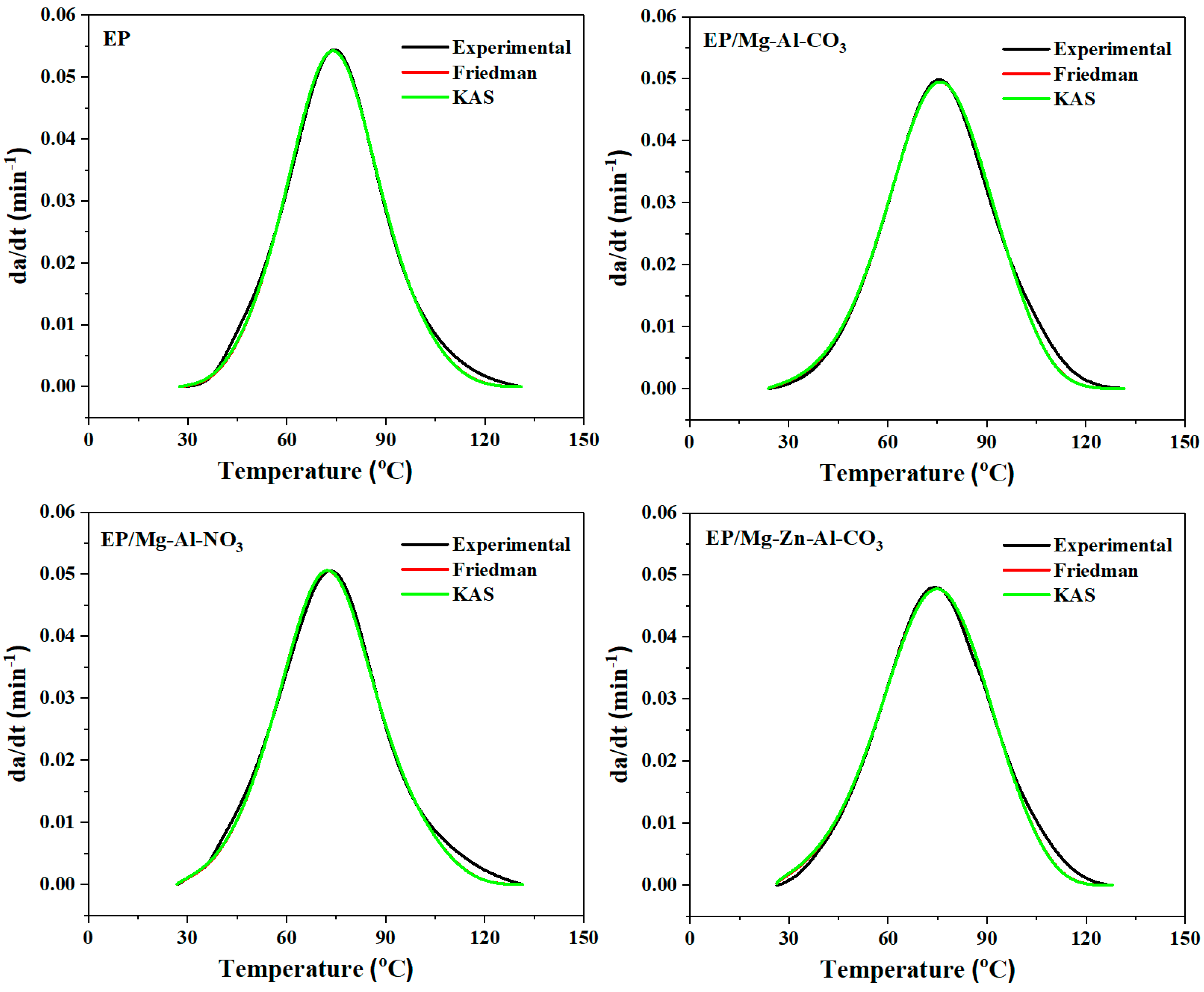
3.2.2. Model Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Materials and Methods
Appendix A.1. Materials
Appendix A.2. Preparation of Epoxy Nanocomposites
Appendix A.3. Characterization of Epoxy Nanocomposites
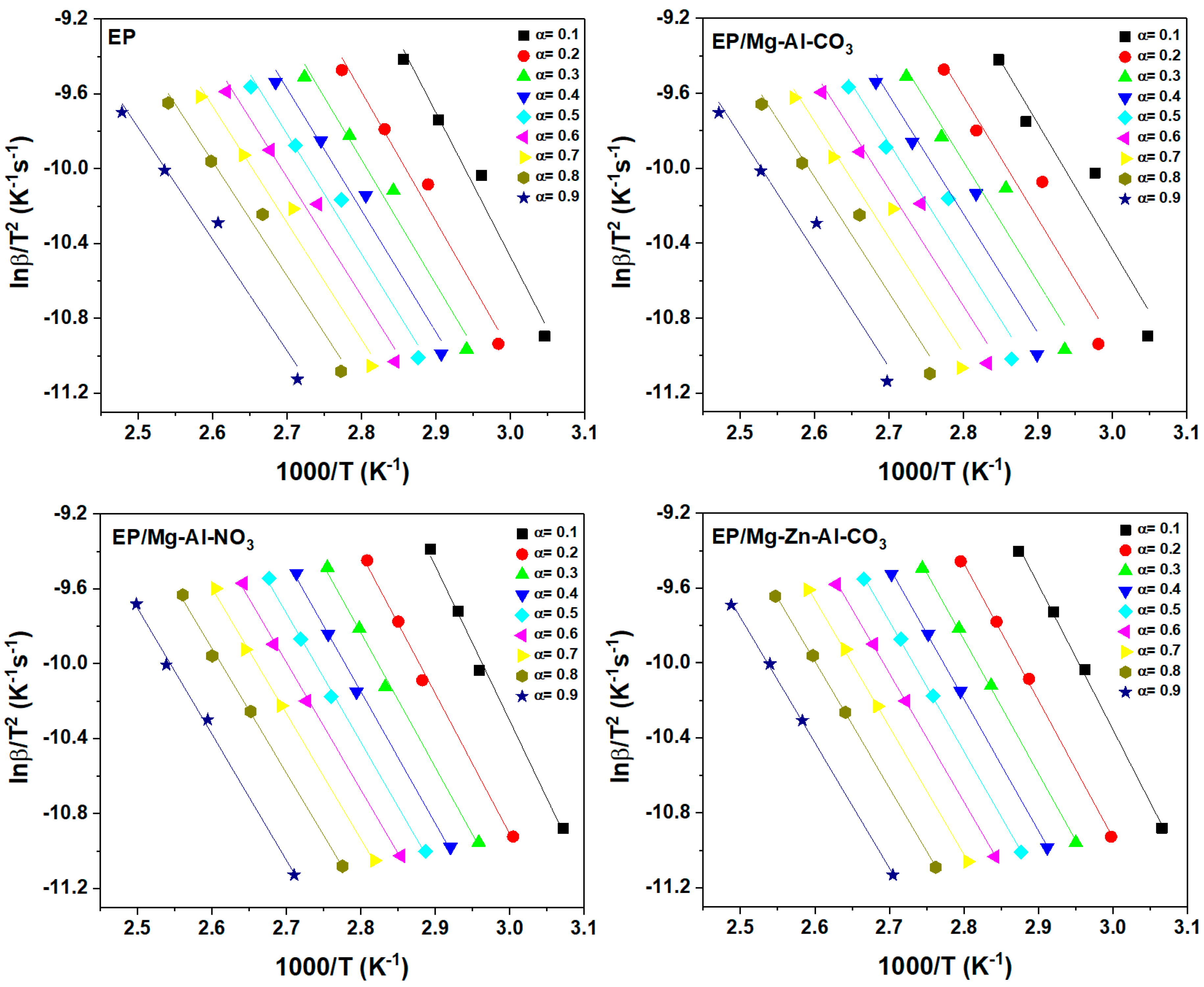
Appendix B. Isoconversional Kinetic Methods
Appendix B.1. Friedman Model
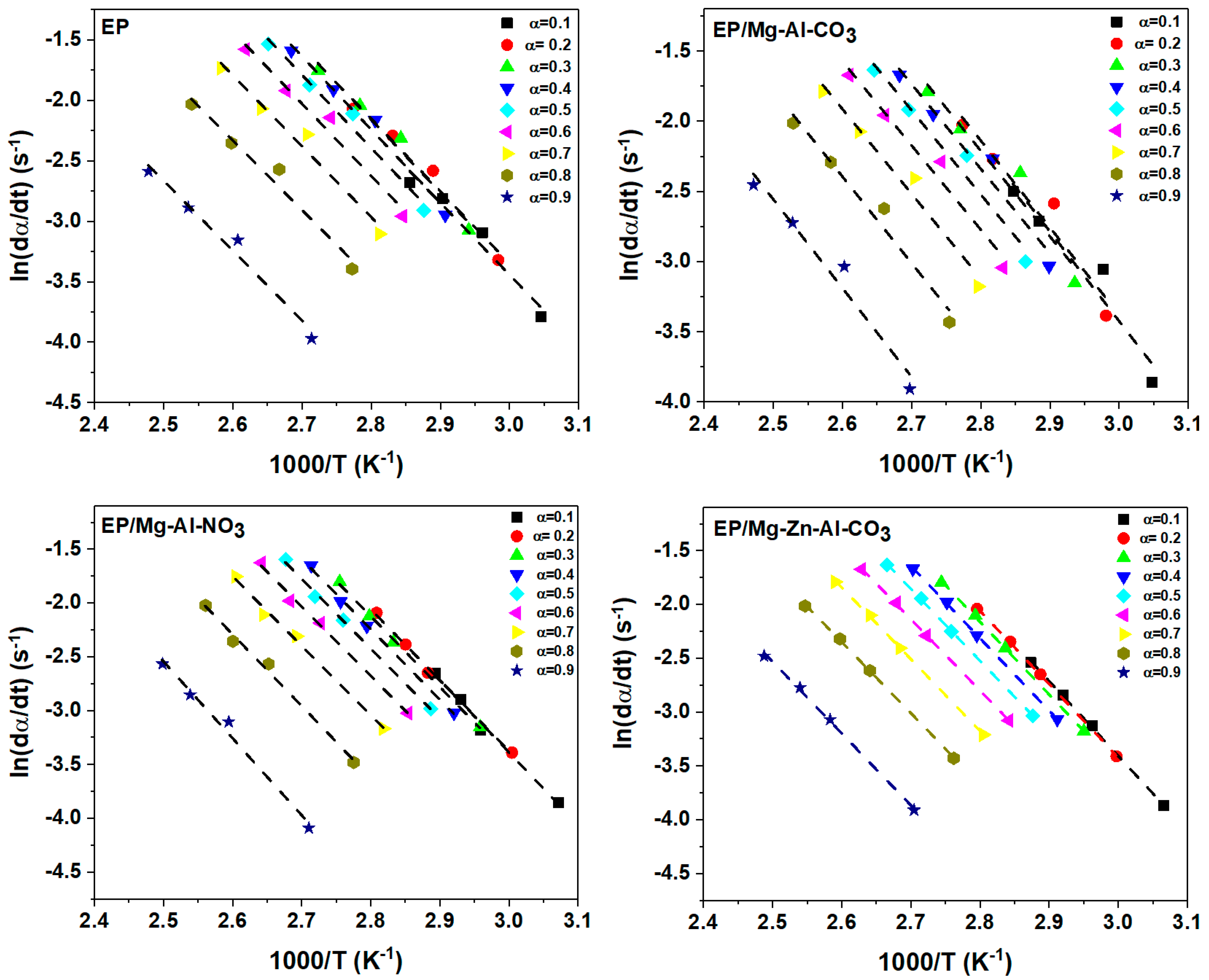
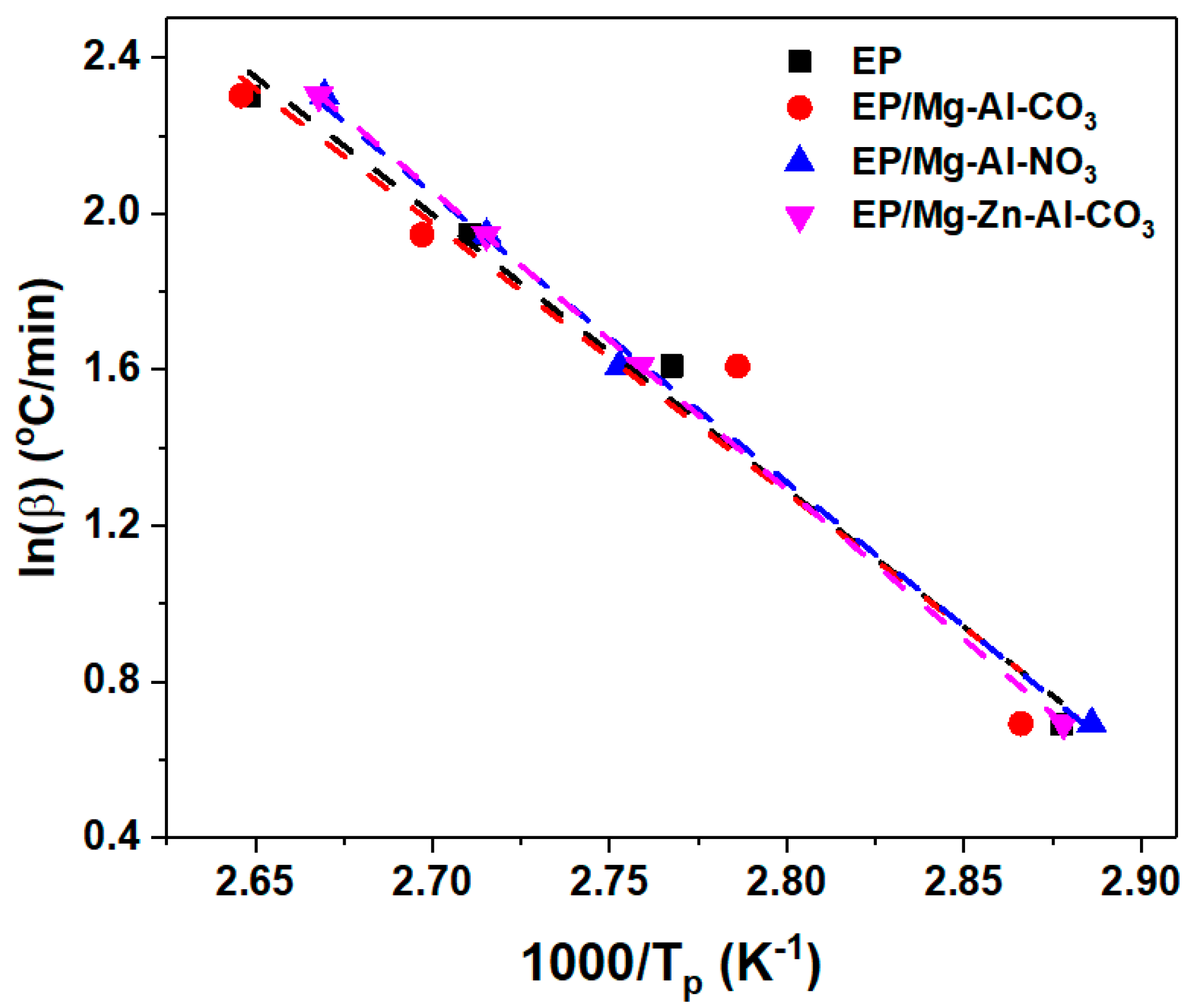
Appendix B.2. KAS Method
Appendix C. Selection of the Cure Reaction Model
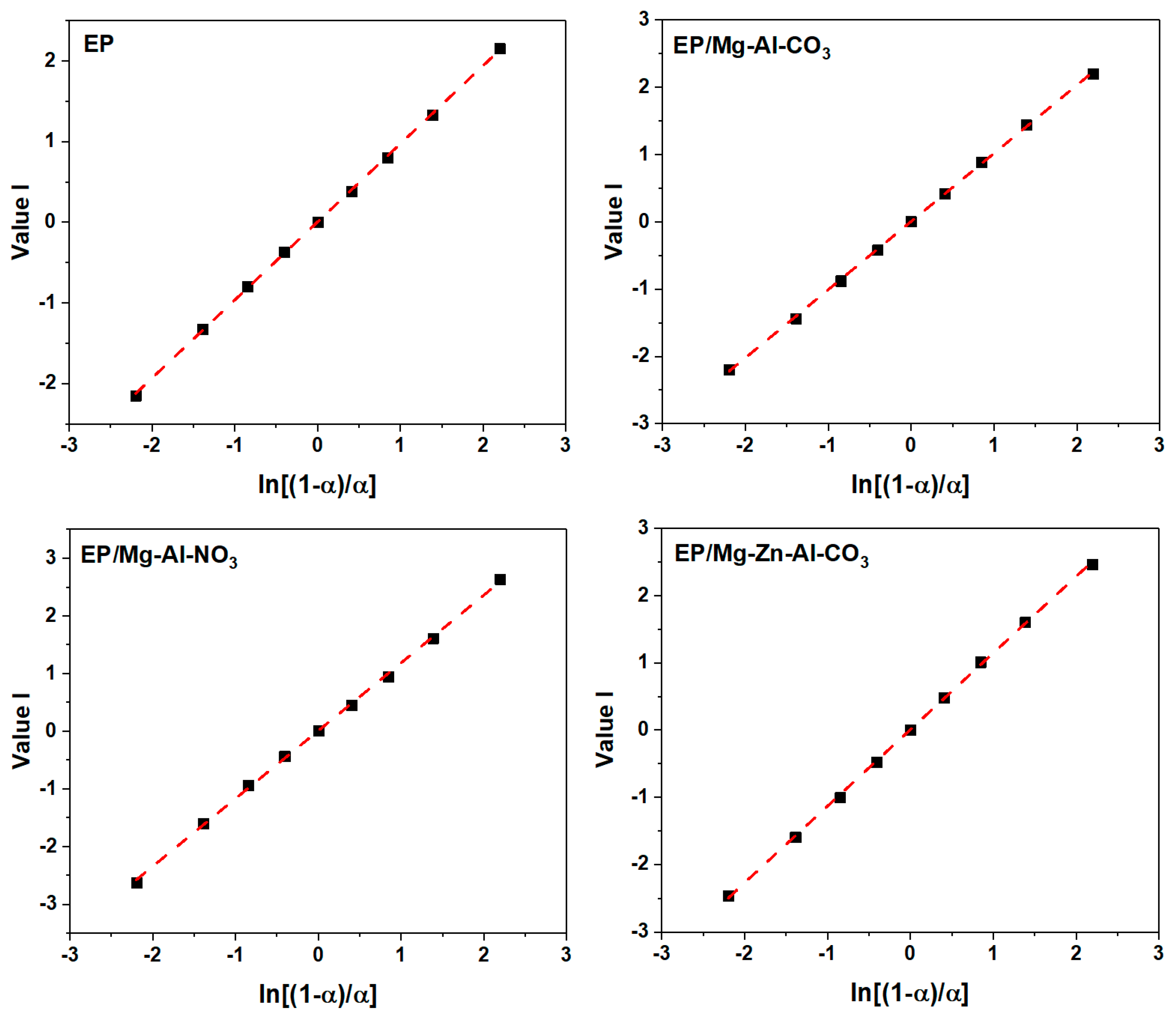
Appendix C.1. Friedman Model
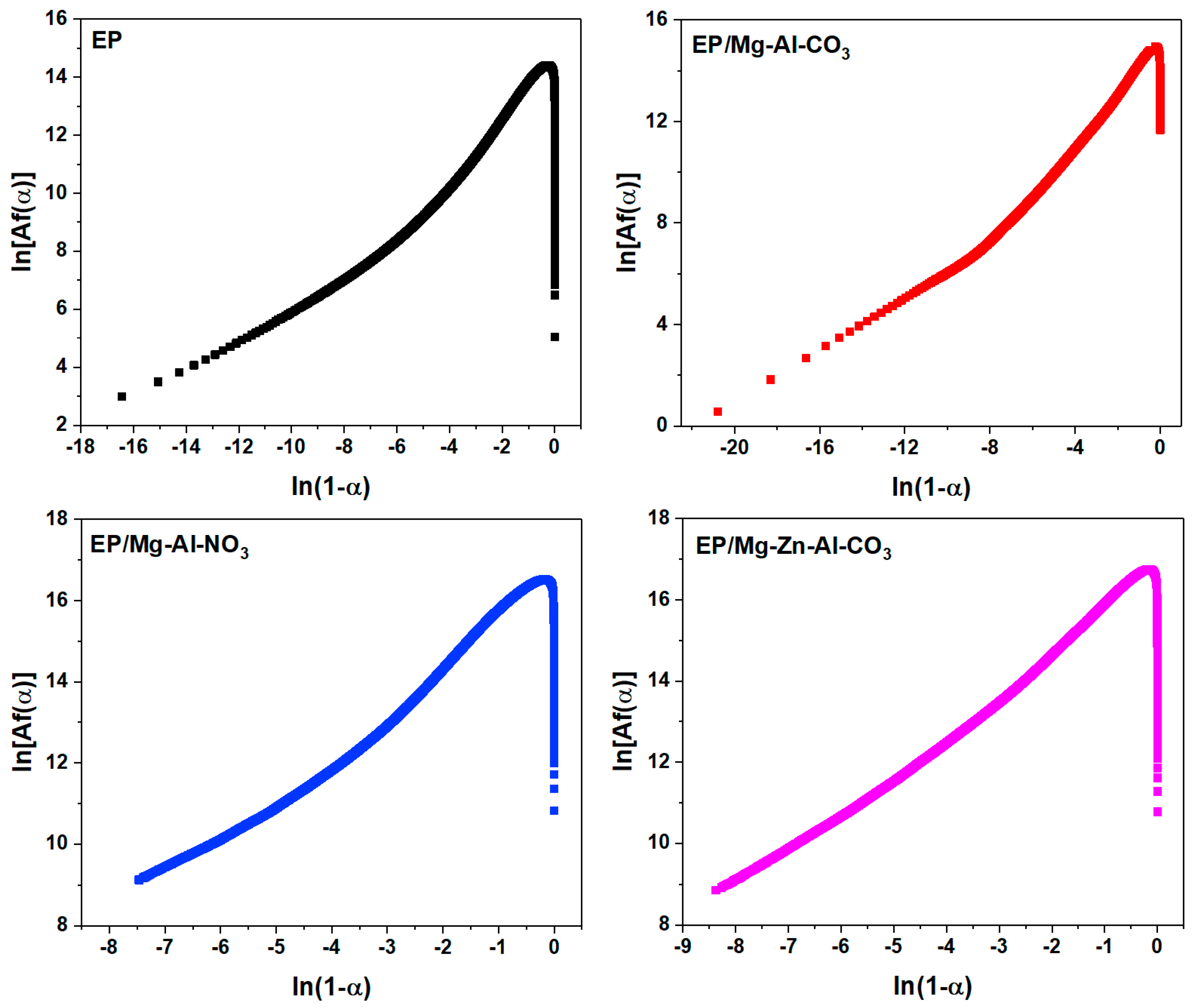
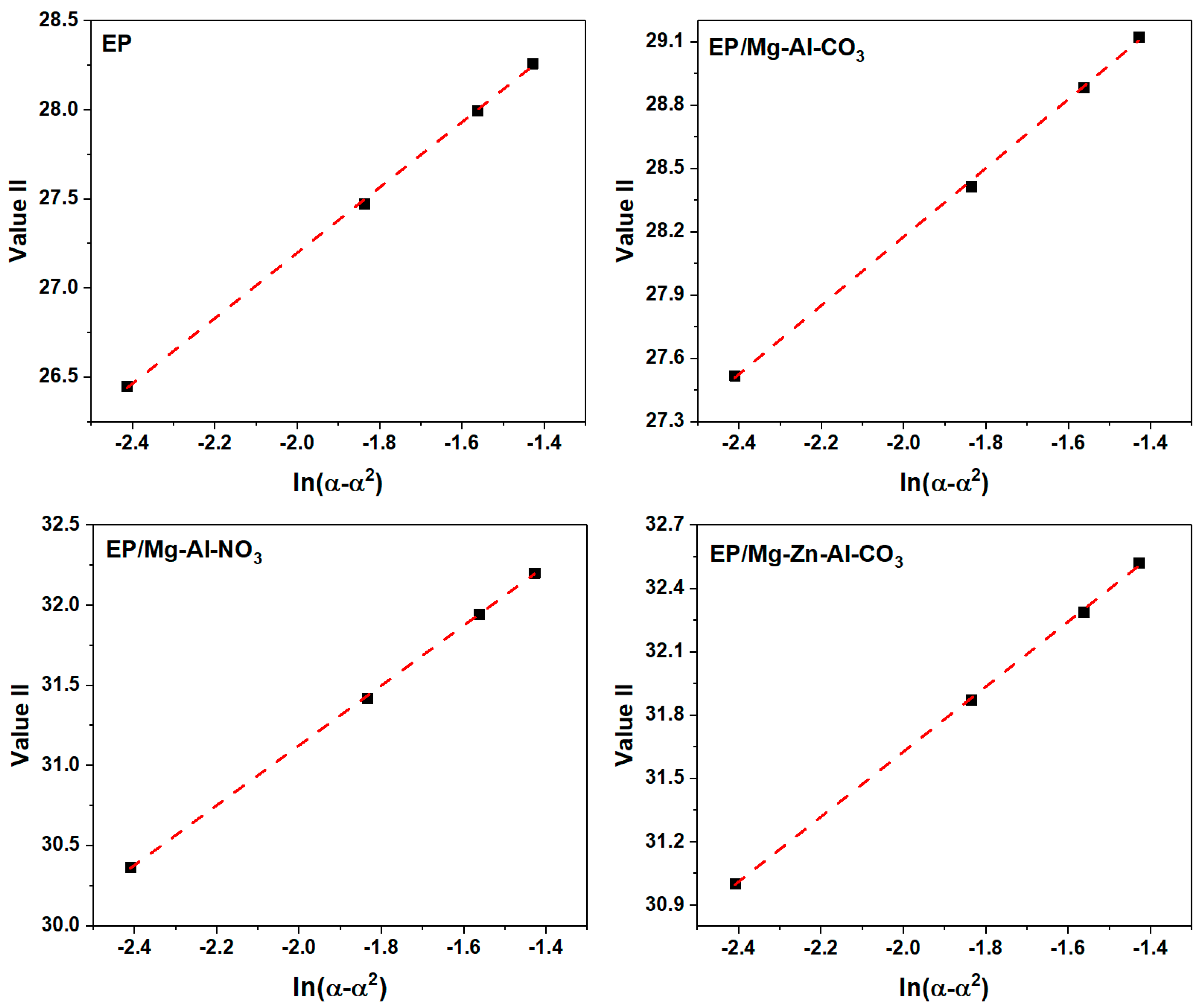
Appendix C.2. Málek Method
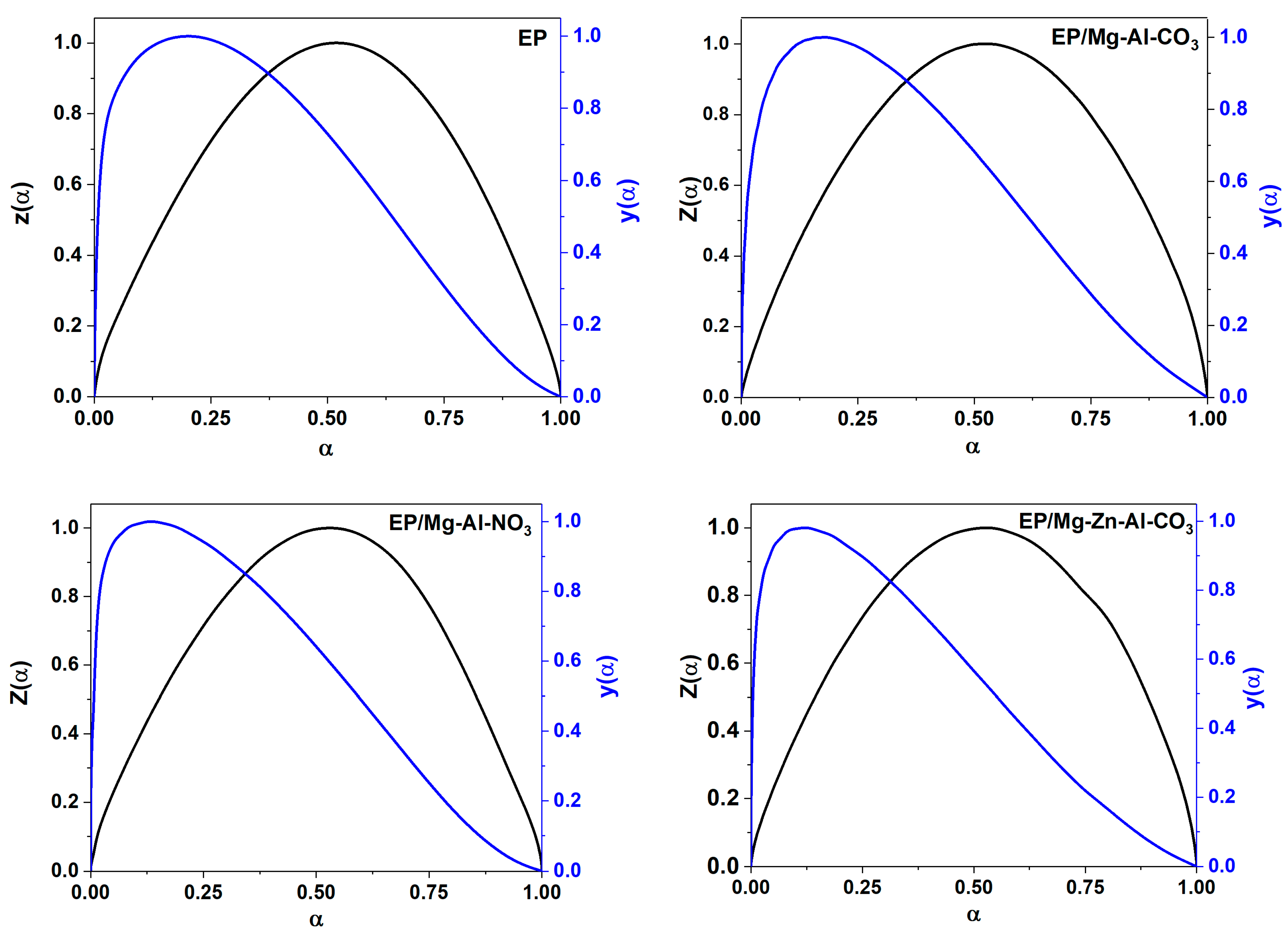
Appendix D. Determination of the Degree of Reaction
References
- Schaming, D.; Remita, H. Nanotechnology: From the ancient time to nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bharadiya, P.; Hansora, D. Layered double hydroxide based bionanocomposites. Appl. Clay Sci. 2019, 177, 19–36. [Google Scholar] [CrossRef]
- Zümreoglu-Karan, B.; Ay, A. Layered double hydroxides—Multifunctional nanomaterials. Chem. Pap. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Wu, M.; Wu, J.; Zhang, J.; Chen, H.; Zhou, J.; Qian, G.; Xu, Z.; Du, Z.; Rao, Q. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Silva, R.A.; Lopes, J.A.; Eulálio, D.; Santos, R.M.M.; Aquino, L.A.; Vergütz, L.; Novais, R.F.; Costa, L.M.; Pinto, F.G. Layered double hydroxides: Nanomaterials for applications in agriculture. Rev. Bras. Ciênc. Solo 2015, 39, 1–13. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J.-P. Polymer interleaved layered double hydroxide: A new emerging class of nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Chhetri, S.; Samanta, P.; Murmu, N.C.; Kuila, T. Anticorrosion Properties of Epoxy Composite Coating Reinforced by Molybdate-Intercalated Functionalized Layered Double Hydroxide. J. Compos. Sci. 2019, 3, 11. [Google Scholar] [CrossRef]
