Factors that Affect Network Formation in Carbon Nanotube Composites and their Resultant Electrical Properties
Abstract
1. Introduction
1.1. Importance of Multiwalled Carbon Nanotube (MWCNT) Composites
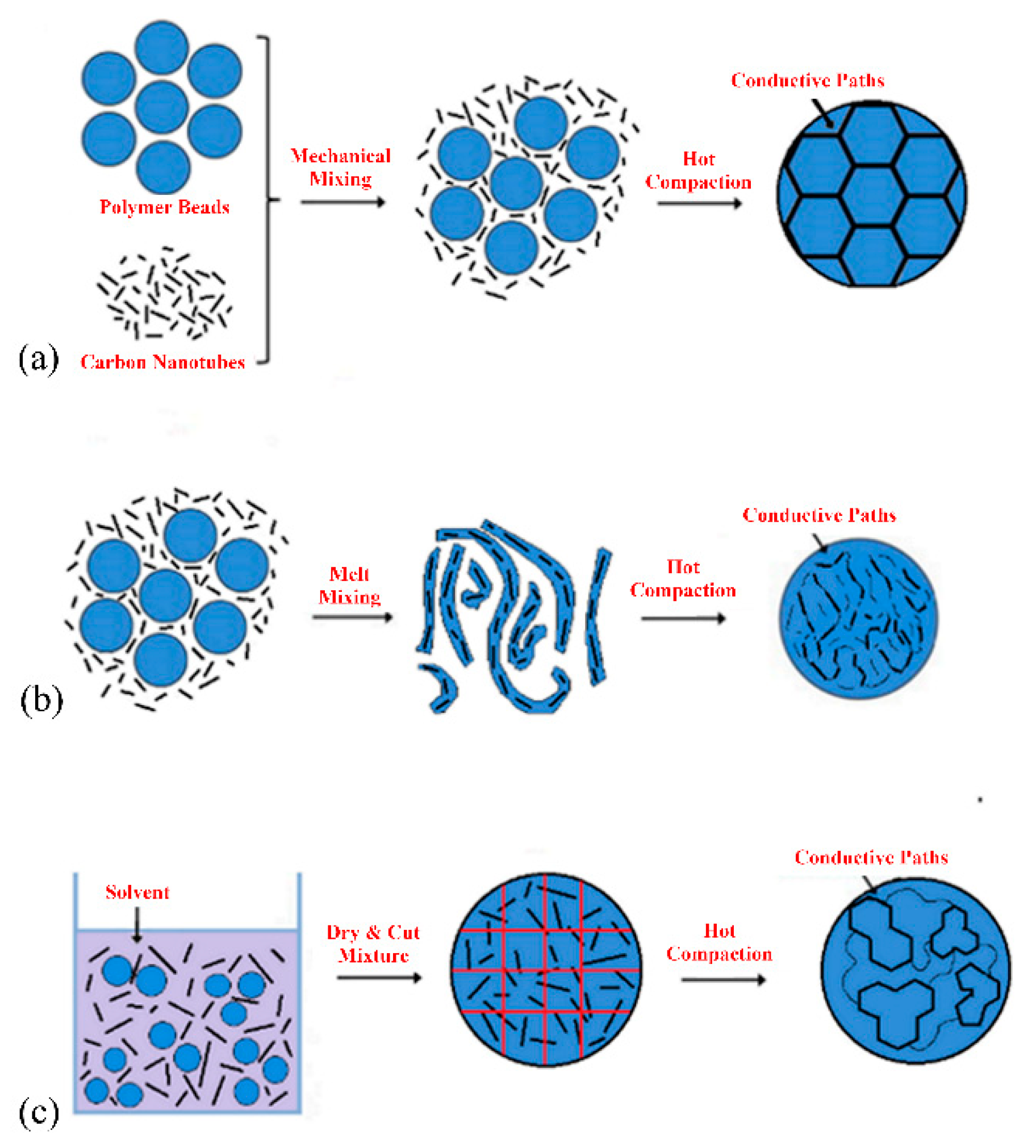
1.2. Methods of Mixing
1.3. Functionalization of MWCNT to Improve Dispersion and/or Conductivity
1.4. Percolation and Electrical Testing
2. Factors that Affect the Electrical Properties of Composites
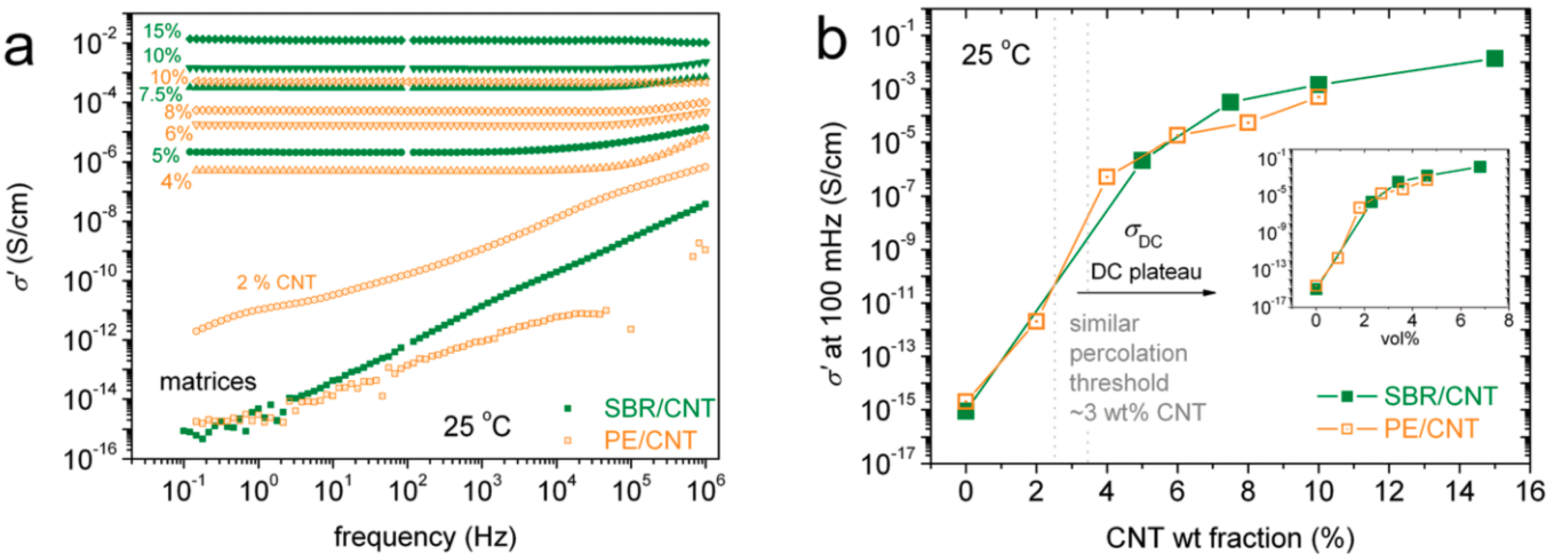
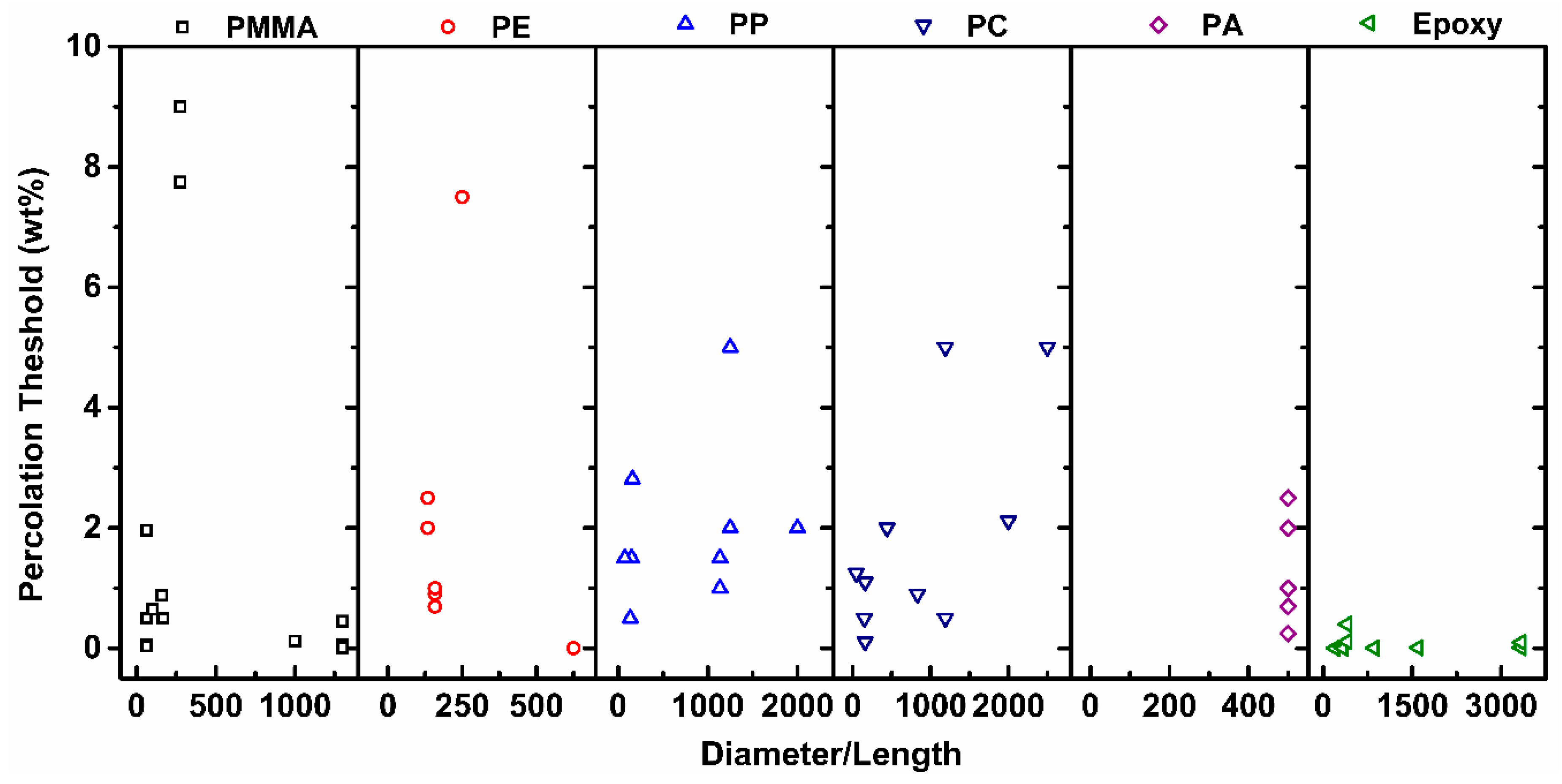
2.1. Differences in Polymer Systems
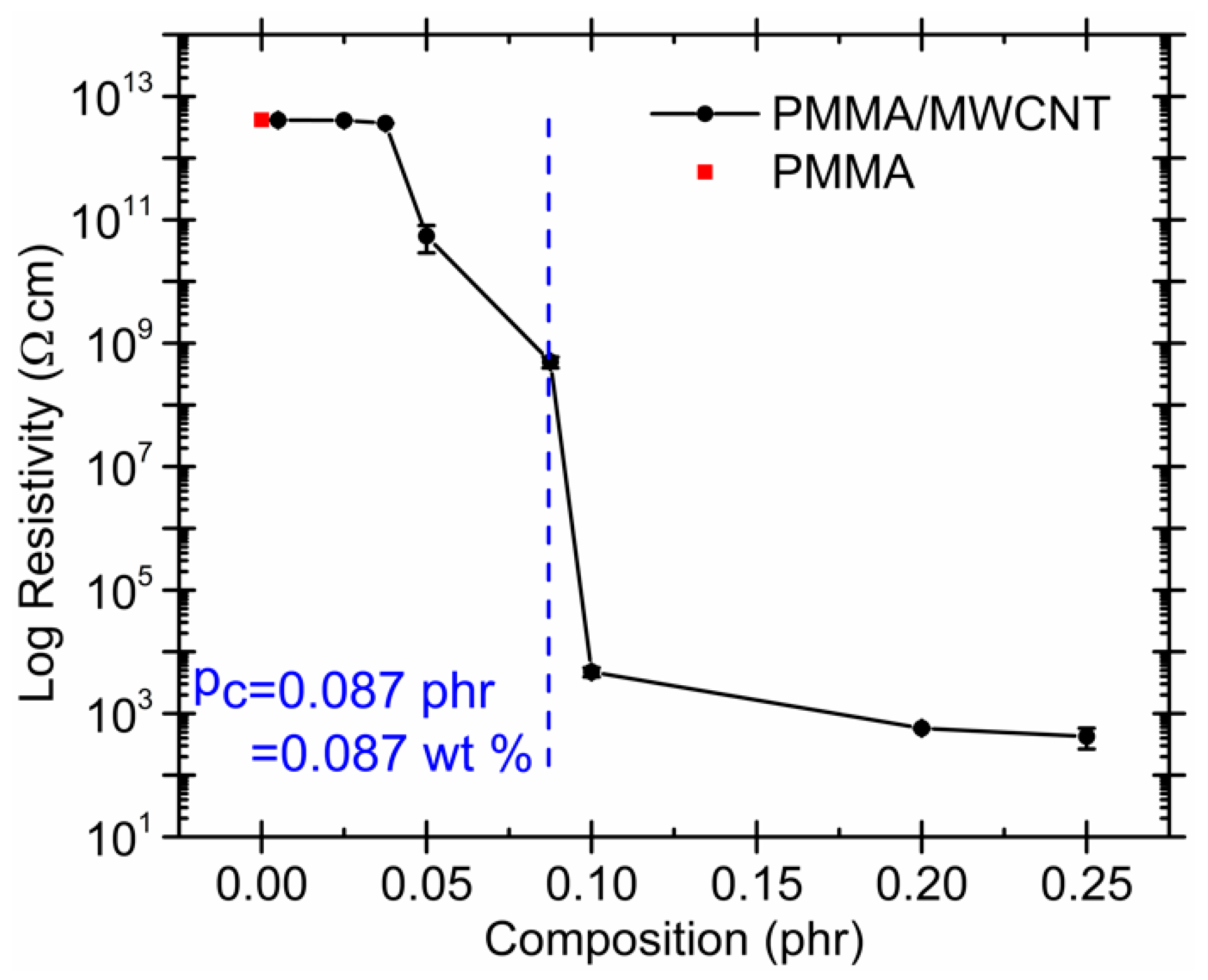
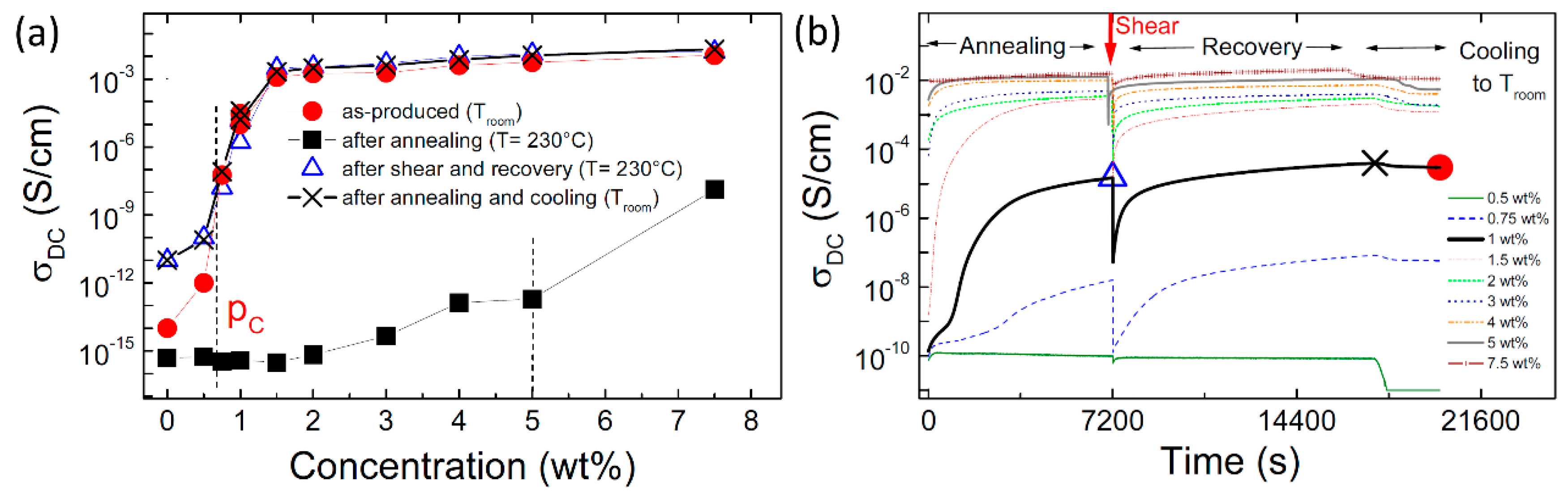
2.2. Effect of Increasing the Amount of MWCNT
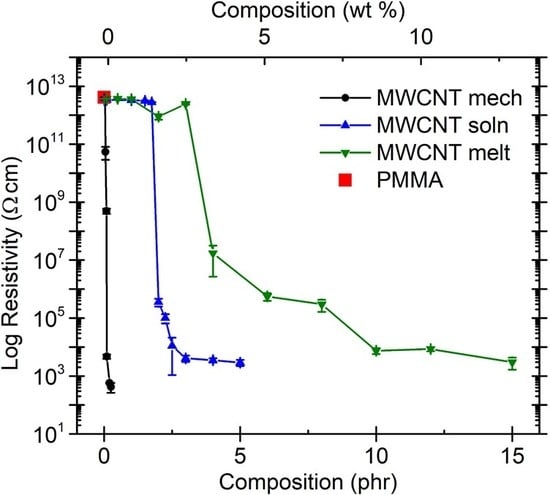
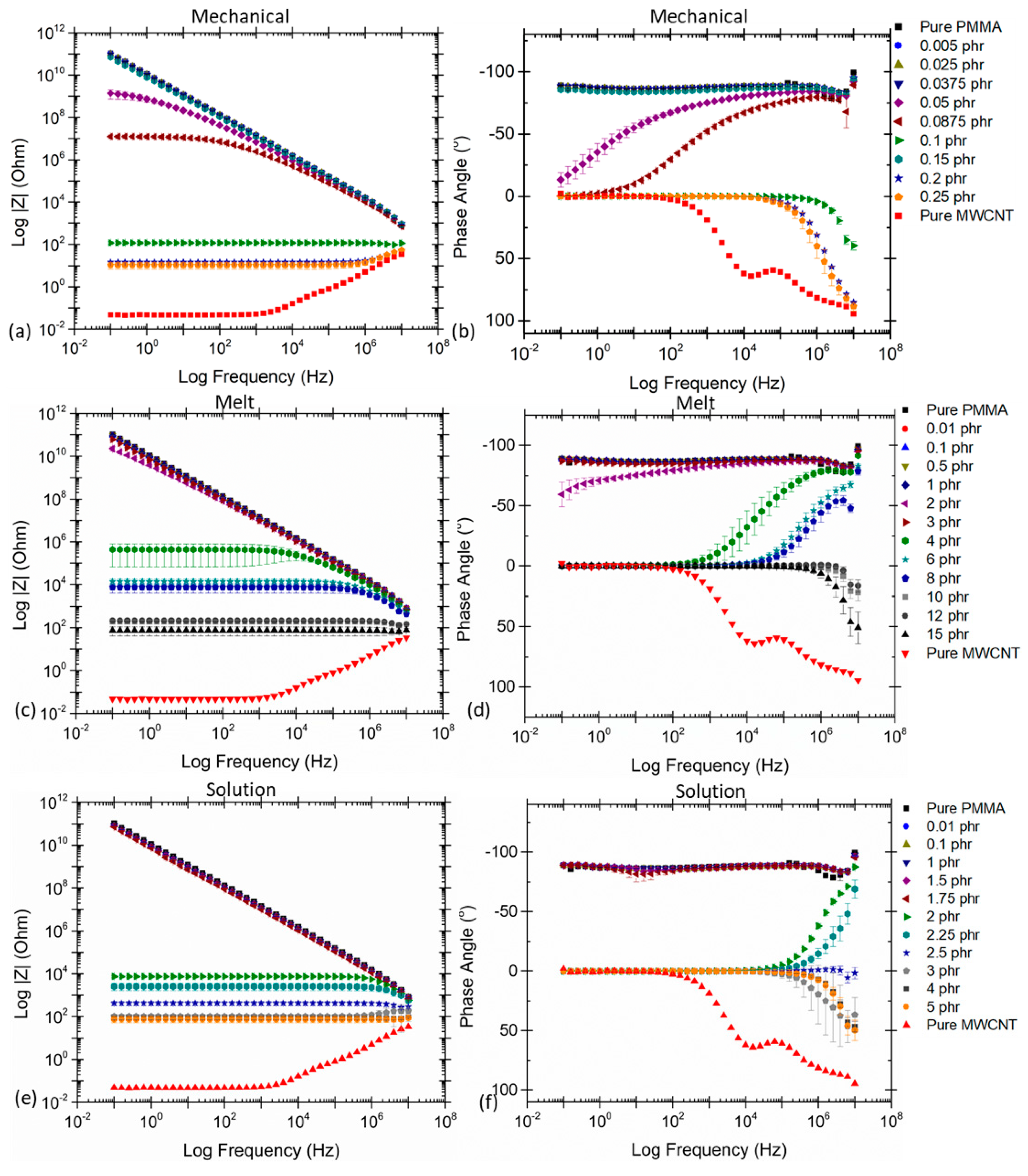
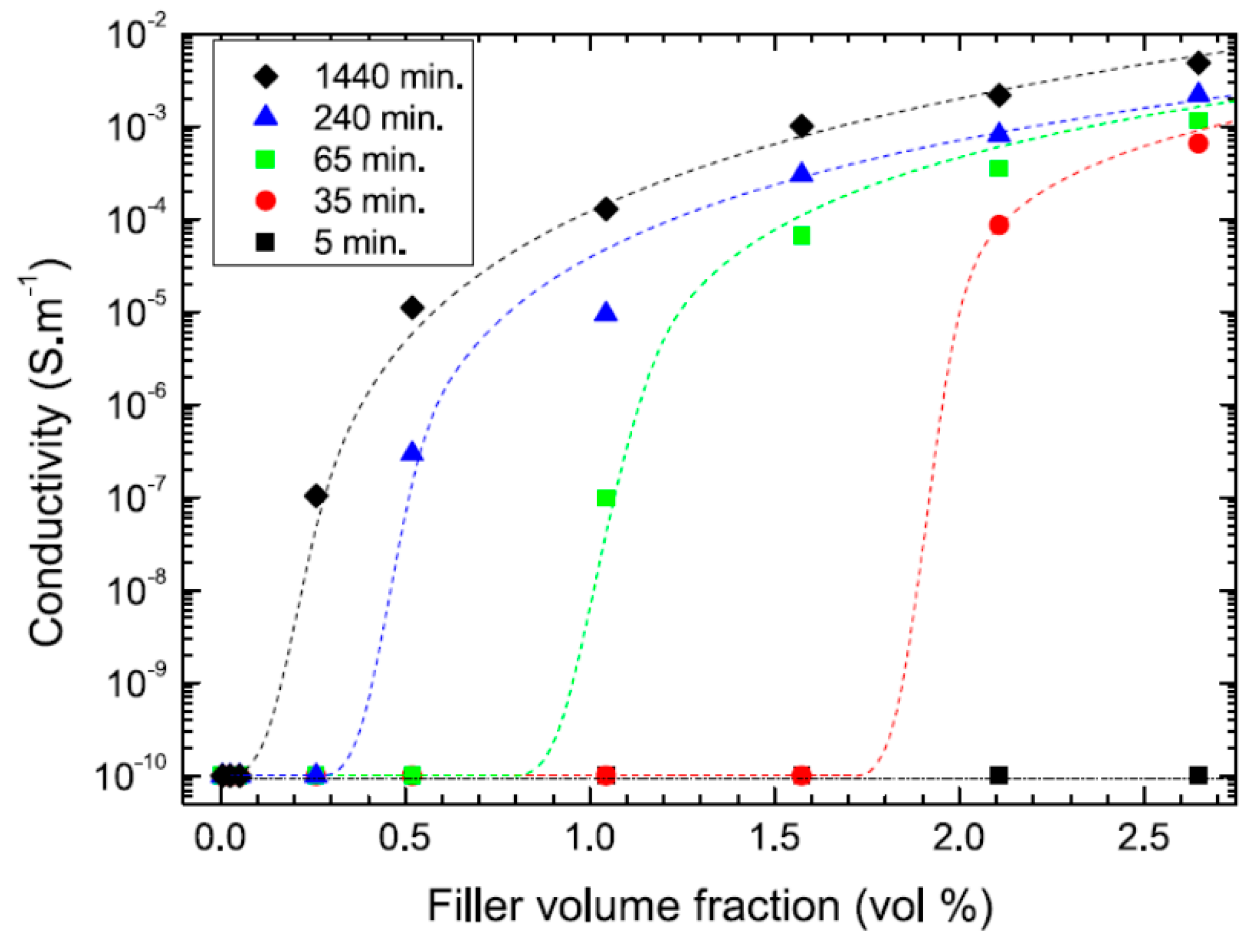
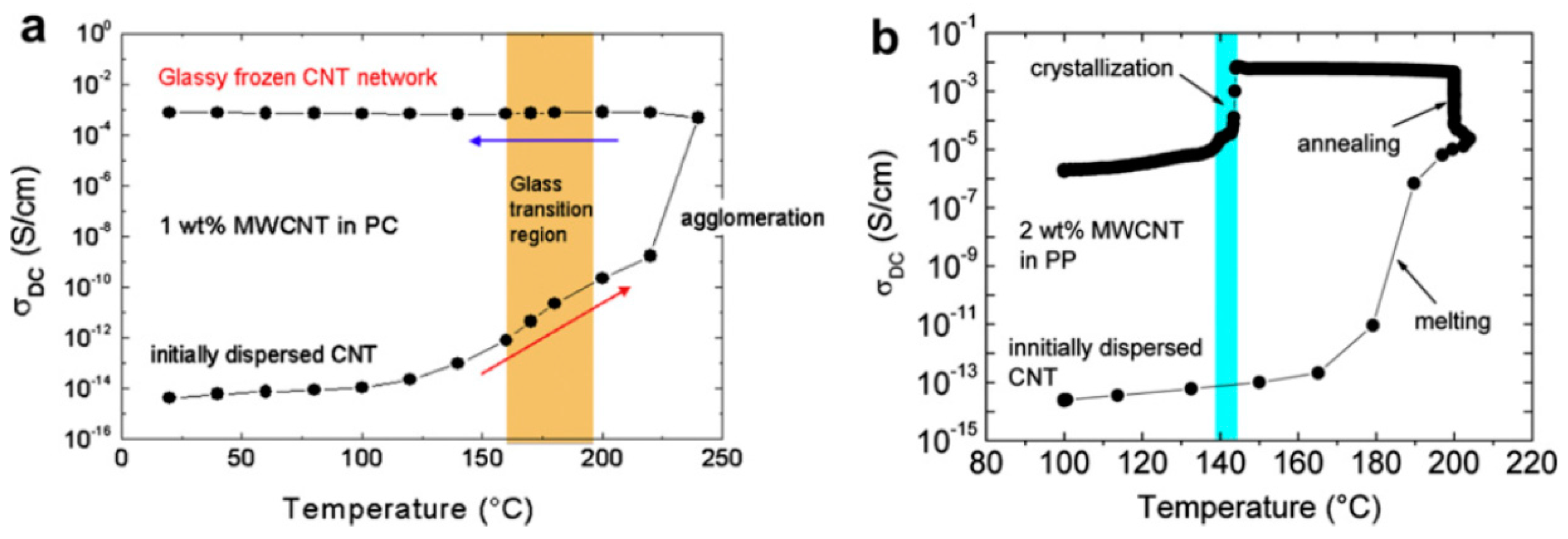
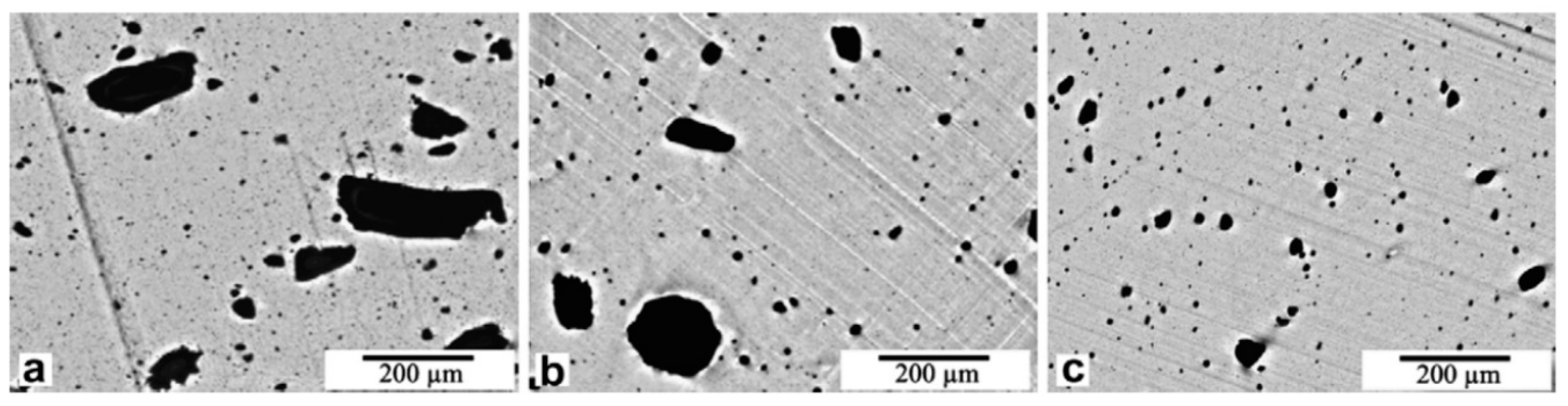
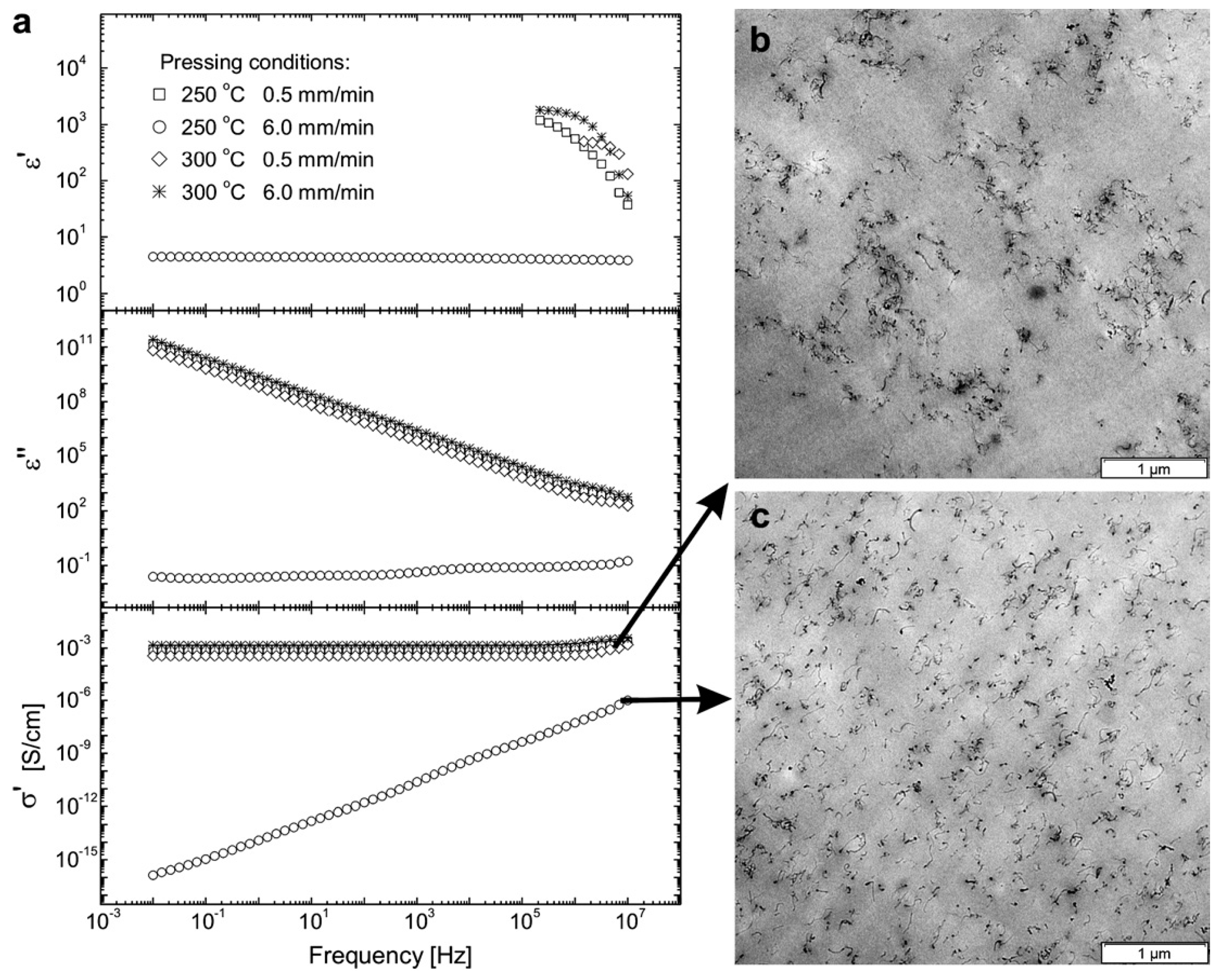
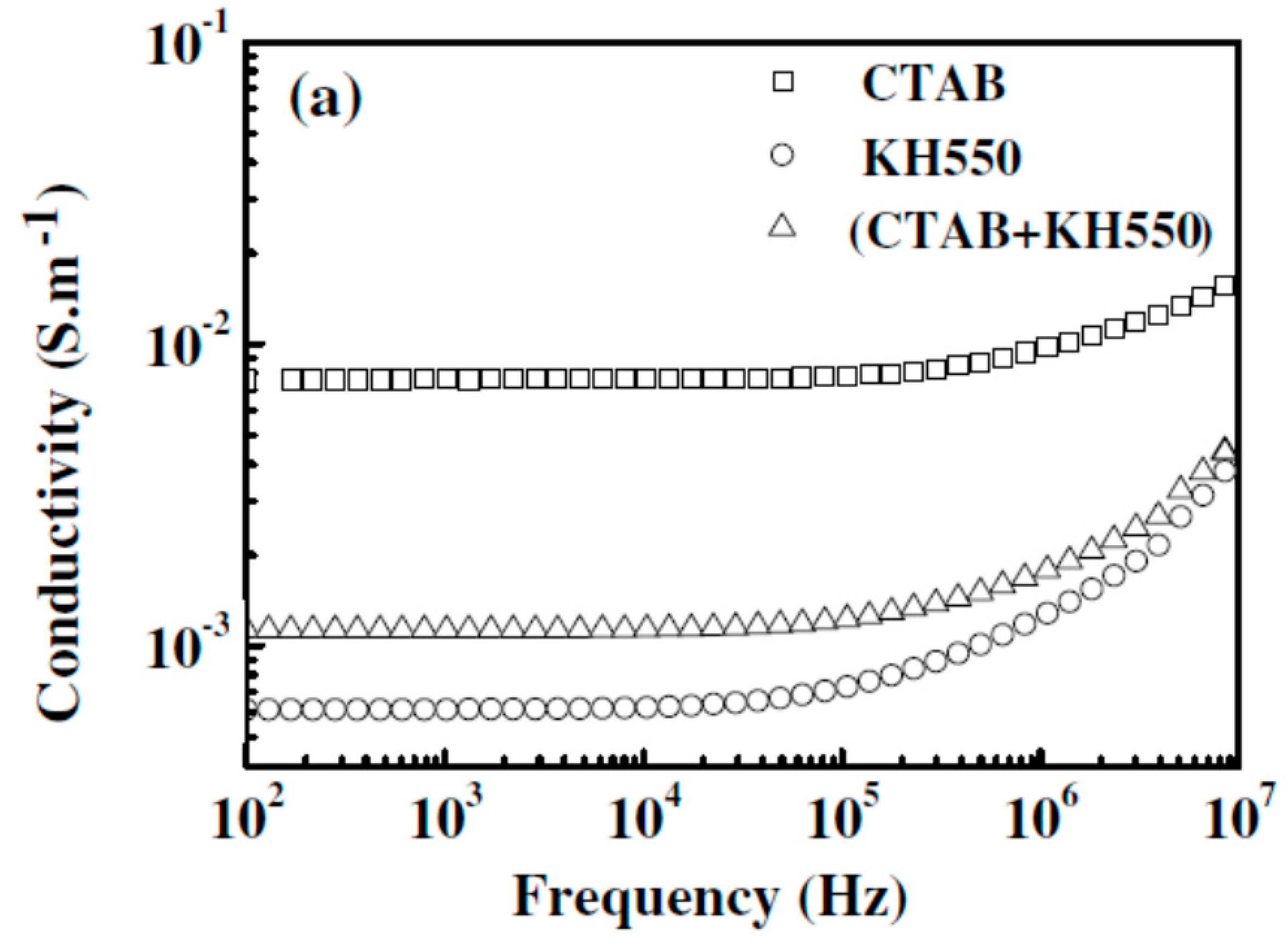
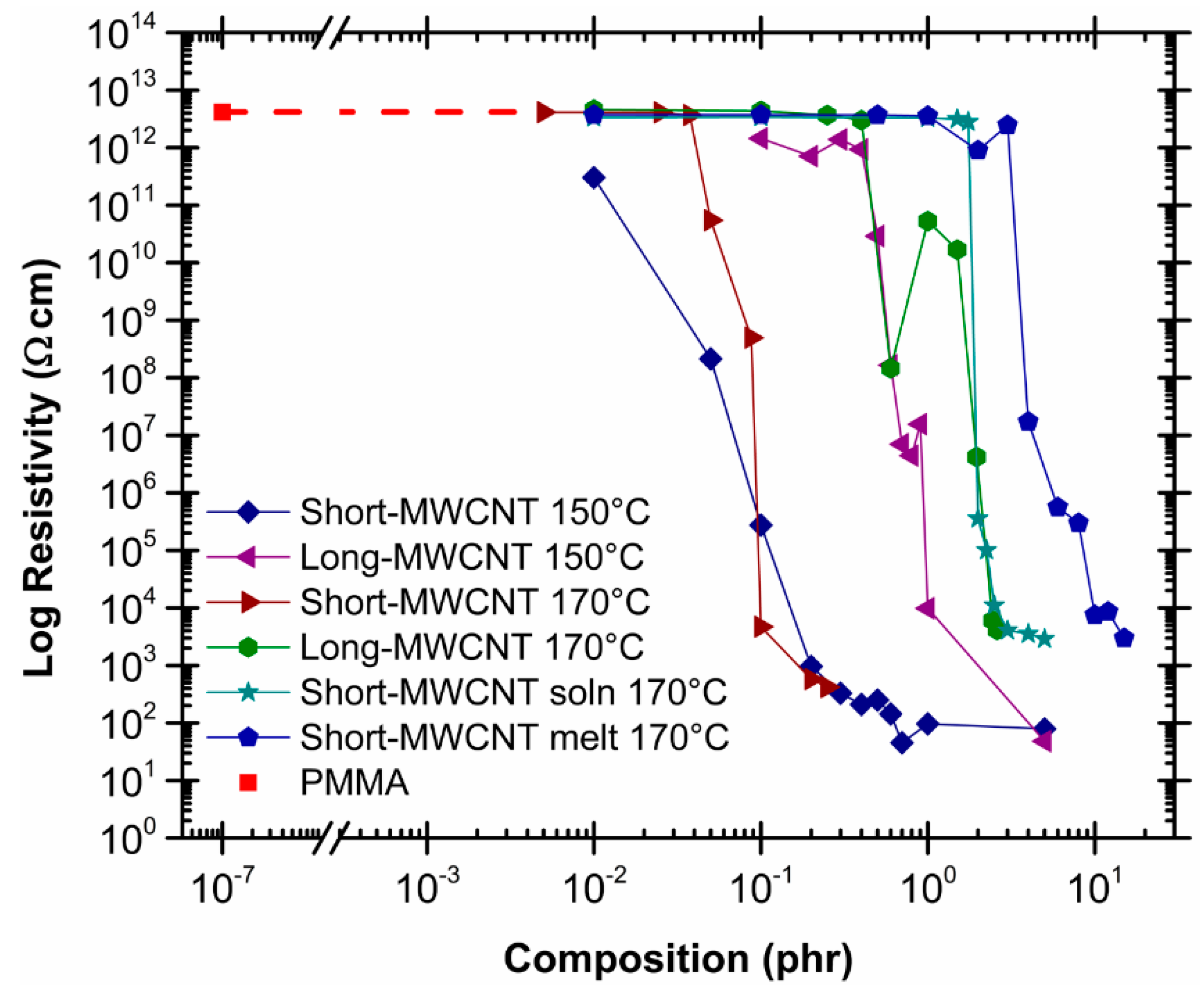
2.3. Effect of Processing Methods
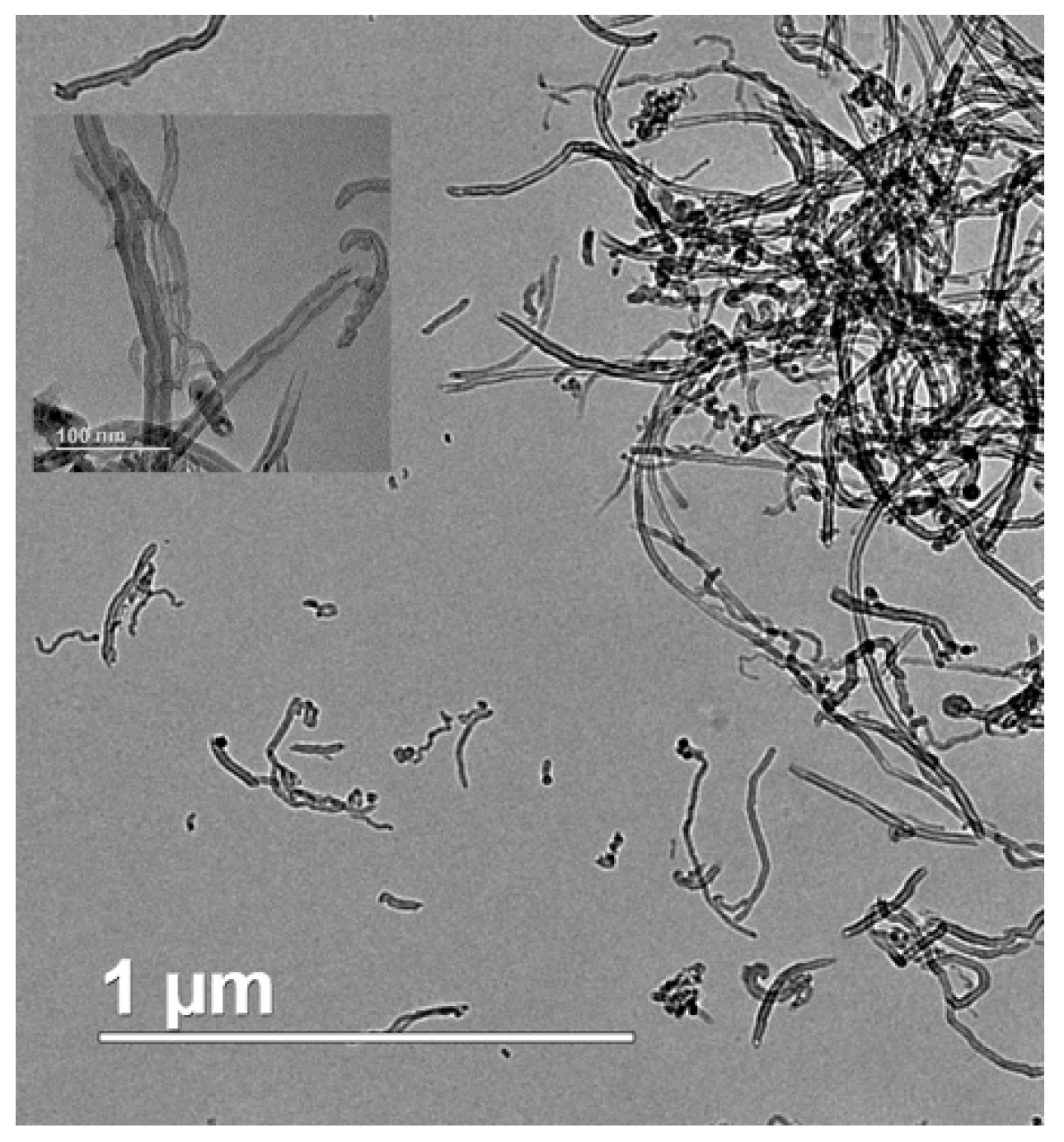
2.4. Modification of the MWCNT
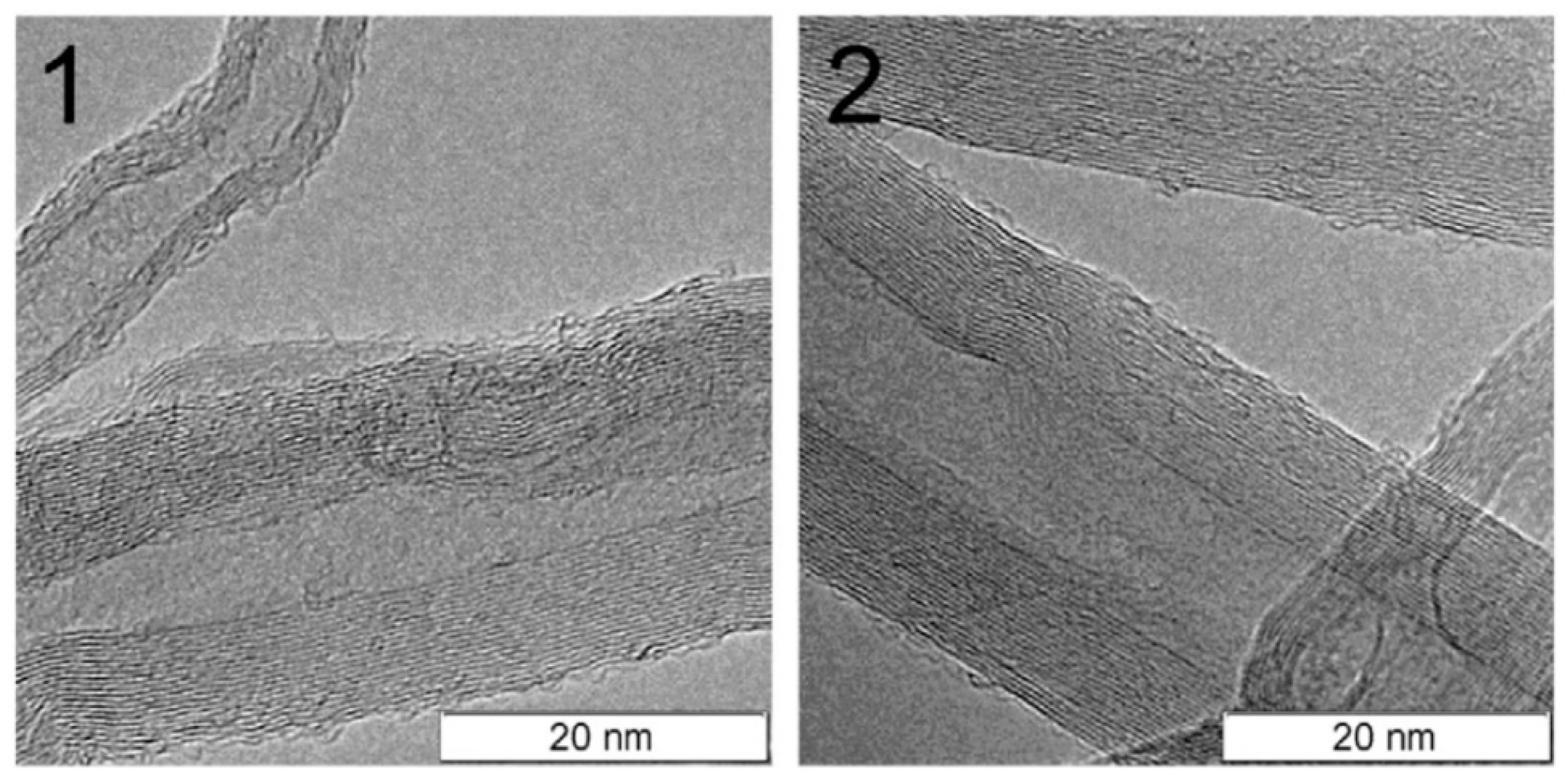
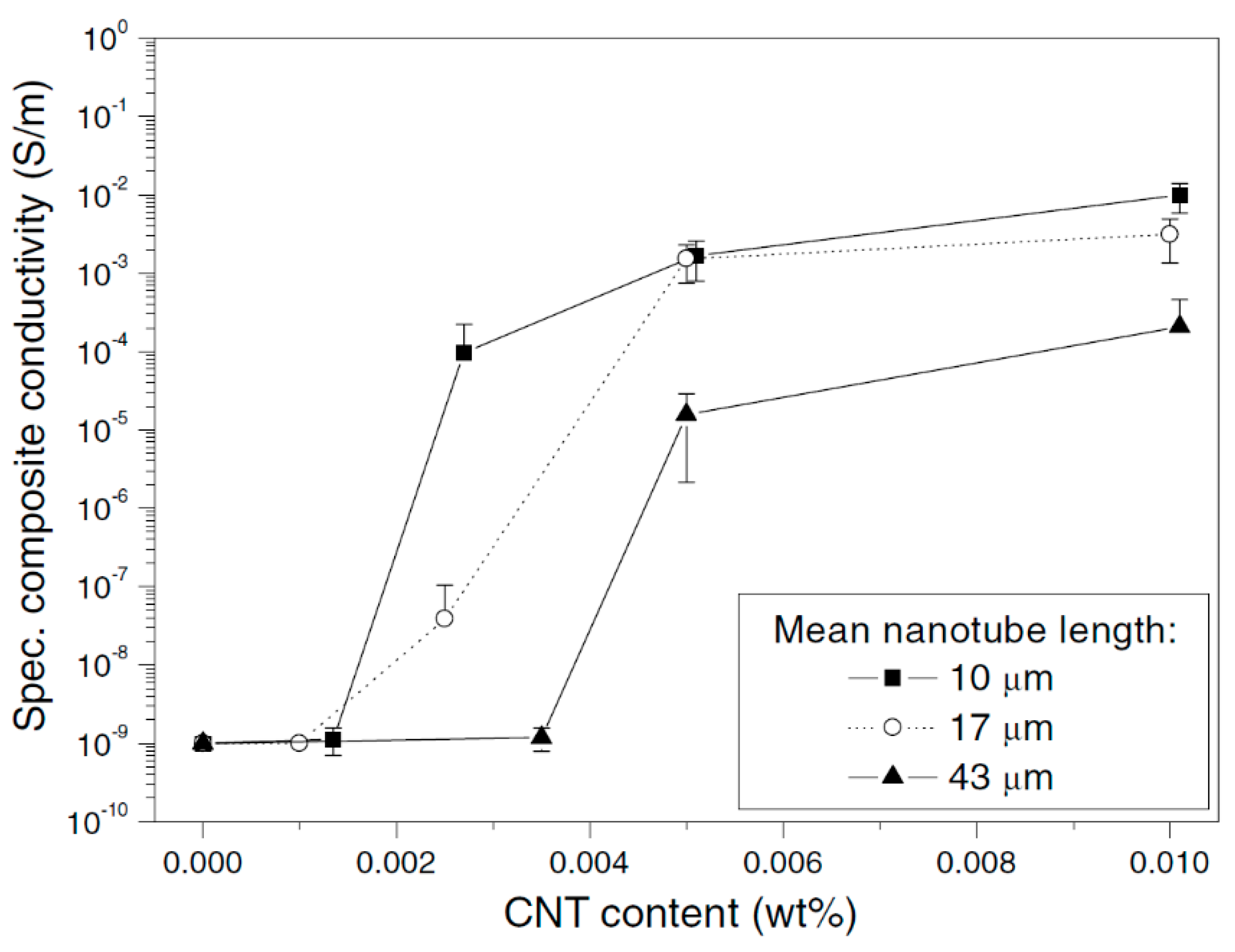
2.5. Effect of Aspect Ratio of MWCNT
2.6. Single-Walled Carbon Nanotube (SWCNT)
3. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Khattari, Z.; Maghrabi, M.; McNally, T.; Abdul Jawad, S. Impedance study of polymethyl methacrylate composites/multi-walled carbon nanotubes (PMMA/MWCNTs). Phys. B Condens. Matter 2012, 407, 759–764. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, N.; Leonhardt, B.; Jolowsky, C.; Hao, A.; Park, J.G.; Liang, R. Carbon-Nanotube-Based Electrical Conductors: Fabrication, Optimization, and Applications. Adv. Electron. Mater. 2019, 5, 1800811. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.; Pissis, P.; Pionteck, J.; Pötschke, P. Highly conducting poly(methyl methacrylate)/carbon nanotubes composites: Investigation on their thermal, dynamic-mechanical, electrical and dielectric properties. Compos. Sci. Technol. 2011, 71, 854–862. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.; Peoglos, V.; Pissis, P.; Pionteck, J.; Pötschke, P.; Mičušík, M.; Omastová, M. Electrical/dielectric properties and conduction mechanism in melt processed polyamide/multi-walled carbon nanotubes composites. Polymer 2009, 50, 5103–5111. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, N.; Park, J.G.; Hao, A.; Liang, R. Chapter 14—Carbon Nanotubes and Their Assemblies: Applications in Electromagnetic Interference Shielding. In Nanotube Superfiber Materials, 2nd ed.; Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 335–357. [Google Scholar]
- Aalaie, J.; Rahmatpour, A.; Maghami, S. Preparation and Characterization of Linear Low Density Polyethylene/Carbon Nanotube Nanocomposites. J. Macromol. Sci. Part B 2007, 46, 877–889. [Google Scholar] [CrossRef]
