Abstract
Disposal of massive amounts of eggshells and seashells from processing industries is a challenge. In recent years, there has been a focus to reuse these waste resources in the production of new thermoplastic and thermoset polymer materials. This paper reviews eggshell and seashell production by country and provides a perspective on the quantity of bio-calcium carbonate that could be produced annually from these wastes. The achievements obtained from the addition of recycled bio-calcium carbonate fillers (uncoated/unmodified) in polymer composites with a focus on tensile strength, flexural strength and impact toughness are discussed. To improve compatibility between calcium carbonate (mineral and bio-based) fillers and polymers, studies on surface modifiers are reviewed. Knowledge gaps and future research and development thoughts are outlined. Developing novel and innovative composites for this waste material could bring additional revenue to egg and seafood processors and at the same time reduce any environmental impact.
1. Introduction
Low-cost fillers are added to polymers to reduce cost and improve their properties. One widely used inorganic filler is mineral limestone or calcium carbonate. An alternative to mined limestone are waste bio-materials such as chicken eggshells and seashells (marine mollusks, mussels, shellfish, oysters, scallops, and cockles) from the egg and seafood processing industries, respectively, which contain high calcium carbonate contents. They are abundant and are discarded by-products which can serve as potential substitutes for limestone derived from sedimentary rocks. A small portion of these bio-calcium carbonate wastes have found niche applications as animal feed supplements, remediation of acidic soils, and treatment of wastewaters [1,2,3]. However, the majority of waste chicken eggshells and seashells around the world are disposed of in landfills which tend to pose environmental problems [4]. Their decomposition produces ammonia, hydrogen sulfide and amines with significant odors [5,6,7]. In addition to a foul smell and attracting mice, rats, flies and insects, pathogens such as Escherichia coli (E. coli) [8] and Salmonella [8,9] are present in eggshell and seashell waste. In addition, untreated seashell waste can lead to leaching of heavy metals from the viscera [10]. In the US, eggshell waste has been placed 15th on the Environmental Protection Agency (EPA) list of food industries creating pollution problems [11], while the European Commission regulations consider industrial eggshell byproducts as hazardous waste [12]. In recent developments, one method to mitigate disposal problems is to find novel applications by reusing and recycling of bio-based calcium carbonate as fillers for polymer matrix composite materials.
The egg processing industry consists of large-scale hatcheries and breaker plants where the latter yields liquid, powdered and frozen eggs for wholesale food producers and bakeries, while seashell waste are derived from various shellfish generated from the seafood processing industry. As the global population increases, the production and consumption of eggshell and seashell products are projected to increase [13]. Greater quantities of waste eggshells (and seashells) suggest higher landfilling costs borne solely by the processors. For example, in the US it can cost one breaker plant about US$100,000 per year to dispose of eggshells into landfills [14], while in the United Kingdom, Just Egg (Chilled foods) Ltd. spends about £50,000 (~US$65,000) a year to dispose of eggshells [15]. In Europe it can costs small- to medium-sized egg processing companies about €100,000 (~US$112,000) per year for disposal [12]. Similarly, seashell producers also consider shellfish a nuisance waste product as it costs them financial losses for proper disposal [16]. The consensus from companies is that they would be satisfied if someone was to take the eggshell waste off their hands for free as this would decrease their landfill tipping fees.
The desire to recycle waste eggshells is in its infancy. In recent years there has been an upswing with several small-scale start-up companies that have separated the membrane from waste eggshells at an industrial scale. For instance, Eggnovo (Spain) utilized calcium carbonate from eggshells for use in food, food supplements, cosmetics and pharmaceutical sectors [17]. American Dehydrated Foods (USA) in conjunction with Georgia Institute of Technology researchers has been developing a separation facility to recover calcium carbonate from eggshells for possible use in plastics in an effort to reduce petroleum-based products [15]. Similarly, Just Egg (United Kingdom) along with Leicester University have designed a pilot plant to produce eggshell powders for possible use as fillers to reinforce plastics [18]. These demonstration plants could be models for recuperating eggshells in other countries as well as adapting them to recover seashells. In a short time, plastic industries may be interested in using eggshell powders as filler materials but would be dependent on price since calcium carbonate from rocks is relatively low cost [18].
To advance the understanding in the field of bio-calcium carbonate fillers combined with polymers, a need to review past studies on the progress made was required in order for users to make well-informed decisions. This work attempts to provide a framework on the important studies in the area of mechanical properties (tensile strength, flexural strength and impact toughness) and outlines the knowledge gaps of calcium carbonate-based particulate-filled thermoplastics and thermoset polymer composites. A review on the use of waste-based calcium carbonate as a sustainable filler has not been published in the literature and will be meaningful for the polymer composite industry.
2. Composition and Sources of Eggshells and Seashells
In nature, calcium carbonate exists as a mineral in sedimentary rocks such as limestone and chalk and in metamorphic marble rocks [19] where they are often extracted via quarry or underground mining. Three crystal polymorphs of calcium carbonate exist in nature in decreasing order of stability: calcite, aragonite and vaterite [20]. Limestone, chalk and marble are primarily composed of calcite. The density of natural limestone ranges from 2.50–2.71 g/cm3 [21,22]. Its major applications in engineering materials have been for fillers in polymer composites [23] as well as raw materials for cement and mortar production [21]. Apart from mineral limestone, there are an abundance of industrial waste materials containing high calcium carbonate contents in the form of chicken eggshells and various seashells.
2.1. Eggshell Structure and Composition
An eggshell is the hard outer layer of a chicken egg and can be brown- or white-coloured [24]. A chicken egg consists of 60% albumen (the liquid white colored substance), 30% yolk (the liquid yellow colored substance) and 10–11% shell (the solid eggshell and organic membrane) [25]. The total weight of an average egg was reported to be between 60.0–60.2 g [26,27], while the empty shell weight ranges between 6.6–7.3 g [25,26]. The eggshell structure shown in Figure 1 is composed of three main layers; an outermost layer surrounding the eggshell called the cuticle, the layer beneath is referred to as the testa (“the shell”) and the innermost layer termed the mammillary layer [28]. Under the mammillary layer are two shell membranes termed outer-shell membrane and inner-shell membrane. The cuticle (dried mucus) layer is a thin film of about 10 µm which protects the embryo from bacterial infection and moisture loss [28]. In the literature, the term testa has also been referred to as the palisade layer as both contain calcite crystals. The palisade (spongy) layer is arranged in columns and is the thickest layer (about 100 µm) of the eggshell. It provides calcium and aids in coloration to the growing egg [28]. In-between the palisade columns are small pores. It is estimated that the shell contains 7000 to 17,000 unevenly distributed circular pore openings which function to allow gas exchange of oxygen from the atmosphere and carbon dioxide release produced by the baby chicken [29,30]. Studies compared scanning electron microscope images at the same magnification of eggshell powders with mineral limestone powders and observed the presence of pores in eggshells and the absence in limestone [31,32]. This suggests that the pores in eggshells are larger than those in limestone. The palisade layer is composed of about 95% calcium carbonate, 3.3% organic proteins and 1.6% moisture [33]. The mammillary layer consists of cones/knobs made from organic protein and are the seeding sites on which the testa/palisade columns grow [34]. The outer-shell membrane (visible to the naked eye and which cannot manually peel-off easily) is thicker than the inner-shell membrane and together have a thickness of about 100 µm. Both membranes are assemblies of a network of protein fibers [35]. To gain a better understanding of the layered structures of a chicken eggshell, researchers have heated eggshells at various elevated temperatures and viewed them under the scanning electron microscope [36,37].
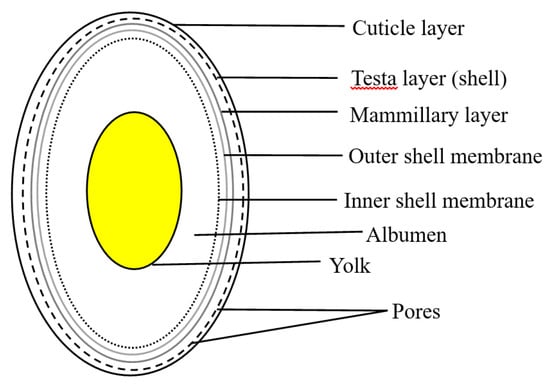
Figure 1.
Structure of an eggshell.
The chemical composition of eggshells are calcium carbonate in the form of calcite ranging from 94–97% [28,38,39] and 3–4.5% organic matter [26,40,41]. In addition, minor traces of other elements have been detected such as; MgO (0.83%), SO3 (0.66%), P2O5 (0.43%), Al2O3 (0.15%), K2O (0.08%), SiO2 (0.07%), Cl2O3 (0.06%) and SrO (0.04%) [42]. There are slight variations in composition from study to study which may be attributed to the type of chicken feed and possible impurities generated by organic proteins and membranes. The color of the shell does not dictate the amount of calcium carbonate. For example, brown eggshells were reported to contain 96–97% calcium carbonate and 3–4% organic membrane [38,43], while white eggshells were found to have 94% calcium carbonate content with 6% organic membrane [27,44]. Based on these findings, the calcium carbonate contents in both brown and white eggshells were considered equivalent [41]. The density of the eggshell has been quantified from various studies to be; 2.50 g/cm3 [41], 2.53 g/cm3 [28], 2.59 g/cm3 [38] and 2.62 g/cm3 [39]. The eggshell density is slightly less than the mineral limestone possibly due to the porous nature of eggshells. The outer-shell membrane density has been reported to be smaller with a value of 1.36 g/cm3 [28].