- Becker, C.M.; Gabbardo, A.D.; Wypych, F.; Amico, S.C. Mechanical and flame-retardant properties of epoxy/Mg–Al LDH composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 196–202. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105198. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Hampp, N.; Akbarzadeh Yazdi, D.; Zarrintaj, P.; Reza Ganjali, M.; Reza Saeb, M. Highly curable self-healing vitrimer-like cellulose-modified halloysite nanotube/epoxy nanocomposite coatings. Chem. Eng. J. 2020, 396, 125196. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Aghazadeh, M.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with electrochemically synthesized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105246. [Google Scholar] [CrossRef]
- Tomić, M.; Dunjić, B.; Nikolić, M.S.; Maletaškić, J.; Pavlović, V.B.; Bajat, J.; Djonlagić, J. Dispersion efficiency of montmorillonites in epoxy nanocomposites using solution intercalation and direct mixing methods. Appl. Clay Sci. 2018, 154, 52–63. [Google Scholar] [CrossRef]
- Zuo, J.; Li, H.; Dong, B.; Wang, L. Effects of metakaolin on the mechanical and anticorrosion properties of epoxy emulsion cement mortar. Appl. Clay Sci. 2020, 186, 105431. [Google Scholar] [CrossRef]
- Bayat, S.; Moini Jazani, O.; Molla-Abbasi, P.; Jouyandeh, M.; Saeb, M.R. Thin films of epoxy adhesives containing recycled polymers and graphene oxide nanoflakes for metal/polymer composite interface. Prog. Org. Coat. 2019, 136, 105201. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Shabanian, M.; Vahabi, H.; Saeb, M.R. Bushy-surface hybrid nanoparticles for developing epoxy superadhesives. Appl. Surf. Sci. 2019, 479, 1148–1160. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Cure Index for labeling curing potential of epoxy/LDH nanocomposites: A case study on nitrate anion intercalated Ni-Al-LDH. Prog. Org. Coat. 2019, 136, 105228. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Akbari, V.; Karami, Z.; Aghazadeh, M.; Zarrintaj, P.; Saeb, M.R. Curing epoxy with polyethylene glycol (PEG) surface-functionalized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105283. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R.; et al. Properties of nano-Fe3O4 incorporated epoxy coatings from Cure Index perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Xie, H.; Liu, B.; Yuan, Z.; Shen, J.; Cheng, R. Cure kinetics of carbon nanotube/tetrafunctional epoxy nanocomposites by isothermal differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3701–3712. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S.; Mititelu, A.; Sladic, C.; Vincent, L. A study of epoxy-amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol. Chem. Phys. 2003, 204, 1815–1821. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized MnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105199. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized CoxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105252. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Dubois, P. Curing epoxy with polyethylene glycol (PEG) surface-functionalized NixFe3-xO4magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105250. [Google Scholar] [CrossRef]
- Akbari, V.; Jouyandeh, M.; Paran, S.M.R.; Ganjali, M.R.; Abdollahi, H.; Vahabi, H.; Ahmadi, Z.; Formela, K.; Esmaeili, A.; Mohaddespour, A. Effect of Surface Treatment of Halloysite Nanotubes (HNTs) on the Kinetics of Epoxy Resin Cure with Amines. Polymers 2020, 12, 930. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Seidi, F.; Xiao, H.; Saeb, M.R. Nonisothermal Cure Kinetics of Epoxy/Polyvinylpyrrolidone Functionalized Superparamagnetic Nano-Fe3O4 Composites: Effect of Zn and Mn Doping. J. Compos. Sci. 2020, 4, 55. [Google Scholar] [CrossRef]