- Macutkevic, J.; Seliuta, D.; Valusis, G.; Adomavicius, R.; Krotkus, A.; Kuzhir, P.; Paddubskaya, A.; Maksimenko, S.; Kuznetsov, V.; Mazov, I.; et al. Multi-walled carbon nanotubes/PMMA composites for THz applications. Diam. Relat. Mater. 2012, 25, 13–18. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Watt, M.R.; Gerhardt, R.A. Effect of processing on the properties and morphology of MWCNT-polymer networks. Mater. Res. Express 2020, 7, 015075. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Dang, Z.-M.; Yao, S.-H.; Bai, J. Effects of surface modification of carbon nanotubes on the microstructure and electrical properties of carbon nanotubes/rubber nanocomposites. Chem. Phys. Lett. 2008, 457, 352–356. [Google Scholar] [CrossRef]
- Deng, H.; Skipa, T.; Zhang, R.; Lellinger, D.; Bilotti, E.; Alig, I.; Peijs, T. Effect of melting and crystallization on the conductive network in conductive polymer composites. Polymer 2009, 50, 3747–3754. [Google Scholar] [CrossRef]
- Bertram, B.D.; Gerhardt, R.A.; Schultz, J.W. Impedance response and modeling of composites containing aligned semiconductor whiskers: Effects of dc-bias partitioning and percolated-cluster length, topology, and filler interfaces. J. Appl. Phys. 2012, 111, 124913. [Google Scholar] [CrossRef]
- Muhlbauer, R.L.; Pruyn, T.L.; Puckett, W.T.; Gerhardt, R.A. Effect of graphitic filler size and shape on the microstructure, electrical percolation behavior and thermal properties of nanostructured multilayered carbon films deposited onto paper substrates. J. Mater. Res. 2014, 29, 472–484. [Google Scholar] [CrossRef]
- Pruyn, T.L.; Gerhardt, R.A. Detection of Different Interfaces in Percolated Networks of Antimony Tin Oxide: Borosilicate Glass Composites by Impedance Spectroscopy. J. Am. Ceram. Soc. 2015, 98, 154–162. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Kinloch, I.A.; Burgess, A.N.; Windle, A.H. The Effect of Aggregation on the Electrical Conductivity of Spin-Coated Polymer/Carbon Nanotube Composite Films. Langmuir 2007, 23, 5707–5712. [Google Scholar] [CrossRef]
- Han, M.S.; Lee, Y.K.; Lee, H.S.; Yun, C.H.; Kim, W.N. Electrical, morphological and rheological properties of carbon nanotube composites with polyethylene and poly(phenylene sulfide) by melt mixing. Chem. Eng. Sci. 2009, 64, 4649–4656. [Google Scholar] [CrossRef]
- McClory, C.; McNally, T.; Baxendale, M.; Pötschke, P.; Blau, W.; Ruether, M. Electrical and rheological percolation of PMMA/MWCNT nanocomposites as a function of CNT geometry and functionality. Eur. Polym. J. 2010, 46, 854–868. [Google Scholar] [CrossRef]
- Chen, H.; Muthuraman, H.; Stokes, P.; Zou, J.; Liu, X.; Wang, J.; Huo, Q.; Khondaker, S.I.; Zhai, L. Dispersion of carbon nanotubes and polymer nanocomposite fabrication using trifluoroacetic acid as a co-solvent. Nanotechnology 2007, 18, 415606. [Google Scholar] [CrossRef]
- Kim, H.M.; Choi, M.-S.; Joo, J.; Cho, S.J.; Yoon, H.S. Complexity in charge transport for multiwalled carbon nanotube and poly(methyl methacrylate) composites. Phys. Rev. B 2006, 74, 054202. [Google Scholar] [CrossRef]