2.2. Sources of Eggshell Waste
Sufficient quantities of waste eggshells would be required for industrializing a recovery process. Households and restaurants would not be a viable route to obtain waste eggshells, rather they would be obtained from egg processors ‘breaker plants’. The first known egg breaker plant was invented in 1928 in the United States before World War II [45]. In this process, the liquid egg is mechanically separated from the shells, which lead to eggshells being obtained in large quantities. For instance, a modern breaker plant can process 188,000 eggs per hour. Eggs are classified into sizes and standards where sub-standard eggs not suitable for the general market are sent to breaker plants while quality medium, large and extra-large eggs are sent to market [46].
The top 10 egg-producing countries are presented in Figure 2 and Table 1. Roughly 30% of eggs are sent to breaker plants for processing into liquids [31]. The amount of calcium carbonate (CaCO3) from eggshell waste that could be recuperated are outlined in Table 1.
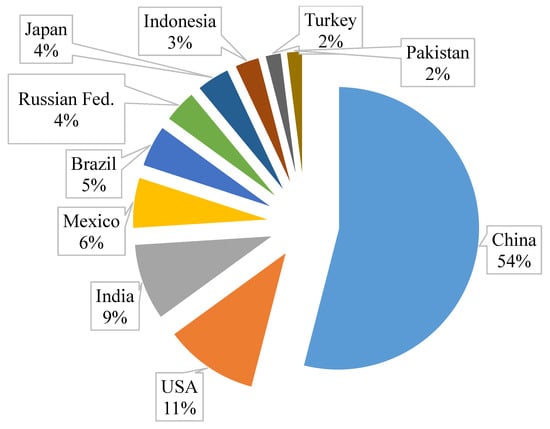
Figure 2.
Global pie chart of eggs produced annually in 2017.

Table 1.
Annual egg production by the top 10 countries in 2017 (* values multiplied by 1000).
The values in kilograms (kg) of recuperated CaCO3 are based on an empty eggshell weight of approximately 6.6 g. As an example, China’s annual production in 2017 was 536,818,007,000 eggs. Of this amount, 30% equates to 161,045,402,000 eggs. Therefore, approximately 1,062,899,654 kg could be recovered. Putting this into perspective, about 35,429,988 bags of CaCO3 powder, each of 30 kg, could be produced from eggshell waste annually. It is important to note, the aim of this review is not to eliminate limestone derived from rocks, but it is to make use of a waste material.
2.3. Marine Seashell Composition
A seashell can be defined as the hard outer layer from the body of an animal that lives in the sea such as marine mollusks; mussels, oysters, scallops, clams, snails and cockles. Sea animals are food proteins extensively consumed by humans and their shells are widely disposed to landfills [48]. Studies determined the chemical composition of various seashells to be calcium carbonate in the range of 92–99% [48,49,50] with about 5% organic matter [51]. The majority of seashell types studied reported calcium oxide (CaO) contents rather than CaCO3 contents [52,53,54]. This suggests the removal of organic material from seashells may be difficult and heating to elevated temperatures results in a purified form. For instance, heated oyster shells have been reported to contain CaO (51.06%), with fractions of SiO2 (2%), Al2O3 (0.50%), Fe2O3 (0.20%), MgO (0.51%), SO3 (0.60%), K2O (0.06%), Na2O (0.58%), TiO2 (0.02%), Mn2O3 (0.02%), P2O5 (0.18%) and SrO (0.09%) as a well as a 44.16% loss of CO2 and organic materials [55]. To identify the amount of calcium carbonate in seashells from CaO contents, estimates were calculated using Equation (1) and results presented in Table 2. This is helpful to compare literature that has only reported calcium carbonate contents.
where, CaCO3 MW, CaO MW and CaO WR are calcium carbonate molecular weights (e.g. 100), calcium oxide molecular weight (e.g. 56), and calcium oxide weight ratio or content in%, respectively. The calculated results are in the range of 91–97% CaCO3. The density of dried mussel shells have been stated to be 1.33 g/cm3 [49], while oyster shells were 1.15–2.62 g/cm3 [7,56].

Table 2.
CaCO3 composition of seashells (*calculated based on Equation (1)).
2.4. Sources of Seashell Waste
Similar to waste eggshells, the number of seashells from homes and restaurants would not be adequate for an industrial calcium carbonate extraction process. Instead, large amounts of seashell waste are generated from canning industries. The top 10 seafood-producing countries are presented in Table 3. The literature source did not differentiate between the types of seashells by country, therefore a general overview is provided. For every oyster shell dozen consumed, about 370–700 g of empty shells are recovered [50,64]. This implies that for one oyster protein consumed, the shell weight recovered is in the range of 30.83–58.33 g. The dry weight of one mussel shell was found to be approximately 0.01–40 g [65], one mollusk shell to be 0.317–2.08 g [66], one empty cockle shell to be 1 g [67] and a sea scallop to be 0.4 g [68]. Based on the above studies, the dry shell weight of oysters, mussels, mollusks, cockles and scallops ranges from 0.01 g to 58.33 g or approximately 52–80% of the whole animal. Table 4 shows the annual mollusk consumption for the top three countries in 2013 [47]. For instance, China consumed 12,537,301 tonnes of mollusk. The amount of CaCO3 that could be recovered in kg are based on one mollusk shell having a dry weight in the range of 0.317–2.08 g [66]. Therefore, in China about 3,974,324–26,077,587 kg of CaCO3 could be recuperated from mollusk waste annually.

Table 3.
Annual seafood production by the top 10 countries in 2016.

Table 4.
Annual mollusk consumption by the top three countries in 2013.
3. Bio-Calcium Carbonate Fillers in Polymer Composites
Several lab-scale manufacturing processes and polymer matrices have been utilized for the fabrication of composites containing bio-calcium carbonate particulate fillers (uncoated/unmodified). Thermoplastic polymers require high processing temperatures to melt polymers while thermoset composites are cured at room temperature and further post-cured at an elevated temperature. To compare numerical results from research papers where only the graphs were available, data points were extracted directly from published article figures with the open source Web Plot Digitizer, version 4.1 [70].
3.1. Thermoplastic Eggshell Fillers
The effect of waste eggshell fillers on the mechanical properties of thermoplastic polymers have been assessed by several studies and are summarized in Table 5. In one study, the tensile Young’s modulus (tensile strength not reported) of eggshells (<38 µm) in a polypropylene (PP) matrix were determined. The eggshells were dried, mechanically triturated into powder, blended using a mixer and hot pressed into specimens. Only one filler loading of 40 w/w (or wt.%) was studied which presented an increase in Young’s modulus of approximately 136% compared to the pure PP resin. The improvement was credited to a good eggshell/PP interface [71].

Table 5.
Summary of thermoplastic polymers containing eggshell filler.
In a related study, the effect of eggshells (<149 µm) on the tensile, flexural and Charpy impact properties of PP were evaluated. The eggshells were dried and sieved (crushing method not reported). PP/eggshell composites with filler loadings of 1, 3, and 5 wt.% were initially homogenized in a mixer, pelletized and manufactured via injection molding. The filler additions did not affect the tensile properties (Young’s modulus not reported). For instance, the tensile strengths did not significantly change with filler content. At 5 wt.%, the tensile strength reduced by only 3%. Similarly, the change in flexural strength and flexural modulus were not remarkable such that at 5 wt.%, the greatest changes were 4% and 10%, respectively. However, improvements were observed with Charpy impact toughness of 38%, 48%, and 48%, respectively. The authors suggested the drop in mechanical properties were due to poor compatibility of the hydrophobic PP with hydrophilic eggshell fillers [72]. Similarly, the effect of eggshell powders (14–157 µm) on the mechanical properties of PP were determined. PP/eggshell composites contained 10, 20, 30 and 40 wt.% loadings. The eggshells were washed in warm water, dried and ball milled into powder. The materials were mixed with a twin screw extruder and further made into plates using a hot press. The tests presented a drop in the tensile strength and an increase in Young’s modulus with filler loading. Pure PP presented a tensile strength of 27 MPa but decreased by approximately 37% at 40 wt.%. The best tensile strength reduced by 4% with 10 wt.% filler. The Young’s modulus improved by 220% at a 40 wt.% loading. The Izod impact results decreased for all loadings, but at 30 wt.% showed the highest value with a 31% reduction in toughness compared to the pure PP. The authors proposed the decrease in tensile strengths were due to poor adhesion of the filler-matrix and agglomeration of eggshell particles while the increase in Young’s modulus was due to the inclusion of rigid and stiff particles into the polymer. The impact toughness reduction was attributed to a composite containing a lower volume of polymer material [73]. In a related study, the effect of adding 5, 10, 20 and 30 wt.% eggshell and mineral calcium carbonate (3 µm) fillers to a PP matrix was analyzed. The eggshells were washed, dried, ball-milled, blended using a twin screw extruder and further injection molded into mechanical test specimens. The PP/eggshell composite tensile strengths decreased by 11–20% with increase in filler loading while Young’s modulus increased by 2–21% compared to pure PP. Similarly, the PP/mineral calcium carbonate composites reduced in tensile strength by 6–14% but improved in Young’s modulus by 2–25%. In general, the mineral calcium carbonate composites had 3–7% and 1–5% higher tensile strengths and Young’s modulus, respectively compared to the eggshell composites. The authors suggested the decrease in tensile strength for the eggshell composites was due to agglomeration of fillers in the matrix, while the improvement of modulus was due to the presence of the fillers. The flexural modulus (flexural strength not reported) increased by 0–38% with eggshell loading, while the Izod impact toughness augmented by 2% at 30 wt.% eggshell loading. The authors suggested that the increase in flexural modulus was due to the strong bonding between the matrix and filler [74]. In another work, eggshells were heated at 500 °C for 2 h, mechanically ground and sieved to a particle size distribution of 100–350 µm. The PP and eggshell powders (30 wt.%) were compounded in a twin screw extruder, pelletized and fed into an extrusion/compression molding process to produce the various specimen shapes. The tensile strength decreased by 13%, while the Young’s modulus, flexural strength, flexural modulus and Izod impact toughness increased by 23%, 4%, 30% and 47%, respectively. The authors advocated that the relatively large particle size of the filler material used served as stress concentrators which could have been responsible for the reduction in the tensile strength. In addition, the improvement in flexural properties may have been due to enhanced adhesion between the filler and the matrix [75].