- Saeb, M.R.; Nonahal, M.; Rastin, H.; Shabanian, M.; Ghaffari, M.; Bahlakeh, G.; Ghiyasi, S.; Khonakdar, H.A.; Goodarzi, V.; Puglia, D. Calorimetric analysis and molecular dynamics simulation of cure kinetics of epoxy/chitosan-modified Fe3O4 nanocomposites. Prog. Org. Coat. 2017, 112, 176–186. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Jazani, O.M.; Formela, K.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. Curing epoxy resin with anhydride in the presence of halloysite nanotubes: The contradictory effects of filler concentration. Prog. Org. Coat. 2019, 126, 129–135. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; Puglia, D.; et al. Development of Mg-Zn-Al-CO3 ternary LDH and its curability in epoxy/amine system. Prog. Org. Coat. 2019, 136, 105264. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ghiyasi, S.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Maadani, M.; Rallini, M.; Luzi, F.; Torre, L.; et al. Exploring curing potential of epoxy nanocomposites containing nitrate anion intercalated Mg–Al–LDH with Cure Index. Prog. Org. Coat. 2020, 139, 105255. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjali, M.R.; Torre, L.; Puglia, D.; Saeb, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- Karami, Z.; Jazani, O.M.; Navarchian, A.H.; Saeb, M.R. State of cure in silicone/clay nanocomposite coatings: The puzzle and the solution. Prog. Org. Coat. 2018, 125, 222–233. [Google Scholar] [CrossRef]
- Dimier, F.; Sbirrazzuoli, N.; Vergnes, B.; Vincent, M. Curing kinetics and chemorheological analysis of polyurethane formation. Polym. Eng. Sci. 2004, 44, 518–527. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.-Y.; Xu, C.-J.; Li, B.-G.; Fan, H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Domínguez, J.; Grivel, J.-C.; Madsen, B. Study on the non-isothermal curing kinetics of a polyfurfuryl alcohol bioresin by DSC using different amounts of catalyst. Thermochim. Acta 2012, 529, 29–35. [Google Scholar] [CrossRef]