- Ryu, S.H.; Cho, H.-B.; Moon, J.W.; Kwon, Y.-T.; Eom, N.S.A.; Lee, S.; Hussain, M.; Choa, Y.-H. Highly conductive polymethly(methacrylate)/multi-wall carbon nanotube composites by modeling a three-dimensional percolated microstructure. Compos. Part A Appl. Sci. Manuf. 2016, 91, 133–139. [Google Scholar] [CrossRef]
- Hermant, M.C.; Smeets, N.M.B.; van Hal, R.C.F.; Meuldijk, J.; Heuts, H.P.A.; Klumperman, B.; van Herk, A.M.; Koning, C.E. Influence of the molecular weight distribution on the percolation threshold of carbon nanotube—Polystyrene composites. E Polym. 2009, 9, 1–13. [Google Scholar] [CrossRef]
- Ryu, S.H.; Cho, H.-B.; Kim, S.; Kwon, Y.-T.; Lee, J.; Park, K.-R.; Choa, Y.-H. The effect of polymer particle size on three-dimensional percolation in core-shell networks of PMMA/MWCNTs nanocomposites: Properties and mathematical percolation model. Compos. Sci. Technol. 2018, 165, 1–8. [Google Scholar] [CrossRef]
- Lisunova, M.O.; Mamunya, Y.P.; Lebovka, N.I.; Melezhyk, A.V. Percolation behaviour of ultrahigh molecular weight polyethylene/multi-walled carbon nanotubes composites. Eur. Polym. J. 2007, 43, 949–958. [Google Scholar] [CrossRef]
- McNally, T.; Pötschke, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene multiwalled carbon nanotube composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Mierczynska, A.; Mayne-L’Hermite, M.; Boiteux, G.; Jeszka, J.K. Electrical and mechanical properties of carbon nanotube/ultrahigh-molecular-weight polyethylene composites prepared by a filler prelocalization method. J. Appl. Polym. Sci. 2007, 105, 158–168. [Google Scholar] [CrossRef]
- Jouni, M.; Boiteux, G.; Massardier, V. New melt mixing polyethylene multiwalled carbon nanotube nanocomposites with very low electrical percolation threshold. Polym. Adv. Technol. 2013, 24, 909–915. [Google Scholar] [CrossRef]
- Jouni, M.; Faure-Vincent, J.; Fedorko, P.; Djurado, D.; Boiteux, G.; Massardier, V. Charge carrier transport and low electrical percolation threshold in multiwalled carbon nanotube polymer nanocomposites. Carbon 2014, 76, 10–18. [Google Scholar] [CrossRef]
- Al-Saleh, M.H. Carbon nanotube-filled polypropylene/polyethylene blends: Compatibilization and electrical properties. Polym. Bull. 2016, 73, 975–987. [Google Scholar] [CrossRef]
- Müller, M.T.; Krause, B.; Pötschke, P. A successful approach to disperse MWCNTs in polyethylene by melt mixing using polyethylene glycol as additive. Polymer 2012, 53, 3079–3083. [Google Scholar] [CrossRef]
- Shi, Y.-D.; Li, J.; Tan, Y.-J.; Chen, Y.-F.; Wang, M. Percolation behavior of electromagnetic interference shielding in polymer/multi-walled carbon nanotube nanocomposites. Compos. Sci. Technol. 2019, 170, 70–76. [Google Scholar] [CrossRef]
- Mičušík, M.; Omastová, M.; Pionteck, J.; Pandis, C.; Logakis, E.; Pissis, P. Influence of surface treatment of multiwall carbon nanotubes on the properties of polypropylene/carbon nanotubes nanocomposites. Polym. Adv. Technol. 2011, 22, 38–47. [Google Scholar] [CrossRef]