Eggshells (<40 µm) have also been added to a polypropylene carbonate (PPC) polymer. The eggshells were washed, dried and made into powder using a kitchen grinder-mixer. The PPC was dissolved in chloroform and the eggshell powder was added in filler contents of 1, 2, 3, 4, and 5 wt.%. The PPC/eggshell composites were fabricated using a film-casting method. The results presented an improvement in both tensile strength and Young’s modulus for all composites where the optimal loading was determined to be 4 wt.%. For instance, the tensile strengths were reported to have increased by approximately 0%, 8%, 38%, 77% and 8%, while the Young’s modulus increased by 18%, 22%, 47%, 70% and 19% for 1, 2, 3, 4, and 5 wt.% loadings, respectively. A better dispersion and interaction between the matrix and the fine eggshell particles benefited the composites. The apparent decrease at 5 wt.% loading may have been due to agglomeration of filler particles with increased filler concentrations [76].
The effect of eggshell particle sizes of 0.2 µm and 7 µm in amounts of 10, 20, 30 and 40 phr on the mechanical properties of polyvinyl chloride (PVC) were studied. In order for the filler loading data in Table 5, Table 6, Table 7 and Table 8 to be consistent, a personal communication with the corresponding author showed the conversion to wt.% used was: wt.% = (phr of component/(total phr of the components) * 100. Therefore 10, 20, 30 and 40 phr corresponds to 7.5, 15, 22.5 and 30 wt.%, respectively. The eggshells were washed, dried and made into smaller pieces using a blender and further ground with a Retsch grinder. The ingredients were blended using a Rheo-Mix mixer and molded using a hot press. The tensile strength was optimal for 7.5 wt.% fillers of 0.2 µm and 0.7 µm where it improved by 26% and 21%, respectively, while at a maximum loading of 30 wt.%, the Young’s modulus increased by approximately 544% and 344%, respectively, compared to pure PVC. Better composite tensile strengths were reported for 0.2 µm fillers over 7 µm particles. The 7 µm particle size fillers had lower surface areas for interaction with the matrix which led to increased particle agglomeration through hydrogen bonding resulting in a higher dispersive resistance. The authors also proposed that the improved Young’s modulus with 0.2 µm fillers was a result of lower particle agglomeration [77].

Table 6.
Summary of thermoplastic polymers containing bio-calcium carbonate filler.

Table 7.
Summary of thermoset polymers containing eggshell fillers.

Table 8.
Summary of thermoset polymers containing seashell fillers (all by solution-mixing technique).
Eggshell fillers have also been added to low-density polyethylene (LDPE) and high density polyethylene (HDPE). For instance, eggshell powders (63 µm) were added to LDPE using filler contents of 5, 10, 15, 20 and 25 wt.%. The eggshells were initially washed, dried and ground into powder using a blender. The powders were then treated with 10% NaOH to remove additional organic membrane. LDPE/eggshell composites were mixed via a Z-blade mixer and further molded in a hot press. At all loadings, the tensile strengths decreased by approximately 14%, 21%, 29%, 50%, and 50% and the Young’s modulus increased by about 27%, 53%, 87%, 107% and 140%, respectively. The authors suggested that the decrease in tensile strengths were due to poor adhesion between the filler and matrix as well as agglomeration of the filler materials while the increase in Young’s modulus was attributed to the calcium carbonate eggshells exhibiting higher stiffness than the pure LDPE [78]. In another study, 20 wt.% eggshells (25 µm) were combined with HDPE. The eggshells were washed, dried and ground using an ultra-centrifugal mill. A twin screw co-rotating extruder was used to blend the components and further made into pellets for use in an injection-molding machine. At this loading level the Young’s modulus, flexural strength, and flexural modulus increased by approximately 8%, 2%, and 3%, respectively, while the tensile strength and Charpy impact toughness decreased by 15% and 42%, respectively. The authors suggested that there were no significant differences in the increase in flexural strength and flexural modulus compared to the pure matrix. Furthermore, the decrease in tensile strength was attributed to poor adhesion of filler-matrix and agglomeration of filler particles [42]. In a recent investigation 7 µm eggshell powder were blended in an HDPE matrix in amounts of 10, 20, 30 and 40 phr which corresponded to 7.5, 15, 22.5 and 30 wt.%, respectively. The waste eggshells were first washed with water and initially crushed using a blender and ground with a Retsch Grinder. The composites were melt blended using an internal mixer, molded with a hydraulic molding press and further cut into tensile dumbbell shaped specimens. At 7.5 wt.% the tensile strength improved by 5% but was inferior by 2%, 6% and 18% with additions of 15, 22.5 and 30 wt.%, respectively compared to pure HDPE. The reduction in strengths above 15 wt.% filler was due to a weaker interfacial adhesion as a result of increased agglomeration. In contrast, the Young’s modulus increased progressively with filler loading by 8%, 20%, 28% and 31%, respectively. The authors believed that the improvement was due to the eggshell powder being stiffer than the HDPE polymer [79].
Waste eggshell powder (<75 µm) was also combined with virgin polystyrene (PS) in an amount of 20 wt.%. The eggshell were washed, dried and ground to size. The ingredients were blended by melt-mixing in an internal mixer and the molded sheet composites were made by compression molding. The tensile strength and modulus of virgin PS (polystyrene 536), obtained from the supplier (Total Petrochemicals, Inc) was 44.8 MPa and 2.96 GPa, respectively (not reported in article). The composite tensile strength and Young’s modulus were approximately 25 MPa and 3.13 GPa, respectively, for a reduction of 44% and an increase of 5%, respectively. This study focused on the amounts of recycled and virgin PS rather than varying the eggshell content [80].
Eggshell (25 µm) loadings of 1, 2, 3, 4 and 5 wt.% were added to polylactic acid (PLA). The eggshells were washed, dried and made into a fine powder using a kitchen grinder-mixer. PLA/eggshell films were produced via a film casting method by initially adding eggshell powders to PLA dissolved in chloroform. Both tensile strength and Young’s modulus improved by 6%, 24%, 53%, 82% and 35% and 9%, 27%, 64%, 82% and 27%, respectively, with increase in filler loading for all composite formulations. The authors proposed that the reductions at higher filler loadings (>4 wt.%) were due to agglomeration of filler particles induced by van der Waals interactions [81]. In a related study, two eggshell particle sizes (63 µm and 32 µm) were incorporated into PLA in amounts of 5, 10 and 20 wt.%. The eggshells were coarse crushed, washed with water, dried and ball milled to fine powders. Pellets for injection molding were produced using a twin screw extruder. Tensile strengths of 49.43 MPa and 47.39 MPa were obtained for 5 wt.% (32 µm) and 5 wt.% (63 µm) composites, respectively compared to pure PLA (51.52 MPa), which suggested slightly better mechanical properties for 32 µm fillers. The Young’s modulus improved with filler loadings and was maximum with 32 µm particles at 20 wt.% (4.4 GPa) which was higher than pure PLA (3.6 GPa). The flexural strength increased up to 10 wt.% and reduced when more filler loadings were added, while flexural modulus presented an improvement with increase in filler loading up to a maximum of 20 wt.%. For both flexural strength and flexural modulus, composites containing 32 µm particle size fillers presented better properties. The flexural modulus increased by approximately 136%. The authors suggested that the decrease in tensile and flexural strengths was due to particle agglomeration induced by electrostatic and van der Waals bonding forces at higher filler loading and larger particle sizes. They also suggested the increase in tensile modulus was due to inclusion of more rigid filler material and the increase in flexural modulus suggested improved resistance to deformation [82].