- Vyazovkin, S. Model-free kinetics. J. Therm. Anal. Calorim. 2006, 83, 45–51. [Google Scholar] [CrossRef]
- Miura, K. A new and simple method to estimate f (E) and k0 (E) in the distributed activation energy model from three sets of experimental data. Energy Fuels 1995, 9, 302–307. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rastin, H.; Shabanian, M.; Ghaffari, M.; Bahlakeh, G. Cure kinetics of epoxy/β-cyclodextrin-functionalized Fe3O4 nanocomposites: Experimental analysis, mathematical modeling, and molecular dynamics simulation. Prog. Org. Coat. 2017, 110, 172–181. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Akbari, V.; Paran, S.M.R.; Livi, S.; Ducos, F.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: Cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym. Eng. Sci. 2020, 6, e03798. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Comparative Structural, thermodynamic and electronic analyses of ZnAlAn− hydrotalcite-like compounds (An− Cl−, F−, Br−, OH−, CO32− or NO3−): An ab initio study. Appl. Clay Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Yarahmadi, E.; Didehban, K.; Ghiyasi, S.; Paran, S.M.R.; Puglia, D.; Ali, J.A.; Jannesari, A.; Saeb, M.R.; Ranjbar, Z.; et al. Cure kinetics of epoxy/graphene oxide (GO) nanocomposites: Effect of starch functionalization of GO nanosheets. Prog. Org. Coat. 2019, 136, 105217. [Google Scholar] [CrossRef]
- Málek, J. A computer program for kinetic analysis of non-isothermal thermoanalytical data. Thermochim. Acta 1989, 138, 337–346. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Lu, Z.; Hu, J.; Zeng, K.; Yang, G. Curing kinetics study on highly efficient thermal synergistic polymerization effect between alicyclic imide moiety and phthalonitrile. Thermochim. Acta 2018, 659, 27–33. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Hamad, S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Kim, S.-J.; Zarrintaj, P.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/ZnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2019, 136, 105290. [Google Scholar] [CrossRef]