- Tjong, S.C.; Liang, G.D.; Bao, S.P. Electrical behavior of polypropylene/multiwalled carbon nanotube nanocomposites with low percolation threshold. Scr. Mater. 2007, 57, 461–464. [Google Scholar] [CrossRef]
- Seo, M.-K.; Park, S.-J. Electrical resistivity and rheological behaviors of carbon nanotubes-filled polypropylene composites. Chem. Phys. Lett. 2004, 395, 44–48. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.W.; Kim, S.H.; Youn, J.R. Rheological and electrical properties of polypropylene/MWCNT composites prepared with MWCNT masterbatch chips. Eur. Polym. J. 2008, 44, 1620–1630. [Google Scholar] [CrossRef]
- Müller, M.T.; Krause, B.; Kretzschmar, B.; Pötschke, P. Influence of feeding conditions in twin-screw extrusion of PP/MWCNT composites on electrical and mechanical properties. Compos. Sci. Technol. 2011, 71, 1535–1542. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Chan, S.H.; Zhao, J. Correlation between dispersion state and electrical conductivity of MWCNTs/PP composites prepared by melt blending. Compos. Part A Appl. Sci. Manuf. 2010, 41, 419–426. [Google Scholar] [CrossRef]
- Pegel, S.; Pötschke, P.; Petzold, G.; Alig, I.; Dudkin, S.M.; Lellinger, D. Dispersion, agglomeration, and network formation of multiwalled carbon nanotubes in polycarbonate melts. Polymer 2008, 49, 974–984. [Google Scholar] [CrossRef]
- Pötschke, P.; Abdel-Goad, M.; Alig, I.; Dudkin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Chen, L.; Pang, X.-J.; Yu, Z.-L. Study on polycarbonate/multi-walled carbon nanotubes composite produced by melt processing. Mater. Sci. Eng. A 2007, 457, 287–291. [Google Scholar] [CrossRef]
- Pötschke, P.; Dudkin, S.M.; Alig, I. Dielectric spectroscopy on melt processed polycarbonate—Multiwalled carbon nanotube composites. Polymer 2003, 44, 5023–5030. [Google Scholar] [CrossRef]
- Pötschke, P.; Fornes, T.D.; Paul, D.R. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- Maiti, S.; Shrivastava, N.; Suin, S.; Khatua, B. A strategy for achieving low percolation and high electrical conductivity in melt-blended polycarbonate (PC)/multiwall carbon nanotube (MWCNT) nanocomposites: Electrical and thermo-mechanical properties. Express Polym. Lett. 2013, 7, 505–518. [Google Scholar] [CrossRef]
- Jin, S.H.; Choi, D.K.; Lee, D.S. Electrical and rheological properties of polycarbonate/multiwalled carbon nanotube nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 242–245. [Google Scholar] [CrossRef]
- Han, M.S.; Lee, Y.K.; Kim, W.N.; Lee, H.S.; Joo, J.S.; Park, M.; Lee, H.J.; Park, C.R. Effect ofF multi-walled carbon nanotube dispersion on the electrical, morphological and rheological properties of polycarbonate/multi-walled carbon nanotube composites. Macromol. Res. 2009, 17, 863–869. [Google Scholar] [CrossRef]