3.2. Thermoplastic Seashell Fillers
The influence of seashell fillers incorporated into thermoplastic polymers on their mechanical properties have been investigated by a number of studies and are summarized in Table 6. For instance, addition of seashell waste have been added to PP. Shellfish (SS) shell powder (<200 nm) was used to reinforce PP in the amounts of 1, 3, 5, and 10 wt.%. The results were compared against commercial calcium carbonate having a particle size of 40–500 nm. The SS were washed with water, coarsely ground, submitted to a 4% (w/v) NaOH solution to eliminate the stratum corneum and further ball milled to produce fine powders. The two composites were compounded separately in a twin screw extruder and injection molded into tensile samples. For both composites the best tensile strengths obtained were with up to 5 wt.% fillers for a reduction of about 13%. The Young’s modulus was optimal at 10 wt.% and 5 wt.% for PP/SS and PP/calcium carbonate composites, respectively which increased by 85% and 79%, respectively. In comparing the overall mechanical properties of both composites, PP/SS composites presented better mechanical properties. The authors suggested that this may be due to organic matter present in the SS, which improved the bonding between the filler and matrix [6]. In a similar study, the properties of PP/SS composites containing SS powders (2–4 µm) in amounts of 5, 7, 10, 15, 20 and 30 wt.% were determined. The SS were washed, coarsely ground and immersed in 4% (w/v) NaOH solution to remove the stratum corneum and then ball milled. The PP and SS powders were mixed in a twin screw extruder, pelletized and injection molded into tensile, flexural and Charpy impact test specimens. The tensile strengths (Young’s modulus not reported) reduced with increase in filler loadings by 3%, 6%, 9%, 12%, 15%, and 21%, respectively, compared to the pure PP. In contrast, the flexural strengths did not present a linear change. For example, strength improvements were observed at filler contents of 7, 10, 15 and 20 wt.% by 2%, 7%, 2%, and 5%, respectively, but tended to decline by 5% and 2%, when 5 wt.% and 30 wt.% filler contents were added, respectively. The flexural modulus improved with additions of filler loadings and was enhanced by 62% with 30 wt.% loading. The Charpy impact energy increased for all composite formulations by 5%, 12%, 17%, 12%, 14%, and 16%, respectively, with the optimum occurring at 10 wt.% loading. The authors attributed the decrease in tensile strength to poor filler–matrix adhesion which created weak interface regions and lower amounts of polymer material as a function of filler. Furthermore, the increase in flexural modulus was attributed to the augmented brittleness and stiffness of the composites [48].
Snail shell fillers were added to PP and the composite mechanical properties were compared with mineral calcium carbonate both with particle sizes less than 10 µm. The snail shells were initially washed with water and pulverized using a grinding disk mill machine. The ingredients were compounded in a twin screw extruder, pelletized and injection molded into tensile specimens. For 5, 10, 15, 20, 25 and 30 wt.% snail shell filler loadings, the tensile strengths decreased by approximately 0%, 3%, 3%, 6%, 9% and 9%, respectively, while the Young’s modulus increased by approximately 30%, 50%, 60%, 70%, 80% and 100%, respectively compared to pure PP. In comparison to bio-CaCO3, mineral calcium carbonate at a 30 wt.% filler loading presented a higher tensile strength and Young’s modulus with a difference of 3%. Since the tensile strength reductions were not substantial, the authors suggested a good dispersion/distribution of the particles and particle-matrix adhesion, while the increase in Young’s modulus may have been due to higher particle rigidity compared to the PP matrix [83].
More recently, 10, 20, 30 and 40 pphr (7.5, 15, 22.5 and 30 wt.%) of cockle shells were blended into LDPE. The cockle shells were washed, dried, crushed and ground using a mill to 100 µm. The ingredients were melt blended using an internal mixer and the test specimens were molded using a hydraulic press. With 7.5 and 15 wt.% of cockle shells, the composites increased in tensile strength by 22% and 8%, respectively, but decreased by 12% and 18% when 22.5 and 30 wt.% were added compared to pure LDPE. Although the composite Young’s modulus is higher than the pure LDPE at all filler contents, it is optimal at 7.5 and 15 wt.%. For example, at 7.5, 15, 22.5 and 30 wt.%, the Young’s modulus improved by 82%, 109%, 69% and 29%, respectively. The improvement was attributed to good dispersion of the fillers which provided a greater surface area for the interaction between the fillers and the matrix. As particles cluster, stress concentrations begin to reduce the stress transfer from polymer chains to the fillers. The agglomeration was due to improper mixing at high loadings and incompatibility of the filler and polymer [84].
3.3. Thermoset Eggshell Fillers
A number of studies have been conducted on the addition of eggshell fillers in thermoset polymers and have evaluated their mechanical properties. The details are summarized in Table 7. The Charpy impact toughness of synthetic epoxy resin was improved by incorporating eggshell fillers (1–9 µm) in amounts of 2, 5, 8 and 10 wt.%. The inner membranes were initially manually removed from the fresh eggshells. The eggshells were washed, vacuum dried and ground into powder using a planetary ball mill. The powders were then immersed in 4% NaOH and dried. The composites were produced using a solution mixing method to disperse the fillers in a mixture of acetone and liquid epoxy. After heating to remove the solvent, the mixture was poured into a steel mold coated with a release agent. The composites were cured at 90 °C for 2 h and at 150 °C for 5 h. The Charpy impact toughness increased by approximately 65%, 75%, 44% and 24%, respectively, compared to pure epoxy. This improvement was due to enhanced interaction between the matrix and the particles [85]. Another investigation determined the effect of nano-eggshell particle (<100 nm) filler loadings of 1, 2, 3, 4, 5 and 10 wt.% on the flexural properties of a bio-epoxy resin. The eggshells were initially boiled at 100 °C for 6 h, reduced in size using a laboratory blender, washed with water, ethanol and dried. The particles were further ball milled, washed with ethanol and centrifuged. Ethanol was again added to the eggshell particles, magnetically stirred and irradiated with ultrasound followed by centrifuging and vacuum drying. The composites were fabricated via a solution-mixing method. The mixtures were poured into silicone molds, cured at room temperature for 2 h and post-cured at 48.8 °C for 2 h. The test results presented an improvement in both flexural strength and flexural modulus for all composites in comparison to the pure bio-resin. For example, the flexural strength and flexural modulus improved by 7–37% and 16–42%, respectively. The composites exhibited similar results in both properties for a filler loading of 4 wt.% as the optimum. The authors reported that the improvement in these properties were due to enhanced interaction between the filler and matrix caused by the hydrogen bonding promoted by amine, carboxylic, and hydroxyl functional groups in eggshells [86].
In another work, nano-eggshell (50 nm) bio-fillers were added in amounts of 1, 2, 3, 4, and 5 wt.% in a synthetic epoxy resin. The eggshells were washed with water and disinfected with sodium hypochlorite followed by vacuum drying. The eggshells were made into a coarse powder using a blender and 5 wt.% sodium lauryl surfactant was added in order to enhance the dispersion and adhesion with the epoxy. The eggshells were then dry ball milled to the final powder size. The composites were made using a resin-casting method, in which the resin and fillers were mixed in an electric shear mixer, and cast onto a glass mold containing a wax release agent. The composites were cured at room temperature for 24 h. The tensile strength, tensile modulus and Izod impact energy improved by 7–93%, 24–112% and 5–15%, respectively. The optimal tensile strength and impact toughness was obtained for 2 wt.% filler loading and the tensile modulus was highest with 5 wt.%. The authors suggested that the increase in tensile strength and impact toughness was due to the nano-sized particles having high surface areas and better adhesion to the matrix material, while the increase in tensile modulus was due to the inclusion of stiffer filler nanoparticles compared to the matrix material [39].