- Bello, R.H.; Coelho, L.A. Curing kinetics of chemically recyclable thermoset and their nanocomposites. Thermochim. Acta 2019, 679, 178317. [Google Scholar] [CrossRef]
- Karami, Z.; Ganjali, M.R.; Zarghami Dehaghani, M.; Aghazadeh, M.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; Mohaddespour, A.; Formela, K.; Haponiuk, J.T. Kinetics of Cross-Linking Reaction of Epoxy Resin with Hydroxyapatite-Functionalized Layered Double Hydroxides. Polymers 2020, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Zhang, J.; Cao, L.; Dong, H.; Zhang, C.; Xu, X.; Zhu, M.; Li, J. Optimizing curing process of graphene oxide/waterborne epoxy blends by curing kinetics simulation considering the coupling of heat conduction and curing reaction. Thermochim. Acta 2019, 672, 60–69. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Res. Report Chiba Inst. Technol. Sci. Technol 1971, 16, 22. [Google Scholar]
- Sbirrazzuoli, N. Is the Friedman method applicable to transformations with temperature dependent reaction heat? Macromol. Chem. Phys. 2007, 208, 1592–1597. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Tao, Q.; Su, L.; Frost, R.L.; He, H.; Theng, B.K.G. Effect of functionalized kaolinite on the curing kinetics of cycloaliphatic epoxy/anhydride system. Appl. Clay Sci. 2014, 95, 317–322. [Google Scholar] [CrossRef]
- Zhou, T.; Gu, M.; Jin, Y.; Wang, J. Studying on the curing kinetics of a DGEBA/EMI-2, 4/nano-sized carborundum system with two curing kinetic methods. Polymer 2005, 46, 6174–6181. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Zou, H.; Liang, M. Curing characteristics of an epoxy resin in the presence of functional graphite oxide with amine-rich surface. Thermochim. Acta 2015, 614, 76–84. [Google Scholar] [CrossRef]
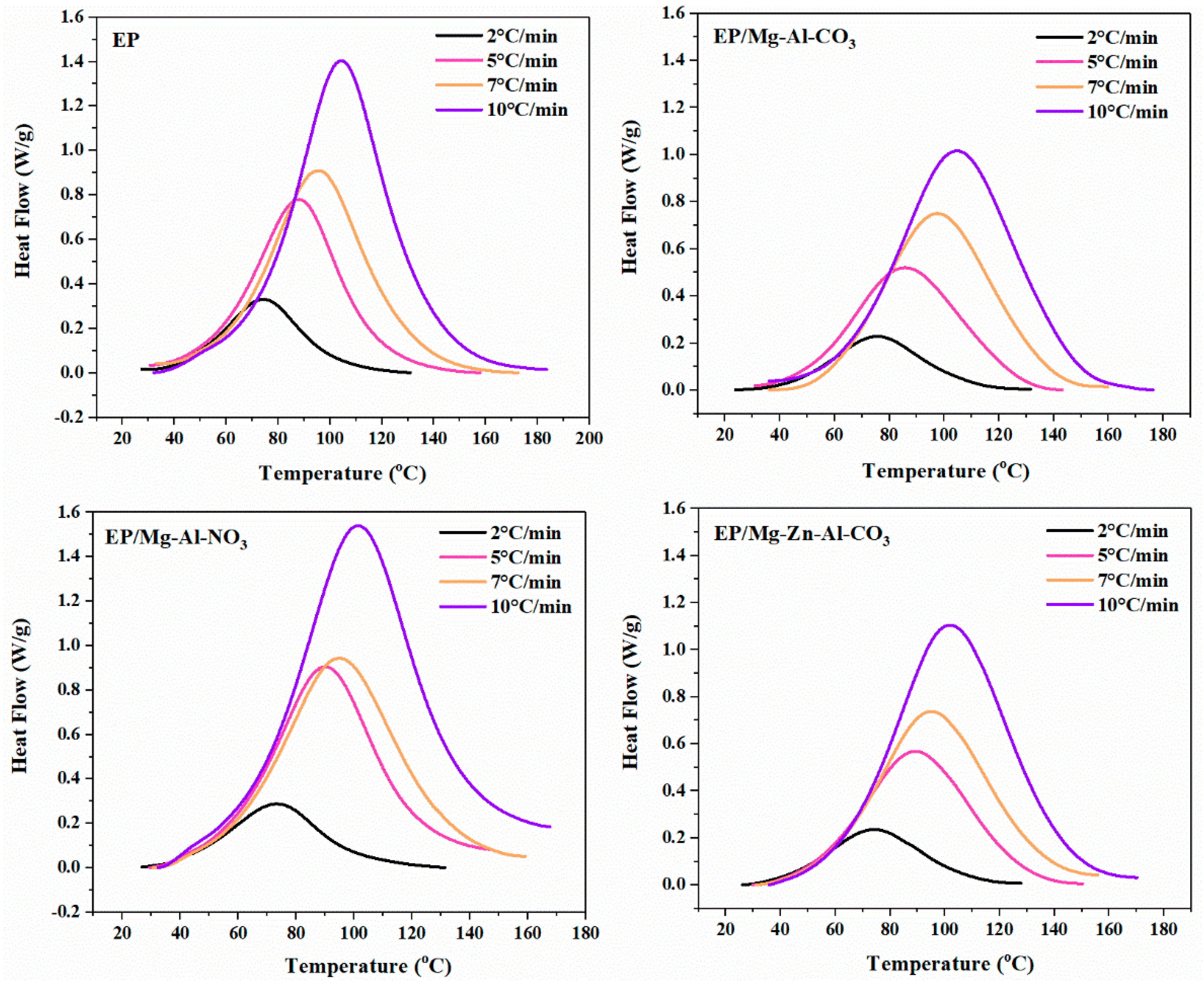
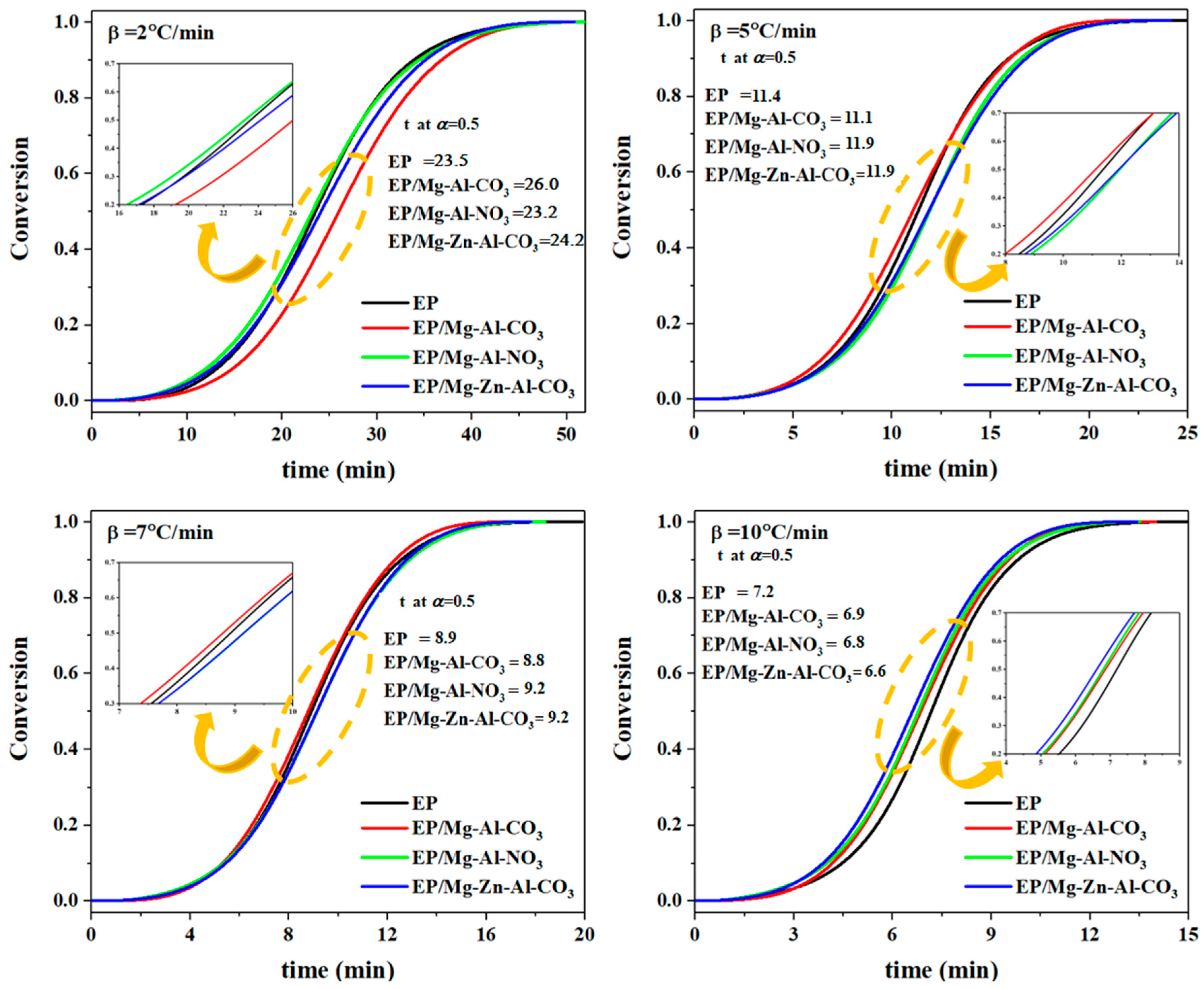