- Kodgire, P.V.; Bhattacharyya, A.R.; Bose, S.; Gupta, N.; Kulkarni, A.R.; Misra, A. Control of multiwall carbon nanotubes dispersion in polyamide6 matrix: An assessment through electrical conductivity. Chem. Phys. Lett. 2006, 432, 480–485. [Google Scholar] [CrossRef]
- Krause, B.; Pötschke, P.; Häußler, L. Influence of small scale melt mixing conditions on electrical resistivity of carbon nanotube-polyamide composites. Compos. Sci. Technol. 2009, 69, 1505–1515. [Google Scholar] [CrossRef]
- Krause, B.; Petzold, G.; Pegel, S.; Pötschke, P. Correlation of carbon nanotube dispersability in aqueous surfactant solutions and polymers. Carbon 2009, 47, 602–612. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Boldt, R.; Hermasch, S.; Wursche, R.; Pötschke, P. Melt mixed nano composites of PA12 with MWNTs: Influence of MWNT and matrix properties on macrodispersion and electrical properties. Compos. Sci. Technol. 2011, 71, 306–314. [Google Scholar] [CrossRef]
- Kovacs, J.Z.; Velagala, B.S.; Schulte, K.; Bauhofer, W. Two percolation thresholds in carbon nanotube epoxy composites. Compos. Sci. Technol. 2007, 67, 922–928. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.-K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Moisala, A.; Li, Q.; Kinloch, I.A.; Windle, A.H. Thermal and electrical conductivity of single- and multi-walled carbon nanotube-epoxy composites. Compos. Sci. Technol. 2006, 66, 1285–1288. [Google Scholar] [CrossRef]
- Martin, C.A.; Sandler, J.K.W.; Shaffer, M.S.P.; Schwarz, M.K.; Bauhofer, W.; Schulte, K.; Windle, A.H. Formation of percolating networks in multi-wall carbon-nanotube—Epoxy composites. Compos. Sci. Technol. 2004, 64, 2309–2316. [Google Scholar] [CrossRef]
- Sandler, J.; Shaffer, M.S.P.; Prasse, T.; Bauhofer, W.; Schulte, K.; Windle, A.H. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer 1999, 40, 5967–5971. [Google Scholar] [CrossRef]
- Yuen, S.-M.; Ma, C.-C.M.; Wu, H.-H.; Kuan, H.-C.; Chen, W.-J.; Liao, S.-H.; Hsu, C.-W.; Wu, H.-L. Preparation and thermal, electrical, and morphological properties of multiwalled carbon nanotubeand epoxy composites. J. Appl. Polym. Sci. 2007, 103, 1272–1278. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Prystaj, L.A. Effect of Carbon Filler Characteristics on the Electrical Properties of Conductive Polymer Composites Possessing Segregated Network Microstructures. Masters Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2008. [Google Scholar]
- He, X.J.; Du, J.H.; Ying, Z.; Cheng, H.M.; He, X.J. Positive temperature coefficient effect in multiwalled carbon nanotube/high-density polyethylene composites. Appl. Phys. Lett. 2005, 86, 062112. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; e-book; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Shrivastava, N.K.; Suin, S.; Maiti, S.; Khatua, B.B. Ultralow Electrical Percolation Threshold in Poly(styrene-co-acrylonitrile)/Carbon Nanotube Nanocomposites. Ind. Eng. Chem. Res. 2013, 52, 2858–2868. [Google Scholar] [CrossRef]