In a recent study, eggshells (5, 10, 15 and 20 wt.%) were blended into a synthetic epoxy matrix. The eggshells were washed with hot water, dried and milled with a planetary ball mill to a size <50 μm. Composites were fabricated using a casting method by first heating the epoxy to 65 °C, adding the eggshells and stirring at 500 rpm for 1 hour. After cooling to room temperature, the catalyst was added, mixed and poured into plastic molds coated with wax for easier removal after two days of curing. The tensile strengths and Young’s modulus improved by 5%, 12%, 36% and 16% as well as by 74%, 63%, 144% and 130%, respectively, while un-notched Izod impact tests increased by 12%, 28%, 13% and 11%, respectively, compared to the pure epoxy resin. Improvements in properties were due to homogeneous dispersion of the shell particles within the epoxy [87]. Similarly, eggshell powder in amounts of 5, 10, 15 and 20 wt.% were combined with a synthetic epoxy resin. The eggshells were washed, dried and crushed to a fine powder (500 μm). The composites were prepared by adding a fixed amount of epoxy and eggshells to a beaker and stirred at 200 rpm. The curing agent was then added, stirred and poured into a wax coated mold. The tensile strength reduced by 13%, 15%, 23% and 33%, respectively, while the tensile modulus improved by 3%, 4%, 8%, respectively but decreased by 1% at 20 wt.% loading. The reduced tensile strength was due to poor adhesion of filler-matrix and agglomeration which creates stress concentrations. This is magnified by the increased filler loadings. Similarly, 5–15 wt.% of eggshell fillers increased the stiffness of the epoxy while reductions were observed with additions of 20 wt.% due to aggregation of particles. The flexural strength decreased by 34%, 35%, 1% and 15%, respectively. The flexural modulus dropped by 30% and 23% for 5 and 10 wt.%, respectively but increased by 13% at a 15 wt.% loading. A further increase to 20 wt.% filler content reduced the modulus by 12%. The reductions are thought to be a result of improper mixing of the fillers within the epoxy which led to a poor dispersion in addition to weak bonding between the filler particles and matrix. The Izod impact strength gradually decreased as the filler loading increased by 17%, 37%, 51% and 58%, respectively, which suggests adding eggshell fillers was not beneficial for improving toughness of the epoxy [88].
Unsaturated polyester/eggshell (1–3 μm) and unsaturated polyester/commercial calcium carbonate (3–8 μm) composite films were produced that contained filler loadings of 5, 10, 15, 20 and 25 wt.%. The eggshells were washed in water, sterilized, dried, crushed in an electric blender and reduce in size using a mortar and pestle. The films were produced via a hand lay-up technique and a hot press. The tensile strength for unsaturated polyester/eggshell films increased by 17%, 83%, 24%, 7% and 14%, respectively, compared to unsaturated polyester/commercial calcium carbonate which increased by 1%, 69%, 19%, 7% and 12%, respectively. The Young’s modulus for unsaturated polyester/eggshell composites increased by approximately 33%, 25%, 58%, 42% and 25%, respectively, in contrast to the unsaturated polyester/commercial calcium carbonate which increased by 27%, 47%, 15%, 13% and 8%, respectively. The flexural strengths (flexural modulus not reported) for the unsaturated polyester/eggshell films increased by approximately 1%, 37% and 18% with 5, 10, and 15 wt.% filler contents, respectively, however, above 15 wt.% the flexural strengths reduced. In a similar way, the unsaturated polyester/calcium carbonate flexural strengths increased by 0.3%, 32% and 10%, respectively, and further decreased above 15 wt.%. For both composites, the Izod impact toughness increased with an increase in filler loading up to 20 wt.% and further decreased at 25 wt.% loading. The authors suggested that improvements in tensile strengths were due to good interfaces and strong bonding between the filler particles and resin, while reductions at higher loadings were a result of increased void contents and increased brittle effect of the filler material leading to toughness reductions [89].
3.4. Thermoset Seashell Fillers
Limited studies have added seashell fillers to thermoset resins as given in Table 8. For instance, bio-epoxy composites were produced with mollusks shells of particle sizes <250 μm and filler amounts of 5, 10, 20, 30 and 40 wt.%. The mollusks were washed with 4% sodium hydroxide, dried and ground using an ultra-centrifugal mill. The powders were then treated with a silane solution to improve the chemical interaction between the filler and the matrix. The composites were made via a solution mixing method, poured in a polytetrafluoroethylene mold and cured at 100 °C for 45 min. The flexural modulus (flexural strength not reported) increased by 5%, 29%, 52%, 57% and 48% with increase in filler loading. In contrast, the Charpy energy drastically decreased at all filler loadings by 85%, 87%, 90%, 90% and 92%, respectively. The flexural modulus improved due to a homogeneous dispersion, polymer chain mobility restriction, but at higher filler loadings the dispersion was reduced and some aggregates were formed leading to the presence of stress concentrators. The dispersion and interaction of the filler particles within the matrix play a role in mechanical properties, but the amount of fillers were not an indicator for the drop in impact energy [63]. In a related study, the effect of cuttlebone shells (15–25 μm) and commercial calcium carbonate (<10 μm) fillers in loadings of 3, 6, 9, 12 and 15 wt.% in a synthetic epoxy were compared. Cuttlebones were washed with water, scrubbed with a steel brush and dried, followed by crushing and grinding using a pulverizer. One batch of cuttlebone powders were used as prepared (CB) and another batch were heated to 400 °C for 3 h. The composites were made via a hand lay-up method. The mixture was poured into a steel mold with an applied release agent and cured at room temperature for 24 h, demolded and post-cured at 80 °C for 4 h. The tensile strengths for epoxy/CB, epoxy/heated CB and epoxy/calcium carbonate composites increased by 17%, 38%, 63%, 46% and 33% and by 25%, 54%, 72%, 67% and 46% as well as by 4%, 8%, 42%, 13% and 8%, respectively. Similarly, the Young’s modulus for epoxy/CB and epoxy/heated CB increased by 2%, 8%, 9%, 18% and 6% and 0.2%, 7%, 12%, 27% and 35%, respectively. However, epoxy/calcium carbonate composite Young’s modulus was less affected by the fillers and increased by 0.2%, 6%, 3%, and 1% but decreased by 7% at 15 wt.%. The authors suggested that the improved tensile strength was due to good interaction between the filler and matrix, while beyond 9 wt.% the fillers had poor dispersion with the matrix. In addition, the Young’s modulus had good adhesion between the filler and matrix but reductions occurred due to agglomeration [22]. More recently, snail shells were mixed into a synthetic epoxy resin. The snail shells were soaked for seven hours in 5% diluted household sodium hypochlorite, rinsed with distilled water and dried. The shells were then ball milled to a size of <50 μm. The composites were made using a conventional casting method as explained earlier. As compared to the unfilled epoxy, the tensile strengths, Young’s modulus and Izod impact tests were improved by 28%, 49%, 32% and 32%, by 79%, 76%, 83% and 119%, and by 11%, 20%, 10% and 7%, respectively. The increase in tensile strengths, Young’s modulus and impact strengths were due to the high carbon content present in the snail shells which produced a tougher and stronger structure as well as the size, shape and interconnectivity formed by the particles [87].
The addition of mineral fillers to polymers is generally to reduce the cost of the composite and may or may not improve the mechanical properties. The hydrophilic property of mineral calcium carbonate renders it incompatible with hydrophobic polymers. In an effort to improve compatibility, reduce agglomeration, improve bonding and enhance the mechanical properties of polymer composites, calcium carbonate surface modifiers have been studied. The modifiers play a role in linking the inorganic filler with the organic polymer. It is important to note that an excess amount of coating could negatively affect the mechanical properties [90]. Differential thermal gravimetric analysis (DTG) can be used to differentiate between a single (thin) coated layer and multiple (thick) layers [91,92]. The most common fatty acid coating applied is stearic acid [91] while other modifiers have been polyacrylic acid [93], phosphate [94], acrylic acid [95], silane [96], dendritic carboxylic acid [97], titanate [98] and zirconate [99]. Several studies exist on bio-calcium carbonate eggshell. For instance, eggshells have been modified with propionic acid (i.e., carboxylic acid) [100], silane [42,85,101], titanate [42], zirconate [42], stearic acid [73,102,103], isophthalic acid [74,78], maleic anhydride [104], pimelic acid [72] and a solid state shear pulverization process (without chemical modification) [105]. Limited studies have been conducted on seashells. For example, seashells have been treated with silane [63,106,107], stearic acid [108], titanate [109] and pimelic acid [110,111]. In general, the majority of studies found improvements in mechanical properties with applications of surface modifiers.
4. Discussion and Knowledge Gaps
Based on this review, the addition of bio-calcium carbonate fillers could successfully be used in polymer composites to improve mechanical properties. Generally, the studies obtained their bio- fillers in powder form by grinding/pulverizing using small-scale laboratory processes generally as a ‘proof of concept”. Although a number of recycling processes for waste eggshells have been proposed [112], medium- to large-scale industrial operations have not been in operation to date to recycle eggshells and seashells into useful powders. The enormous amounts of eggshells and seashells generated everyday by their respective industries are difficult to compost and for the most part are considered waste for landfills. In addition, the unpleasant smell generated from decaying organic material in the shells makes it difficult to distribute on the land. As nearby lands become saturated, hauling of the waste comes at a cost to the company. More importantly, in certain countries (e.g., the US) this form of disposal may not be permitted [113] and landfilling may be the only option.