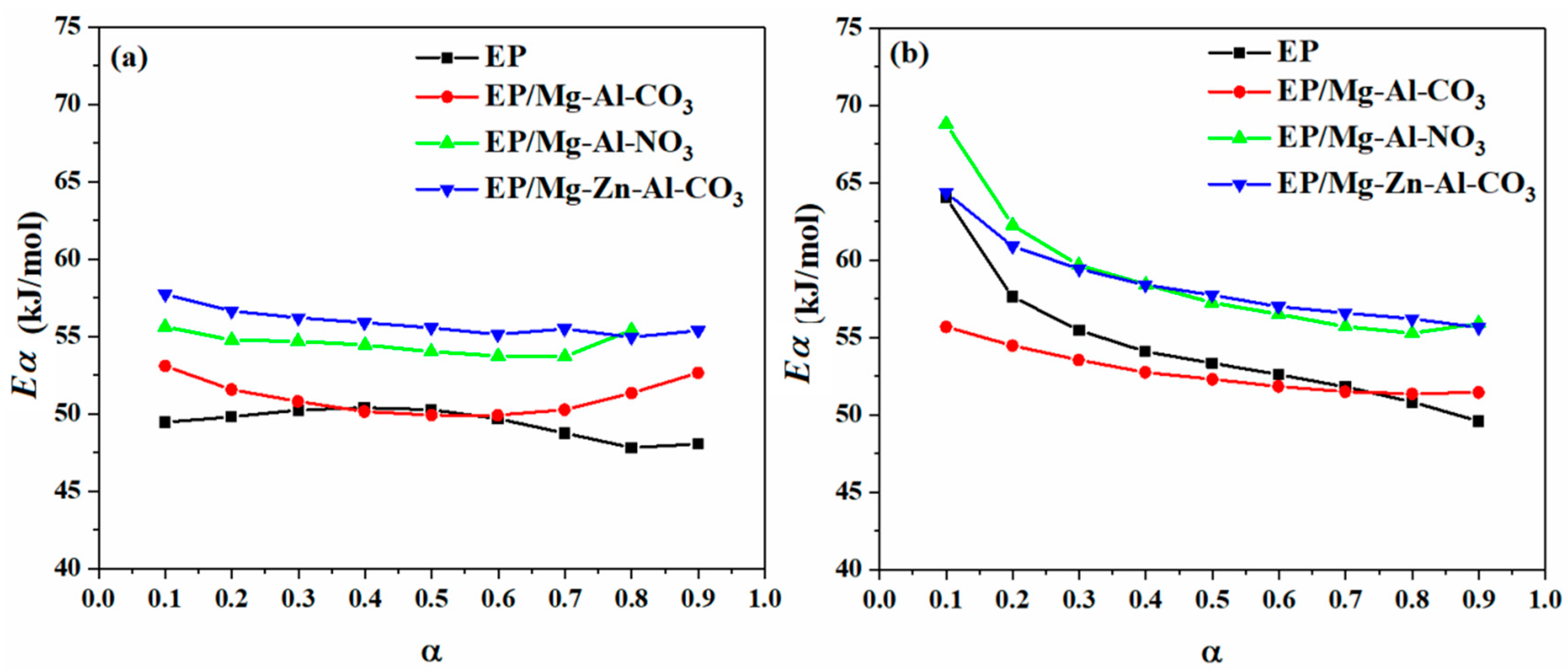
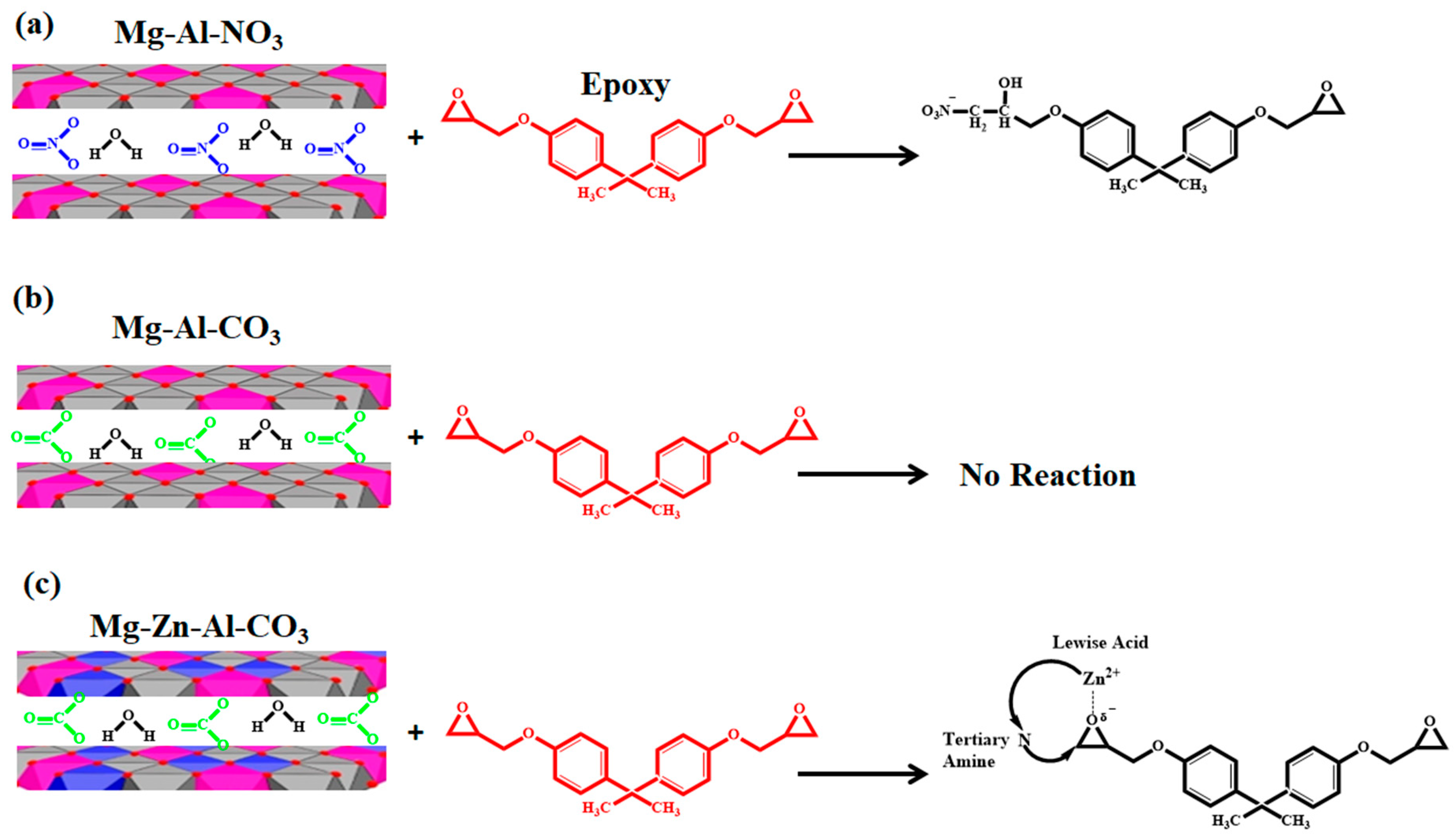
| Designation | Heating Rate (°C·min−1) | αp∞ | αm | αp |
|---|---|---|---|---|
| EP | 2 | 0.493 | 0.199 | 0.519 |
| 5 | 0.489 | 0.182 | 0.544 | |
| 7 | 0.481 | 0.186 | 0.529 | |
| 10 | 0.561 | 0.215 | 0.532 | |
| EP/Mg-Al-CO3 | 2 | 0.496 | 0.179 | 0.529 |
| 5 | 0.513 | 0.149 | 0.523 | |
| 7 | 0.536 | 0.147 | 0.531 | |
| 10 | 0.516 | 0.137 | 0.537 | |
| EP/Mg-Al-NO3 | 2 | 0.502 | 0.134 | 0.531 |
| 5 | 0.684 | 0.136 | 0.546 | |
| 7 | 0.648 | 0.095 | 0.530 | |
| 10 | 0.668 | 0.009 | 0.545 | |
| EP/Mg-Zn-Al-CO3 | 2 | 0.494 | 0.122 | 0.527 |
| 5 | 0.544 | 0.092 | 0.532 | |
| 7 | 0.592 | 0.095 | 0.532 | |
| 10 | 0.536 | 0.092 | 0.533 |
| Designation | Heating Rate (°C·min−1) | Ēα (kJ/mol) | ln(A) (1/s) | Mean (1/s) | m | Mean | n | Mean |
|---|---|---|---|---|---|---|---|---|
| Friedman method | ||||||||
| EP | 2 | 49.38 | 15.44 | 15.53 | 0.437 | 0.441 | 1.406 | 1.426 |
| 5 | 15.64 | 0.446 | 1.410 | |||||
| 7 | 15.48 | 0.390 | 1.422 | |||||
| 10 | 15.55 | 0.491 | 1.465 | |||||
| EP/Mg-Al-CO3 | 2 | 51.07 | 15.73 | 15.70 | 0.313 | 0.231 | 1.327 | 1.27 |
| 5 | 15.83 | 0.189 | 1.259 | |||||
| 7 | 15.63 | 0.205 | 1.230 | |||||
| 10 | 15.63 | 0.216 | 1.265 | |||||
| EP/Mg-Al-NO3 | 2 | 55.08 | 17.44 | 17.31 | 0.348 | 0.300 | 1.527 | 1.436 |
| 5 | 17.30 | 0.324 | 1.380 | |||||
| 7 | 17.19 | 0.228 | 1.396 | |||||
| 10 | 17.33 | 0.298 | 1.440 | |||||
| EP/Mg-Zn-Al-CO3 | 2 | 55.88 | 17.36 | 17.33 | 0.206 | 0.176 | 1.345 | 1.329 |
| 5 | 17.30 | 0.158 | 1.310 | |||||
| 7 | 17.33 | 0.169 | 1.321 | |||||
| 10 | 17.32 | 0.172 | 1.341 | |||||
| KAS method | ||||||||
| EP | 2 | 54.37 | 17.16 | 17.17 | 0.389 | 0.386 | 1.450 | 1.469 |
| 5 | 17.29 | 0.391 | 1.452 | |||||
| 7 | 17.10 | 0.335 | 1.468 | |||||
| 10 | 17.13 | 0.430 | 1.507 | |||||
| EP/Mg-Al-CO3 | 2 | 52.76 | 16.31 | 16.26 | 0.295 | 0.212 | 1.342 | 1.285 |
| 5 | 16.39 | 0.169 | 1.275 | |||||