- Du, J.; Zhao, L.; Zeng, Y.; Zhang, L.; Li, F.; Liu, P.; Liu, C. Comparison of electrical properties between multi-walled carbon nanotube and graphene nanosheet/high density polyethylene composites with a segregated network structure. Carbon 2011, 49, 1094–1100. [Google Scholar] [CrossRef]
- Gao, J.-F.; Li, Z.-M.; Meng, Q.-j.; Yang, Q. CNTs/ UHMWPE composites with a two-dimensional conductive network. Mater. Lett. 2008, 62, 3530–3532. [Google Scholar] [CrossRef]
- Pang, H.; Yan, D.-X.; Bao, Y.; Chen, J.-B.; Chen, C.; Li, Z.-M. Super-tough conducting carbon nanotube/ultrahigh-molecular-weight polyethylene composites with segregated and double-percolated structure. J. Mater. Chem. 2012, 22, 23568–23575. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Dang, Z.-M.; Bozlar, M.; Miomandre, F.; Bai, J. Broad-frequency dielectric behaviors in multiwalled carbon nanotube/rubber nanocomposites. J. Appl. Phys. 2009, 106, 084902. [Google Scholar] [CrossRef]
- Alig, I.; Skipa, T.; Lellinger, D.; Bierdel, M.; Meyer, H. Dynamic percolation of carbon nanotube agglomerates in a polymer matrix: Comparison of different model approaches. Phys. Status Solidi (b) 2008, 245, 2264–2267. [Google Scholar] [CrossRef]
- Combessis, A.; Bayon, L.; Flandin, L. Effect of filler auto-assembly on percolation transition in carbon nanotube/polymer composites. Appl. Phys. Lett. 2013, 102, 011907. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Pegel, S.; Dudkin, S.; Lellinger, D. Plastic composites containing carbon nanotubes: Optimisation of processing conditions and properties. Rubber Fibre Plast. 2008, 3, 92–95. [Google Scholar]
- Klonos, P.A.; Tegopoulos, S.N.; Koutsiara, C.S.; Kontou, E.; Pissis, P.; Kyritsis, A. Effects of CNTs on thermal transitions, thermal diffusivity and electrical conductivity in nanocomposites: Comparison between an amorphous and a semicrystalline polymer matrix. Soft Matter 2019, 15, 1813–1824. [Google Scholar] [CrossRef]
- Schlea, M.R.; Meree, C.E.; Gerhardt, R.A.; Mintz, E.A.; Shofner, M.L. Network behavior of thermosetting polyimide/multiwalled carbon nanotube composites. Polymer 2012, 53, 1020–1027. [Google Scholar] [CrossRef]
- Kasaliwal, G.R.; Villmow, T.; Pegel, S.; Pötschke, P. 4 - Influence of material and processing parameters on carbon nanotube dispersion in polymer melts. In Polymer–Carbon Nanotube Composites; McNally, T., Pötschke, P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 92–132. [Google Scholar]
- Zhu, Y.-F.; Ma, C.; Zhang, W.; Zhang, R.-P.; Koratkar, N.; Liang, J. Alignment of multiwalled carbon nanotubes in bulk epoxy composites via electric field. J. Appl. Phys. 2009, 105, 054319. [Google Scholar] [CrossRef]
- Janoff, A.; Gerhardt, R.A. Comparison of Short and Long Multi Wall Carbon Nanotube and Polymethyl Methacrylate Composites. In Proceedings of the MRS, San Francisco, CA, USA, 6–10 April 2015; Available online: http://hdl.handle.net/1853/62965 (accessed on 9 July 2020).