Bio-fillers could potentially replace and/or substitute mineral limestone added to polymers to reduce their cost while still improving their mechanical properties. This review illustrated the bio-calcium carbonate filler (uncoated/unmodified) loadings played an important role on the mechanical properties of the matrix polymers. The trend for thermoplastics composites containing bio-fillers tended to reduce in tensile strength with filler loading but were acceptable with 5wt.% bio-fillers, while the flexural strengths were ideal with 5–10 wt.% loadings. Both Young’s modulus and flexural modulus improved with filler loadings and were optimal at the higher contents. The impact toughness showed 10 wt.% loadings were the best. Similarly, thermoset composite tensile and flexural strengths decreased with filler loading but were ideal with not more than 10 wt.% fillers. The Young’s modulus and flexural modulus were ideal up to 15 wt.% fillers. The impact toughness decreased at all filler loadings but recommendations of less than 10 wt.% were suitable. Common improvements in properties were credited to homogeneous particle dispersion/distribution, polymer chain mobility restrictions, enhanced interaction, good interface and better adhesion/bonding between the filler particles and matrix. In contrast, the general observations for the deterioration of mechanical properties were attributed to poor compatibility of the hydrophobic polymer with hydrophilic bio-calcium carbonate fillers, poor adhesion/bonding of the filler-matrix, and agglomeration induced by electrostatic and van der Waals bonding forces of bio-calcium carbonate particles which amplified at higher filler loadings. The composite modulus improvements were a result of inclusion of stiffer and more rigid particles than the polymer matrix. The particle size were very different from study to study and ranged from micro- to nano-meter scale where properties largely improved for smaller sizes. A few studies conducted on eggshell and seashell fillers suggested hydrogen bonding between the matrix and filler were promoted by amine, carboxylic, and hydroxyl functional groups from the membrane on eggshells and organic matter in the seashells which may have contributed/improved the bonding between the filler and matrix.
Bio-calcium carbonate has not been commercialized to date possibly due to an absence of proper management systems for recuperating and processing waste eggshells or seashells. The lack of recycling facilities in many countries may also be hindered from end-user demand. The quick decay of organic material within the eggshells and seashells may limit the locations of recovery units to the processors. A map of eggshell and seashell processors within each country would help identify key actors and link smaller companies to better manage this waste into central locations. The driving force for adoption of new materials such as bio-calcium carbonates by industry is their cost, availability, continuous supply and performance which is unknown at this time. Additions of mineral limestone fillers in polymers has been the norm in the plastics industry for many years and changing to new materials can be difficult without its familiarity. Generating eggshell and seashell processing procedures and standards may help implement the use of bio-calcium carbonate, particularly in composite materials.
Universally, the literature is not clear if organic membranes should be removed from eggshells/seashells by heating or by use of chemicals or do they interact favorably with the polymers. Particle surface modifiers could be applied to eggshell and seashell fillers but more work should be conducted in this area to optimize the coating thickness relative to cost. A study could be conducted on the viability of a closed system that could heat eggshells and/or seashells above 750 °C in order to form into calcium oxide and further react with carbon dioxide (collected from CaO formation) to form a purified form of calcium carbonate without any organic membranes. Purified forms of calcium carbonate could be used in many other markets/industries such as pet food, pharmaceutical, mortars and concrete, production of lime and hydroxyapatite for dentistry and medical applications. Another challenge is to investigate the possibility of a calcium carbonate recovery unit for the seashell industry similar to current egg breaking plant undertakings. A final knowledge gap in this area is to carry out techno-economic analysis and life-cycle analysis studies on the recovery of waste eggshells and seashells for strategic regions.
5. Conclusions
The review of bio-calcium carbonate fillers in polymers has gained the attention of industry and researchers. The analyses showed improvements in composite materials with the addition of fillers (even without surface modifications) compared to pure polymer matrices. Upcoming research and development should focus on improving the properties of bio-polymers with bio-calcium carbonate fillers to make them competitive with synthetic polymers. Recently, there has been small-scale egg-breaking plants partnering with universities in an effort to industrialize waste eggshell recuperation at their facilities. With suitable cleaning and grinding equipment, waste eggshells and seashells have potential to be recycled for a variety of applications. Processing these bio-materials could have minimal impact on the environment provided a closed-loop recovery system is used. At present recycling is not an option for waste eggshell and seashell industry producers. Once investments are made in research, facilities and infrastructure and the supply of bio-calcium carbonate becomes accessible, perhaps market demand will follow.
Author Contributions
The following statements should be used conceptualization, S.O. and D.C.; methodology, D.C.; formal analysis, S.O.; investigation, S.O.; resources, D.C.; data curation, S.O and D.C..; writing—original draft preparation, S.O.; writing—review and editing, D.C.; supervision, D.C.; project administration and funding acquisition, D.C., please turn to the CRediT taxonomy for the term explanation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2020-06701. The APC was funded by MDPI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morris, J.P.; Thierry, B.; Gauthier, C. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Cordeiro, M.M.C.; Maxwell, T.H. Recent patents on eggshell: Shell and membrane applications. Recent Pat. Food Nutr. Agric. 2011, 3, 1–8. [Google Scholar] [CrossRef]
- Jovic, M.; Mandic, M.; Sljivic-Ivanovic, M.; Smiciklas, I. Recent trends in application of shell waste from mariculture. Stud. Mar. 2019, 32, 47–62. [Google Scholar] [CrossRef]
- Hart, A. Mini-review of waste shell-derived materials’ applications. Waste Manag. Res. 2020. [Google Scholar] [CrossRef]
- Felipe-Sesé, M.; Eliche-Quesada, D.; Corpas-Iglesias, F.A. The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceram. Int. 2011, 37, 3019–3028. [Google Scholar] [CrossRef]
- Li, H.Y.; Tan, Y.Q.; Zhang, L.; Zhang, Y.X.; Song, Y.H.; Ye, Y.; Xia, M.S. Bio-filler from waste shellfish shell: Preparation, characterization, and its effect on the mechanical properties on polypropylene composites. J. Hazard. Mater. 2012, 217, 256–262. [Google Scholar] [CrossRef]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Das, K.C.; Minkara, M.Y.; Melear, N.D.; Tollner, E.W. Effect of poultry litter amendment on hatchery waste composting. J. Appl. Poult. Res. 2002, 11, 282–290. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdul, H.Z.A. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Sawai, J. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J. Eggshells break into collagen market. New Sci. 1999, 161, 6. [Google Scholar]
- Shellbrane Project. Co-Funded by the European Commission through the Seventh Framework Programme (FP7) through the Funding Scheme “Research for the Benefit of SME-s” under Grant Agreement No. 286910. 2012. Available online: https://cordis.europa.eu/error500 (accessed on 3 February 2020).
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for world population and global food availability for global health. In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, MA, USA, 2019; pp. 3–24. [Google Scholar] [CrossRef]
- Sonenklar, C. Famous for egg waste. In Research/Penn State News; Penn State University: State College, PA, USA, 1999; Volume 20, pp. 1–2. Available online: https://news.psu.edu/story/140891/1999/09/01/research/famous-egg-waste (accessed on 15 March 2020).
- Hseih, J. Researchers develop process to recover eggshell waste for alternative uses. Poult. Tech. 2007, 19, 1. [Google Scholar]
- Morris, J.P.; Wang, Y.; Backeljau, T.; Chapelle, G. Biomimetic and bio-inspired uses of mollusc shells. Mar. Genom. 2016, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Fenaux, M.; Nuez, M.L.; Molero, A.; Aguirre, A. Short-term effects of ovomet®, eggshell membrane. Joint pain: A double-blind and placebo study. J. Osteopor. Phys. Act. 2018, 6, 2. [Google Scholar] [CrossRef]
- Shearman, S. Scotch egg company claims to have cracked problem of eggshell waste. Guard 2016, 1. Available online: https://www.theguardian.com/sustainable-business/2016/jun/30/scotch-egg-company-cracked-eggshell-waste-problem-recycling-plastic (accessed on 19 March 2020).