| 7 | 16.17 | 0.187 | 1.245 | |||||
| 10 | 16.17 | 0.195 | 1.280 | |||||
| EP/Mg-Al-NO3 | 2 | 58.86 | 18.75 | 18.56 | 0.309 | 0.254 | 1.562 | 1.468 |
| 5 | 18.54 | 0.279 | 1.410 | |||||
| 7 | 18.41 | 0.181 | 1.430 | |||||
| 10 | 18.54 | 0.248 | 1.472 | |||||
| EP/Mg-Zn-Al-CO3 | 2 | 58.47 | 18.25 | 18.18 | 0.178 | 0.145 | 1.368 | 1.352 |
| 5 | 18.15 | 0.127 | 1.333 | |||||
| 7 | 18.17 | 0.137 | 1.344 | |||||
| 10 | 18.14 | 0.140 | 1.364 |
| Sample Code | Cure State | Ēα (kJ/mol) (Friedman) | Ēα (kJ/mol) (KAS) | Eα (kJ/mol) (FWO) |
|---|---|---|---|---|
| EP | - | 49.38 | 54.37 | 55.74 |
| EP/Mg-Al-CO3 | Poor | 51.07 | 52.76 | 54.60 |
| EP/Mg-Al-NO3 | Excellent | 55.08 | 58.86 | 58.47 |
| EP/Mg-Zn-Al-CO3 | Poor | 55.88 | 58.47 | 60.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, Z.; Paran, S.M.R.; Vijayan P., P.; Ganjali, M.R.; Jouyandeh, M.; Esmaeili, A.; Habibzadeh, S.; J. Stadler, F.; Saeb, M.R. A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites. J. Compos. Sci. 2020, 4, 111. https://doi.org/10.3390/jcs4030111
Karami Z, Paran SMR, Vijayan P. P, Ganjali MR, Jouyandeh M, Esmaeili A, Habibzadeh S, J. Stadler F, Saeb MR. A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites. Journal of Composites Science. 2020; 4(3):111. https://doi.org/10.3390/jcs4030111
Chicago/Turabian StyleKarami, Zohre, Seyed Mohammad Reza Paran, Poornima Vijayan P., Mohammad Reza Ganjali, Maryam Jouyandeh, Amin Esmaeili, Sajjad Habibzadeh, Florian J. Stadler, and Mohammad Reza Saeb. 2020. "A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites" Journal of Composites Science 4, no. 3: 111. https://doi.org/10.3390/jcs4030111
APA StyleKarami, Z., Paran, S. M. R., Vijayan P., P., Ganjali, M. R., Jouyandeh, M., Esmaeili, A., Habibzadeh, S., J. Stadler, F., & Saeb, M. R. (2020). A Comparative Study on Cure Kinetics of Layered Double Hydroxide (LDH)/Epoxy Nanocomposites. Journal of Composites Science, 4(3), 111. https://doi.org/10.3390/jcs4030111