- Pötschke, P.; Hornbostel, B.; Roth, S.; Vohrer, U.; Dudkin, S.M.; Alig, I. Purification and Percolation—Unexpected Phenomena in Nanotube Polymer Composites. AIP Conf. Proc. 2005, 786, 596–601. [Google Scholar]
- Kymakis, E.; Amaratunga, G.A.J. Electrical properties of single-wall carbon nanotube-polymer composite films. J. Appl. Phys. 2006, 99, 084302. [Google Scholar] [CrossRef]
- Liu, L.; Matitsine, S.; Gan, Y.B.; Chen, L.F.; Kong, L.B.; Rozanov, K.N. Frequency dependence of effective permittivity of carbon nanotube composites. J. Appl. Phys. 2007, 101, 094106. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Q.; Li, W.; Wei, Z.; Xu, G. Properties of well aligned SWNT modified poly (methyl methacrylate) nanocomposites. Mater. Lett. 2007, 61, 27–29. [Google Scholar] [CrossRef]
- Chauvet, O.; Benoit, J.M.; Corraze, B. Electrical, magneto-transport and localization of charge carriers in nanocomposites based on carbon nanotubes. Carbon 2004, 42, 949–952. [Google Scholar] [CrossRef]
- Nogales, A.; Broza, G.; Roslaniec, Z.; Schulte, K.; Šics, I.; Hsiao, B.S.; Sanz, A.; García-Gutiérrez, M.C.; Rueda, D.R.; Domingo, C.; et al. Low Percolation Threshold in Nanocomposites Based on Oxidized Single Wall Carbon Nanotubes and Poly(butylene terephthalate). Macromolecules 2004, 37, 7669–7672. [Google Scholar] [CrossRef]
- Zhang, Q.; Rastogi, S.; Chen, D.; Lippits, D.; Lemstra, P.J. Low percolation threshold in single-walled carbon nanotube/high density polyethylene composites prepared by melt processing technique. Carbon 2006, 44, 778–785. [Google Scholar] [CrossRef]
- Bryning, M.B.; Islam, M.F.; Kikkawa, J.M.; Yodh, A.G. Very Low Conductivity Threshold in Bulk Isotropic Single-Walled Carbon Nanotube–Epoxy Composites. Adv. Mater. 2005, 17, 1186–1191. [Google Scholar] [CrossRef]
- Barrau, S.; Demont, P.; Peigney, A.; Laurent, C.; Lacabanne, C. DC and AC Conductivity of Carbon Nanotubes−Polyepoxy Composites. Macromolecules 2003, 36, 5187–5194. [Google Scholar] [CrossRef]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube Networks in Polymer Nanocomposites: Rheology and Electrical Conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]
- Li, N.; Huang, Y.; Du, F.; He, X.; Lin, X.; Gao, H.; Ma, Y.; Li, F.; Chen, Y.; Eklund, P.C. Electromagnetic Interference (EMI) Shielding of Single-Walled Carbon Nanotube Epoxy Composites. Nano Lett. 2006, 6, 1141–1145. [Google Scholar] [CrossRef]
- Du, F.; Fischer, J.E.; Winey, K.I. Effect of nanotube alignment on percolation conductivity in carbon nanotube/polymer composites. Phys. Rev. B 2005, 72, 121404. [Google Scholar] [CrossRef]
- Du, F.; Fischer, J.E.; Winey, K.I. Coagulation method for preparing single-walled carbon nanotube/poly(methyl methacrylate) composites and their modulus, electrical conductivity, and thermal stability. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 3333–3338. [Google Scholar] [CrossRef]
- Du, F.; Guthy, C.; Kashiwagi, T.; Fischer, J.E.; Winey, K.I. An infiltration method for preparing single-wall nanotube/epoxy composites with improved thermal conductivity. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1513–1519. [Google Scholar] [CrossRef]
- Dettlaff-Weglikowska, U.; Kaempgen, M.; Hornbostel, B.; Skakalova, V.; Wang, J.; Liang, J.; Roth, S. Conducting and transparent SWNT/polymer composites. Phys. Status Solidi (b) 2006, 243, 3440–3444. [Google Scholar] [CrossRef]
- Hornbostel, B.; Pötschke, P.; Kotz, J.; Roth, S. Single-walled carbon nanotubes/polycarbonate composites: Basic electrical and mechanical properties. Phys. Status Solidi (b) 2006, 243, 3445–3451. [Google Scholar] [CrossRef]
- Grossiord, N.; Wouters, M.E.L.; Miltner, H.E.; Lu, K.; Loos, J.; Mele, B.V.; Koning, C.E. Isotactic polypropylene/carbon nanotube composites prepared by latex technology: Electrical conductivity study. Eur. Polym. J. 2010, 46, 1833–1843. [Google Scholar] [CrossRef]