- Gettens, R.J.; FitzHugh, E.W.; Feller, R.L. Calcium carbonate whites. Stud. Conserv. 1974, 19, 157–184. [Google Scholar] [CrossRef]
- Cölfen, H. Precipitation of carbonates: Recent progress in controlled production of complex shapes. Curr. Opin. Colloid Interface Sci. 2003, 8, 23–31. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; ISBN 0-07-049439-8. [Google Scholar]
- Periasamy, K.; Mohankumar, G.C. Sea coral-derived cuttlebone reinforced epoxy composites: Characterization and tensile properties evaluation with mathematical models. J. Compos. Mater. 2016, 50, 807–823. [Google Scholar] [CrossRef]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Tyler, C.; Simkiss, K.A. Study of the Egg Shells of Ratite Birds. In Proceedings of the Zoological Society of London, Regent’s Park, London, UK, 8 December 1959; Blackwell Publishing Ltd.: Oxford, UK, 1959; Volume 133, pp. 201–243. [Google Scholar] [CrossRef]
- DSM Nutritional Products Ltd. A Practical Guide to the Efficient Evaluation of Egg Quality at Farm Level in: DSM Egg Quality Manual; DSM Nutritional Products Ltd.: Basel, Switzerland, 2018; pp. 1–16. [Google Scholar]
- John-Jaja, S.A.; Udoh, U.H.; Nwokolo, S.C. Repeatability estimates of egg weight and egg-shell weight under various production periods for bovan nera black laying chicken. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 389–394. [Google Scholar] [CrossRef]
- Stadelman, W.J. Eggs and Egg Products. In Encyclopedia of Food Science and Technology; Francis, F.J., Ed.; Wiley: New York, NY, USA, 2000; pp. 593–599. [Google Scholar]
- Mittal, A.; Teotia, M.; Soni, R.K.; Mittal, J. Applications of egg shell and egg shell membrane as adsorbents: A review. J. Mol. Liq. 2016, 223, 376–387. [Google Scholar] [CrossRef]
- Stadelman, W.J.; Cotterill, O. Egg Science and Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 1995; ISBN 9781560228554. [Google Scholar]
- Solomon, S.E. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cree, D.; Rutter, A. Sustainable bio-Inspired limestone eggshell powder for potential industrialized applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Betancourt, N.; Cree, D. Mechanical properties of poly (lactic acid) composites reinforced with CaCO3 eggshell based fillers. Mater. Res. Soc. 2017, 3, 6–11. [Google Scholar] [CrossRef]
- Parsons, A.H. Structure of the eggshell. Poult. Sci. 1982, 61, 2013–2021. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Cree, D.; Pliya, P. Effect of elevated temperature on eggshell, eggshell powder and eggshell powder mortars for masonry applications. J. Build. Eng. 2019, 100852. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Koga, N. Thermal decomposition of biomineralized calcium carbonate: Correlation between the thermal behavior and structural characteristics of avian eggshell. ACS Sustain. Chem. Eng. 2018, 6, 5283–5295. [Google Scholar] [CrossRef]
- Intharapat, P.; Kongnoo, A.; Kateungngan, K. The potential of chicken eggshell waste as a bio-filler filled epoxidized natural rubber (ENR) composite and its properties. J. Polym. Environ. 2013, 21, 245–258. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Thermal, mechanical and physical properties of nano egg shell particle-filled epoxy nanocomposites. J. Compos. Mater. 2018, 52, 3989–4000. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Siddharthan, A.; Seshadri, S.K.; Kumar, T.S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 2007, 18, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Pliya, P.; Cree, D. Limestone derived eggshell powder as a replacement in portland cement mortar. Constr. Build. Mater. 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a biocomposite based on green polyethylene biopolymer and eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Beck, K.; Brunetaud, X.; Mertz, J.D.; Al-Mukhtar, M. On the use of eggshell lime and tuffeau powder to formulate an appropriate mortar for restoration purposes. Geol. Soc. Lond. Spec. Publ. 2010, 331, 137–145. [Google Scholar] [CrossRef]
- Okonkwo, U.N.; Odiong, I.C.; Akpabio, E.E. The effects of eggshell ash on strength properties of cement-stabilized lateritic. Int. J. Sustain. Constr. Eng. Technol. 2012, 3, 18–25. [Google Scholar]
- Koudele, J.W.; Edwin, C.H. The egg products industry of the United States: Part I-historical highlights, 1900–1959. Kans. Agric. Expt. Stn. Bull. 1960, 423, 1–46. [Google Scholar]
- Wu, J. Eggs and Egg Products Processing. In Food Processing: Principles and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 437–455. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Corporate Statistical Database. 2017. Available online: http://www.fao.org/faostat/en/#data/CL (accessed on 6 May 2020).
- Yao, Z.T.; Chen, T.; Li, H.Y.; Xia, M.S.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with modified shell waste. J. Hazard. Mater. 2013, 262, 212–217. [Google Scholar] [CrossRef]
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Duquesne, S.; Darwish, N.A. Characterization of bio-filler derived from seashell wastes and its effect on the mechanical, thermal, and flame retardant properties of ABS composites. Polym. Compos. 2017, 38, 2788–2797. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Li, H.; Chen, T.; Ye, Y.; Zheng, H. Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2502–2530. [Google Scholar] [CrossRef]
- Jung, J.H.; Shon, B.H.; Yoo, K.S.; Oh, K.J. Physicochemical characteristics of waste sea shells for acid gas cleaning absorbent. Korean J. Chem. Eng. 2000, 17, 585–592. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Olivia, M.; Oktaviani, R. Properties of concrete containing ground waste cockle and clam seashells. Procedia Eng. 2017, 171, 658–663. [Google Scholar] [CrossRef]
- Zhong, B.Y.; Zhou, Q.; Chan, C.F.; Yu, Y. Structure and property characterization of oyster shell cementing material. Jiegou Huaxue 2012, 31, 85–92. [Google Scholar]
- Tudor, H.E.; Gryte, C.C.; Harris, C.C. Seashells: Detoxifying agents for metal-contaminated waters. Water Air Soil Pollut. 2006, 173, 209–242. [Google Scholar] [CrossRef]
- Mohamed, M.; Yusup, S.; Maitra, S. Decomposition study of calcium carbonate in cockle shell. J. Eng. Sci. Technol. 2012, 7, 1–10. [Google Scholar]
- Olivia, M.; Mifshella, A.A.; Darmayanti, L. Mechanical properties of seashell concrete. Procedia Eng. 2015, 125, 760–764. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Martínez-Abella, F.; Carro-López, D. Performance of mussel shell as aggregate in plain concrete. Constr. Build. Mater. 2017, 139, 570–583. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Lee, K.; Park, J. Oyster shell as substitute for aggregate in mortar. Waste Manag. Res. 2004, 22, 158–170. [Google Scholar] [CrossRef]
- Safi, B.; Saidi, M.; Daoui, A.; Bellal, A.; Mechekak, A.; Toumi, K. The use of seashells as a fine aggregate (by sand substitution) in self-compacting mortar (SCM). Constr. Build. Mater. 2015, 78, 430–438. [Google Scholar] [CrossRef]
- Yang, E.I.; Yi, S.T.; Leem, Y.M. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Fombuena, V.; Bernardi, L.; Fenollar, O.; Boronat, T.; Balart, R. Characterization of green composites from biobased epoxy matrices and bio-fillers derived from seashell a wastes. Mater. Des. 2014, 57, 168–174. [Google Scholar] [CrossRef]
- De Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using life cycle assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H. Shell weight as an independent variable in relation to cadmium content of mollusks. Mar. Ecol. Prog. Ser. Oldendorf. 1983, 12, 59–75. [Google Scholar] [CrossRef]
- Palmer, A.R. Calcification in marine molluscs: How costly is it? Proc. Natl. Acad. Sci. USA 1992, 89, 1379–1382. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Bayne, B.L. Seasonal changes in the physiology, reproductive condition and carbohydrate content of the cockle cardium (=cerastoderma) edule (bivalvia: Cardiidae). Mar. Biol. 1980, 56, 11–19. [Google Scholar] [CrossRef]
- Kleinman, S.; Hatcher, B.G.; Scheibling, R.E.; Taylor, L.H.; Hennigar, A.W. Shell and tissue growth of juvenile sea scallops (placopecten magellanicus) in suspended and bottom culture in lunenburg bay, nova scotia. Aquaculture 1996, 142, 75–97. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Fishery and Aquaculture Statistics. 2018. Available online: http://faostat.fao.org (accessed on 6 May 2020).
- Rohatgi, A. WebPlotDigitalizer: HTML5 based Online Tool to Extract Numerical Data from Plot Images. Version 4.1. Available online: https://automeris.io/WebPlotDigitizer (accessed on 10 February 2020).
- Toro, P.; Quijada, R.; Yazdani-Pedram, M.; Arias, J.L. Eggshell, a new bio-filler for polypropylene composites. Mater. Lett. 2007, 61, 4347–4350. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Z.; Mai, K. Preparation and properties of eggshell/β-polypropylene bio-composites. J. Appl. Polym. Sci. 2012, 125, 61–66. [Google Scholar] [CrossRef]
- Ghabeer, T.; Dweiri, R.; Al-Khateeb, S. Thermal and mechanical characterization of polypropylene/eggshell biocomposites. J. Reinf. Plast. Compos. 2013, 32, 402–409. [Google Scholar] [CrossRef]
- Kumar, R.; Dhaliwal, J.S.; Kapur, G.S. Mechanical properties of modified biofiller-polypropylene composites. Polym. Compos. 2014, 35, 708–714. [Google Scholar] [CrossRef]
- Hassen, A.A.; Dizbay-Onat, M.; Bansal, D.; Bayush, T.; Vaidya, U. Utilization of chicken eggshell waste as a bio-filler for thermoplastic polymers: Thermal and mechanical characterization of polypropylene filled with naturally derived CaCO3. Polym. Polym. Compos. 2015, 23, 653–662. [Google Scholar] [CrossRef]
- Feng, Y.; Ashok, B.; Madhukar, K.; Zhang, J.; Zhang, J.; Reddy, K.O.; Rajulu, A.V. Preparation and characterization of polypropylene carbonate bio-filler (eggshell powder) composite films. Int. J. Polym. Anal. Charact. 2014, 19, 637–647. [Google Scholar] [CrossRef]
- Murugan, S.; Munusamy, Y.; Ismail, H. Effects of chicken eggshell filler size on the processing, mechanical and thermal properties of PVC matrix composite. Plast. Rubber Compos. 2017, 46, 42–51. [Google Scholar] [CrossRef]
- Shuhadah, S.; Supri, A.G. LDPE-isophthalic acid modified egg shell powder composites (LDPE/ESPI). J. Phys. Sci. 2009, 20, 87–98. [Google Scholar]
- Murugan, S.; Munusamy, Y.; Muniandy, M.; Ismail, H. Development of HDPE-modified eggshell composite. Polym. Compos. 2018, 39, 1630–1637. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Lim, W.S.; Hanafi, I. Sustainable use of eggshell powder in the composite based on recycled polystyrene and virgin polystyrene mixture. Int. J. Polym. Anal. Charact. 2019, 24, 266–275. [Google Scholar] [CrossRef]
- Ashok, B.; Naresh, S.; Reddy, K.O.; Madhukar, K.; Cai, J.; Zhang, L.; Rajulu, A.V. Tensile and thermal properties of poly (lactic acid)/eggshell powder composite films. Int. J. Polym. Anal. Charact. 2014, 19, 245–255. [Google Scholar] [CrossRef]
- Betancourt, N.; Cree, D. Investigation on the properties of brown eggshell powder filled poly (lactic acid) composites. In Proceedings of the 2017 Canadian International Conference on Composites (CANCOM), Ottawa, ON, Canada, 17–20 July 2017; pp. 1–8. [Google Scholar]
- Essabir, H.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; El Kacem Qaiss, A. A comparison between bio-and mineral calcium carbonate on the properties of polypropylene composites. Constr. Build. Mater. 2017, 134, 549–555. [Google Scholar] [CrossRef]
- Munusamy, Y.; Sumathi, S.; Chi, H.C. Potential use of waste cockle shell as filler for thermoplastic composite. J. Mater. Cycles Waste Manag. 2019, 21, 1063–1074. [Google Scholar] [CrossRef]
- Ji, G.; Zhu, H.; Qi, C.; Zeng, M. Mechanism of interactions of eggshell microparticles with epoxy resins. Polym. Eng. Sci. 2009, 49, 1383–1388. [Google Scholar] [CrossRef]
- Tiimob, B.J.; Jeelani, S.; Rangari, V.K. Eggshell reinforced biocomposite-an advanced “green” alternative structural material. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B.; Awogbemi, O. Studies on the mechanical and absorption properties of achatina fulica snail and eggshells reinforced composite materials. Compos. Struct. 2020, 239, 112043. [Google Scholar] [CrossRef]
- Azman, N.A.N.; Islam, M.R.; Parimalam, M.; Rashidi, N.M.; Mupit, M. Mechanical, structural, thermal and morphological properties of epoxy composites filled with chicken eggshell and inorganic CaCO3 particles. Polym. Bull. 2020, 77, 805–821. [Google Scholar] [CrossRef]
- Rahman, G.S.; Aftab, H.; Islam, M.S.; Mukhlish, M.Z.B.; Ali, F. Enhanced physico-mechanical properties of polyester resin film using CaCO3 filler. Fibers Polym. 2016, 17, 59–65. [Google Scholar] [CrossRef]
- Osman, M.A.; Ayman, A.; Ulrich, W.S. Influence of excessive filler coating on the tensile properties of LDPE–calcium carbonate composites. Polymer 2004, 45, 1177–1183. [Google Scholar] [CrossRef]
- Osman, M.A.; Ulrich, W.S. Surface treatment of calcite with fatty acids: Structure and properties of the organic monolayer. Chem. Mater. 2002, 14, 4408–4415. [Google Scholar] [CrossRef]
- Lin, Y.; Haibin, C.; Chi, M.C.; Jingshen, W. High impact toughness polypropylene/CaCO3 nanocomposites and the toughening mechanism. Macromolecules 2008, 41, 9204–9213. [Google Scholar] [CrossRef]
- Shui, M. Polymer surface modification and characterization of particulate calcium carbonate fillers. Appl. Surf. Sci. 2003, 220, 359–366. [Google Scholar] [CrossRef]
- Liu, Z.; Gilbert, M. Structure and properties of talc-filled polypropylene: Effect of phosphate coating. J. Appl. Polym. Sci. 1996, 59, 1087–1098. [Google Scholar] [CrossRef]
- Tabtiang, A.; Venables, R. The performance of selected unsaturated coatings for calcium carbonate filler in polypropylene. Eur. Polym. J. 2000, 36, 137–148. [Google Scholar] [CrossRef]
- Zoltán, D.; Pukánszky, B.; Nagy, J. Evaluation of interfacial interaction in polypropylene/surface treated CaCO3 composites. Compos. Part A Appl. Sci. Manuf. 1998, 29, 323–329. [Google Scholar] [CrossRef]
- Guo, Z.X.; Jian, Y. Surface modification of CaCO3 with dendritic carboxylic acids. Chin. J. Polym. Sci. 2002, 20, 231–235. [Google Scholar]
- Doufnoune, R.; Chebira, F.; Haddaoui, N. Effect of titanate coupling agent on the mechanical properties of calcium carbonate filled polypropylene. Int. J. Polym. Mater. 2003, 52, 967–984. [Google Scholar] [CrossRef]
- Doufnoune, R.; Haddaoui, N.; Riahi, F. The Interactions of silane and zirconate coupling agents with calcium carbonate. Int. J. Polym. Mater. 2007, 56, 227–246. [Google Scholar] [CrossRef]
- Threepopnatkul, P.; Amnard, S.; Kanpurassakorn, A.; Onanong, M.; Alongkorn, K.; Chanin, K.; Hesham, M. Preparation and properties of polylactide reinforced with eggshell modified with different fatty acids. Key Eng. Mater. 2019, 824, 16–22. [Google Scholar] [CrossRef]
- Abdul Wahab, N.M.; Md Hanafiah, M.A.; Samsudin, D.; Mustafa, M.S.; Ahmad, Z.; Syed Ismail, S.N. Mechanical properties of silane-treated eggshell powder on unsaturated polyester composite. In Charting the Sustainable Future of ASEAN in Science and Technology; Alias, N., Yusof, R., Eds.; Springer: Singapore, 2020; pp. 435–445. [Google Scholar]
- Shah, A.H.; Zhang, Y.; Xu, X.; Dayo, A.Q.; Li, X.; Wang, S.; Liu, W. Reinforcement of stearic acid treated egg shell particles in epoxy thermosets: Structural, thermal, and mechanical characterization. Materials 2018, 11, 1872. [Google Scholar] [CrossRef]
- Villarreal-Lucio, D.S.; Rivera-Armenta, J.L.; Martínez-Hernández, A.L.; Estrada-Moreno, I.A. Effect of eggshell particle size in thermal and thermomechanical properties of pp/eggshell composites. Int. J. Eng. Sci. Res. Technol. 2018, 7, 82–88. [Google Scholar]
- Supri, A.G.; Ismail, H.; Shuhadah, S. Effect of polyethylene-grafted maleic anhydride (PE-g-MAH) on properties of low density polyethylene/eggshell powder (LDPE/ESP) composites. Polym. Plast. Technol. Eng. 2010, 49, 347–353. [Google Scholar] [CrossRef]
- Iyer, K.A.; Torkelson, J.M. Green composites of polypropylene and eggshell: Effective biofiller size reduction and dispersion by single-step processing with solid-state shear pulverization. Compos. Sci. Technol. 2014, 102, 152–160. [Google Scholar] [CrossRef]
- Rajan, B.S.; Balaji, M.A.S.; Noorani, A.B.M.A. Effect of silane surface treatment on the physico-mechanical properties of shell powder reinforced epoxy modified phenolic friction composite. Mater. Res. Express 2019, 6, 065315. [Google Scholar] [CrossRef]
- Ji, G.; Zhu, H.; Jiang, X.; Qi, C.; Zhang, X.M. Mechanical strengths of epoxy resin composites reinforced by calcined pearl shell powders. J. Appl. Polym. Sci. 2009, 114, 3168–3176. [Google Scholar] [CrossRef]
- Serife, Y.; Chateigner, D.; Le Pluart, L.; Gascoin, S.; Eve, S. Investigation of structural and mechanical properties of bioCaCO3-LDPE composites. Met. Mater. Int. 2019, 1, 29–43. [Google Scholar] [CrossRef]
- Melo, P.M.A.; Macêdo, O.B.; Barbosa, G.P.; Ueki, M.M.; Silva, L.B. High-density polyethylene/mollusk shell-waste composites: Effects of particle size and coupling agent on morphology, mechanical and thermal properties. J. Mater. Res. Technol. 2019, 8, 1915–1925. [Google Scholar] [CrossRef]
- Zhidan, L.; Guan, Z.; Chen, C.; Cao, L.; Wang, Y.; Gao, S.; Xu, B.; Li, W. Preparation, structures and properties of shell/polypropylene biocomposites. Thermochim. Acta 2013, 551, 149–154. [Google Scholar] [CrossRef]
- Xian, J.; He, Z.; Li, M.; Lin, Z.; Chen, J.; Yang, Q.; Xiao, L.; Li, W. Preparation and properties of coral/β-polypropylene biocomposites. J. Therm. Anal. Calorim. 2015, 122, 1005–1011. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Frontng, G.W.; Bergquist, D. Research note: Utilization of inedible eggshells and technical egg white using extrusion technology. Poult. Sci. 1990, 69, 2051–2053. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).