- Jeon, K.; Lumata, L.; Tokumoto, T.; Steven, E.; Brooks, J.; Alamo, R. Low electrical conductivity threshold and crystalline morphology of single-walled carbon nanotubes—High density polyethylene nanocomposites characterized by SEM, Raman spectroscopy and AFM. Polymer 2007, 48, 4751–4764. [Google Scholar] [CrossRef]
- Krause, B.; Pötschke, P.; Ilin, E.; Predtechenskiy, M. Melt mixed SWCNT-polypropylene composites with very low electrical percolation. Polymer 2016, 98, 45–50. [Google Scholar] [CrossRef]
- Benoit, J.M.; Corraze, B.; Lefrant, S.; Blau, W.J.; Bernier, P.; Chauvet, O. Transport properties of PMMA-Carbon Nanotubes composites. Synth. Met. 2001, 121, 1215–1216. [Google Scholar] [CrossRef]
- Skákalová, V.; Dettlaff-Weglikowska, U.; Roth, S. Electrical and mechanical properties of nanocomposites of single wall carbon nanotubes with PMMA. Synth. Met. 2005, 152, 349–352. [Google Scholar] [CrossRef]


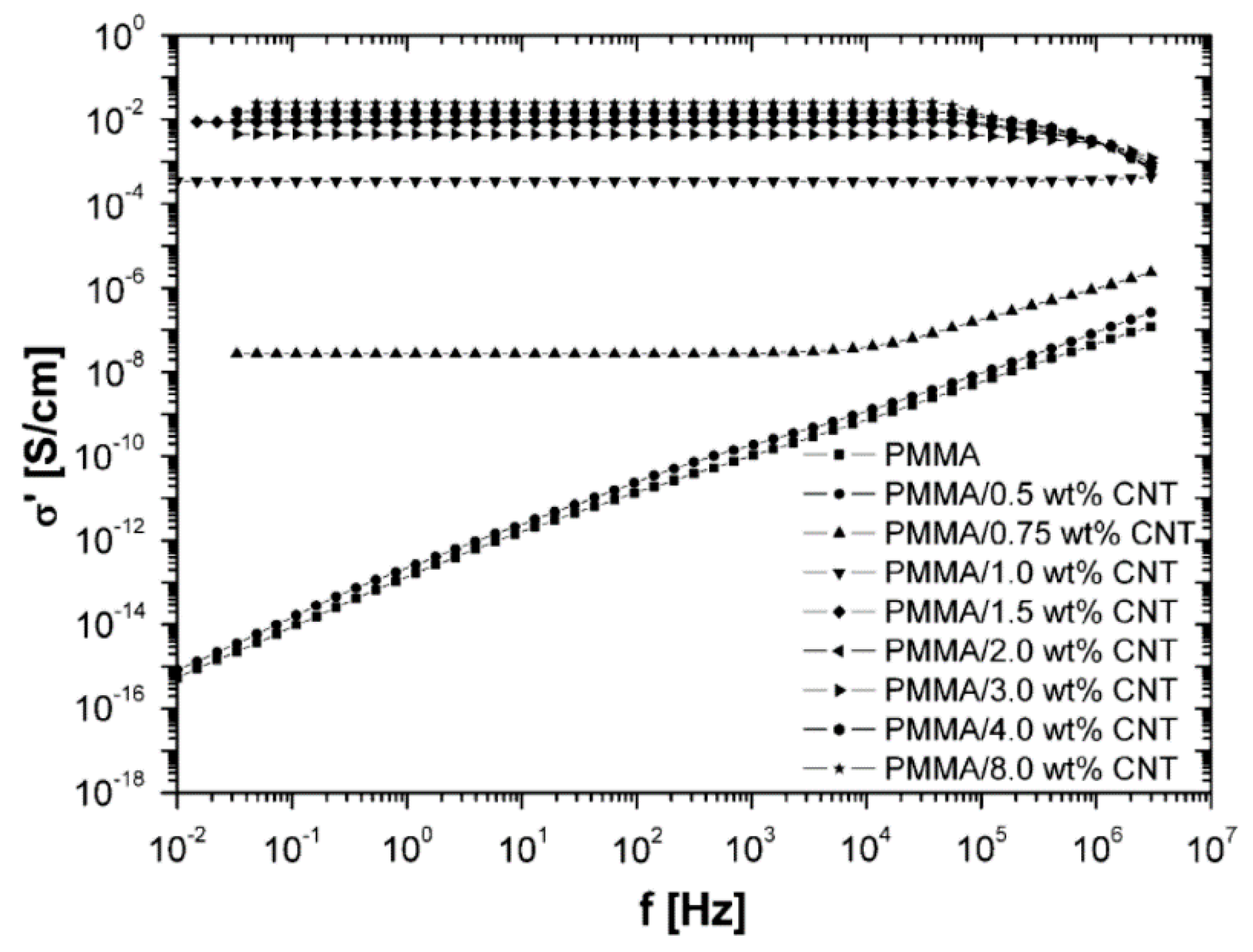
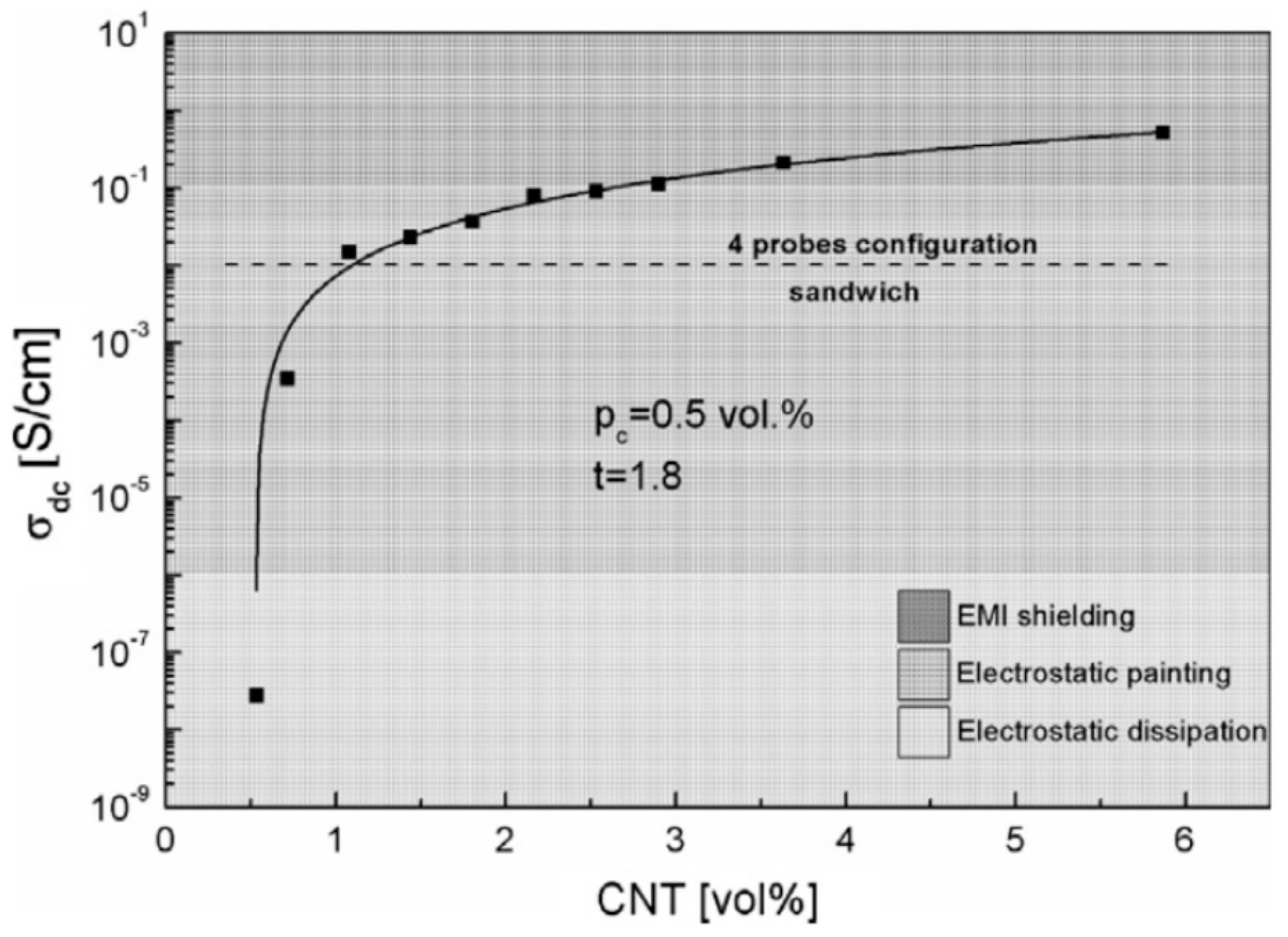
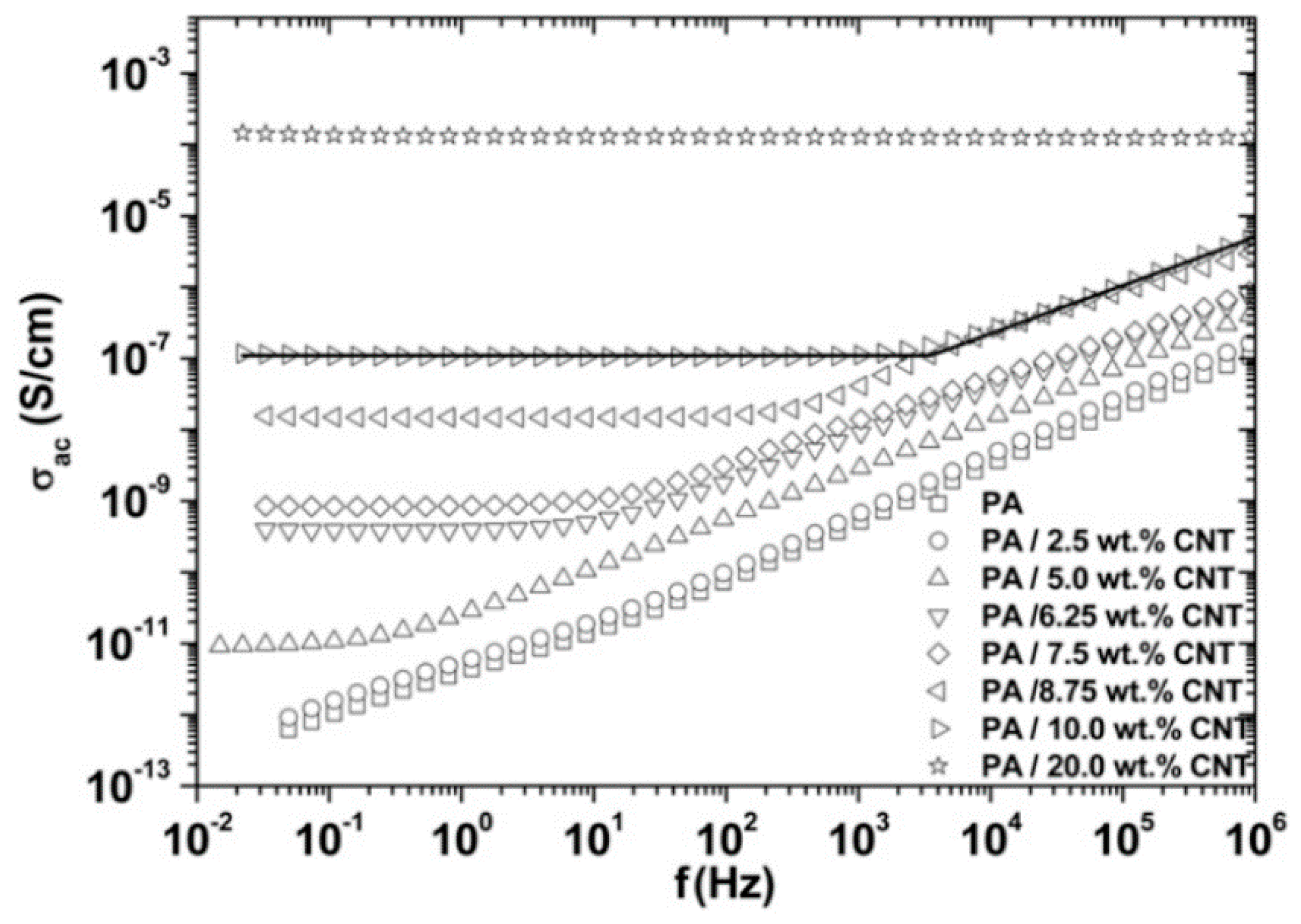
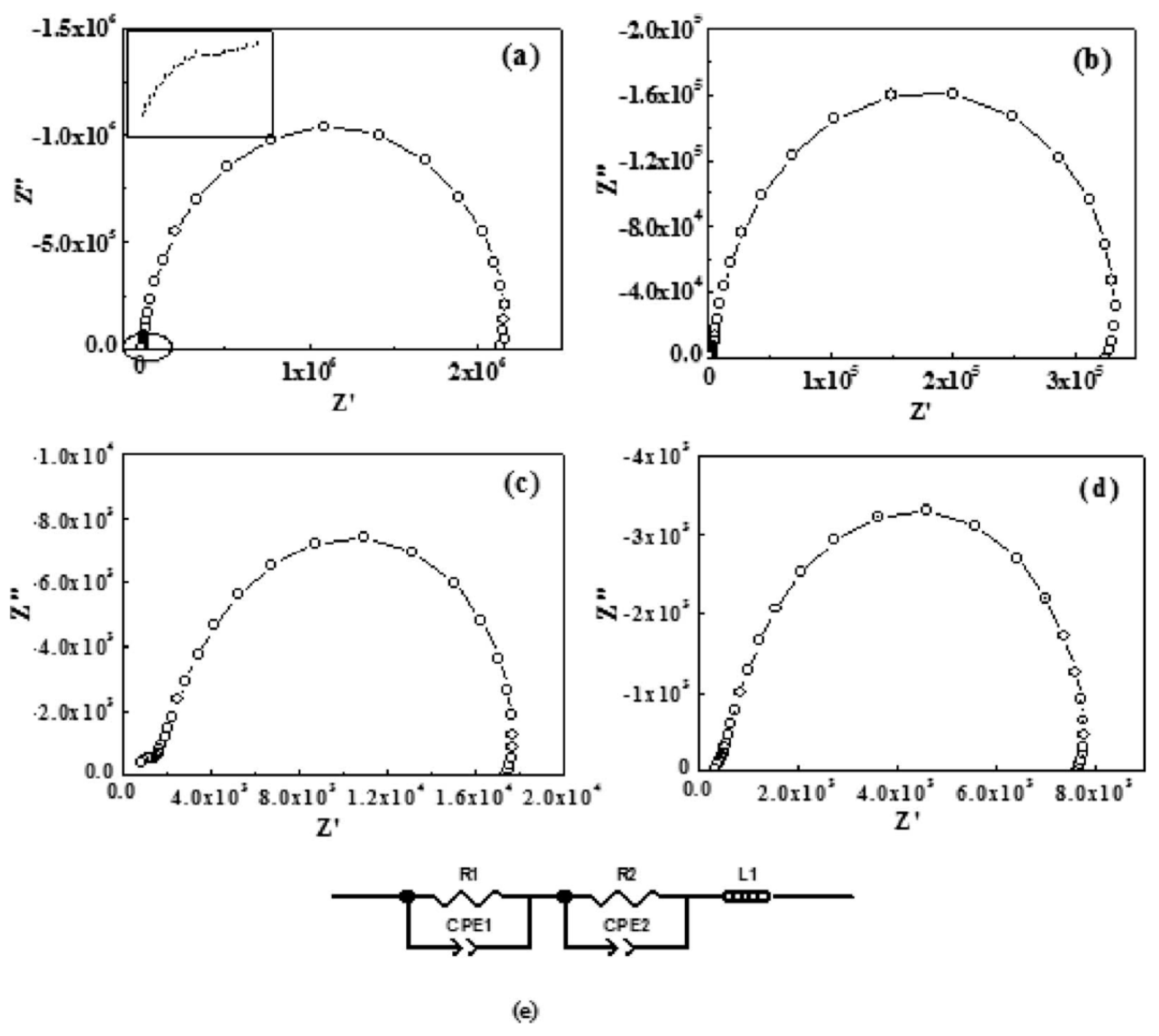





| Polymer Type | Filler Type | Filler Structure | Percolation Threshold (Wt%) | Ref. |
|---|---|---|---|---|
| HDPE a | MWCNT | Randomly distributed | 2 | [58] |
| PMMA | SWCNT | Randomly distributed | 0.3 | [59] |
| UHMWPE b | MWCNT | Randomly distributed | 3.1 | [60] |
| HDPE a | MWCNT | Segregated | 0.311 | [61] |
| SAN particles c | MWCNT | Segregated | 0.032 | [62] |
| UHMWPE b/PMMA | CNT | Segregated | 0.3 | [63] |
| PMMA | MWCNT_DMF | Segregated | 0.0095 | [20] |
| PMMA | MWCNT_MeOH | Segregated | 0.051 | [20] |
| PMMA | MWCNT (blended) | Randomly distributed | 0.45 | [20] |
| PMMA | MWCNT d | Segregated | 0.05 | [9] |
| PMMA | MWCNT e | Randomly distributed | 3.85 | [9] |
| PMMA | MWCNT f | Agglomerated | 1.96 | [9] |
| Differences in Polymer Systems | Percolation threshold is higher for randomly distributed structures and lower for segregated |
| Effect of increasing the amount of MWCNT | Even using the same polymer system, different percolation thresholds may be caused by differences in MWCNT characteristics and processing conditions |
| Effect of Processing Methods | Melt—evenly distributes Solution—may cause any microstructure Mechanical—segregated grain-like microstructure Annealing—increase conductivity in both PC, PP and PE composites, decreased percolation threshold in PE composites Curing—uncured PETI composite had higher conductivity than cured before percolation. Little difference above percolation Melt viscosity—higher melt viscosity results in smaller initial agglomerates Compression molding temperature: higher molding temperature resulted in more conductive composites |
| Modification of CNT | The effect of functionalizing CNT composites varies widely depending on the modifiers, additives, and solvents used. Functionalization may be a solution to obtain even distribution of CNT |
| Effect of Aspect Ratio of MWCNT | Shorter CNT results in lower percolation—due to increase in the difference in size between CNT and matrix |
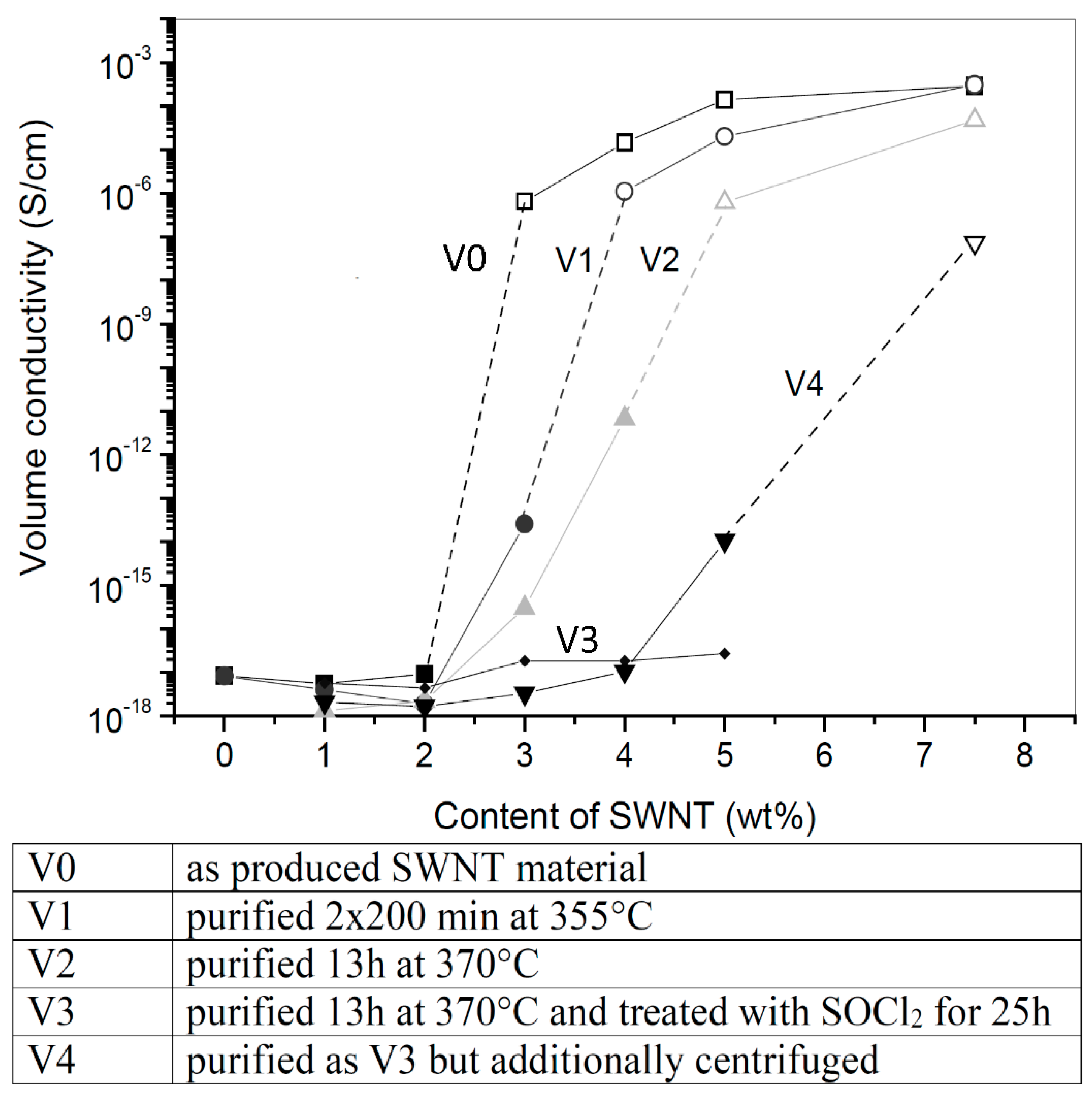
| SWCNT | Purity—increased purity decreased conductivity and increased the percolation threshold |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watt, M.R.; Gerhardt, R.A. Factors that Affect Network Formation in Carbon Nanotube Composites and their Resultant Electrical Properties. J. Compos. Sci. 2020, 4, 100. https://doi.org/10.3390/jcs4030100
Watt MR, Gerhardt RA. Factors that Affect Network Formation in Carbon Nanotube Composites and their Resultant Electrical Properties. Journal of Composites Science. 2020; 4(3):100. https://doi.org/10.3390/jcs4030100
Chicago/Turabian StyleWatt, Morgan R., and Rosario A. Gerhardt. 2020. "Factors that Affect Network Formation in Carbon Nanotube Composites and their Resultant Electrical Properties" Journal of Composites Science 4, no. 3: 100. https://doi.org/10.3390/jcs4030100
APA StyleWatt, M. R., & Gerhardt, R. A. (2020). Factors that Affect Network Formation in Carbon Nanotube Composites and their Resultant Electrical Properties. Journal of Composites Science, 4(3), 100. https://doi.org/10.3390/jcs4030100