Hydrothermal Carbon/Carbon Nanotube Composites as Electrocatalysts for the Oxygen Reduction Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Materials Preparation
2.3. Physicochemical Characterization
2.4. Electrode Preparation
2.5. Electrochemical Measurements
3. Results and Discussion
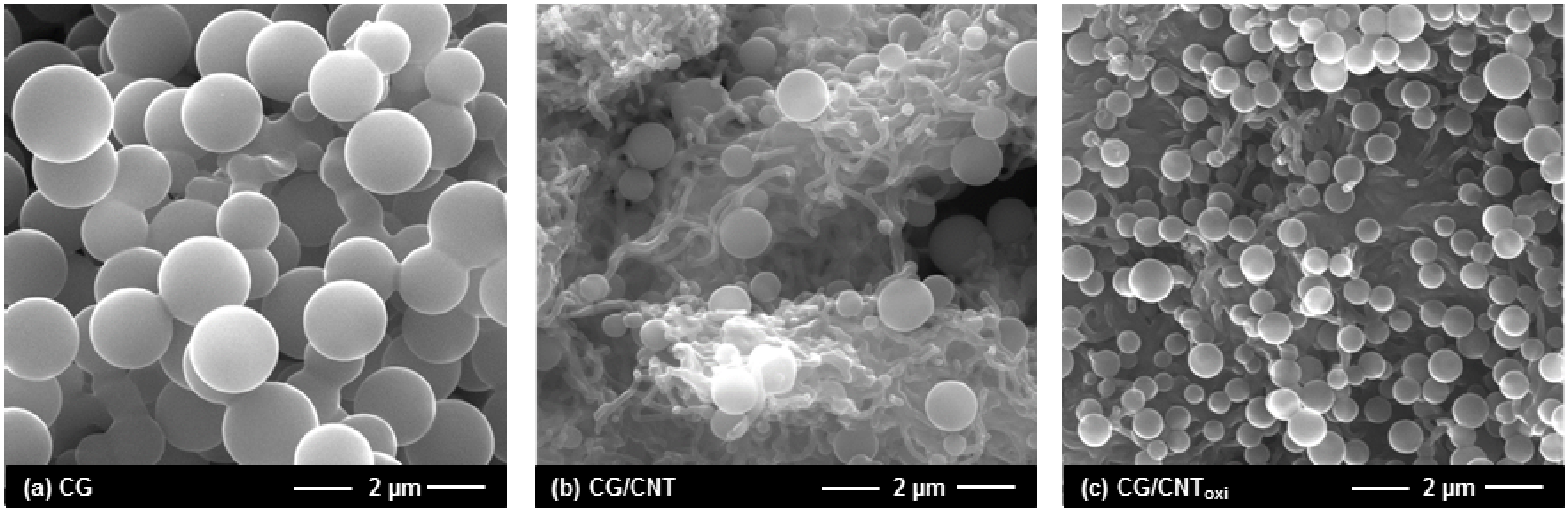
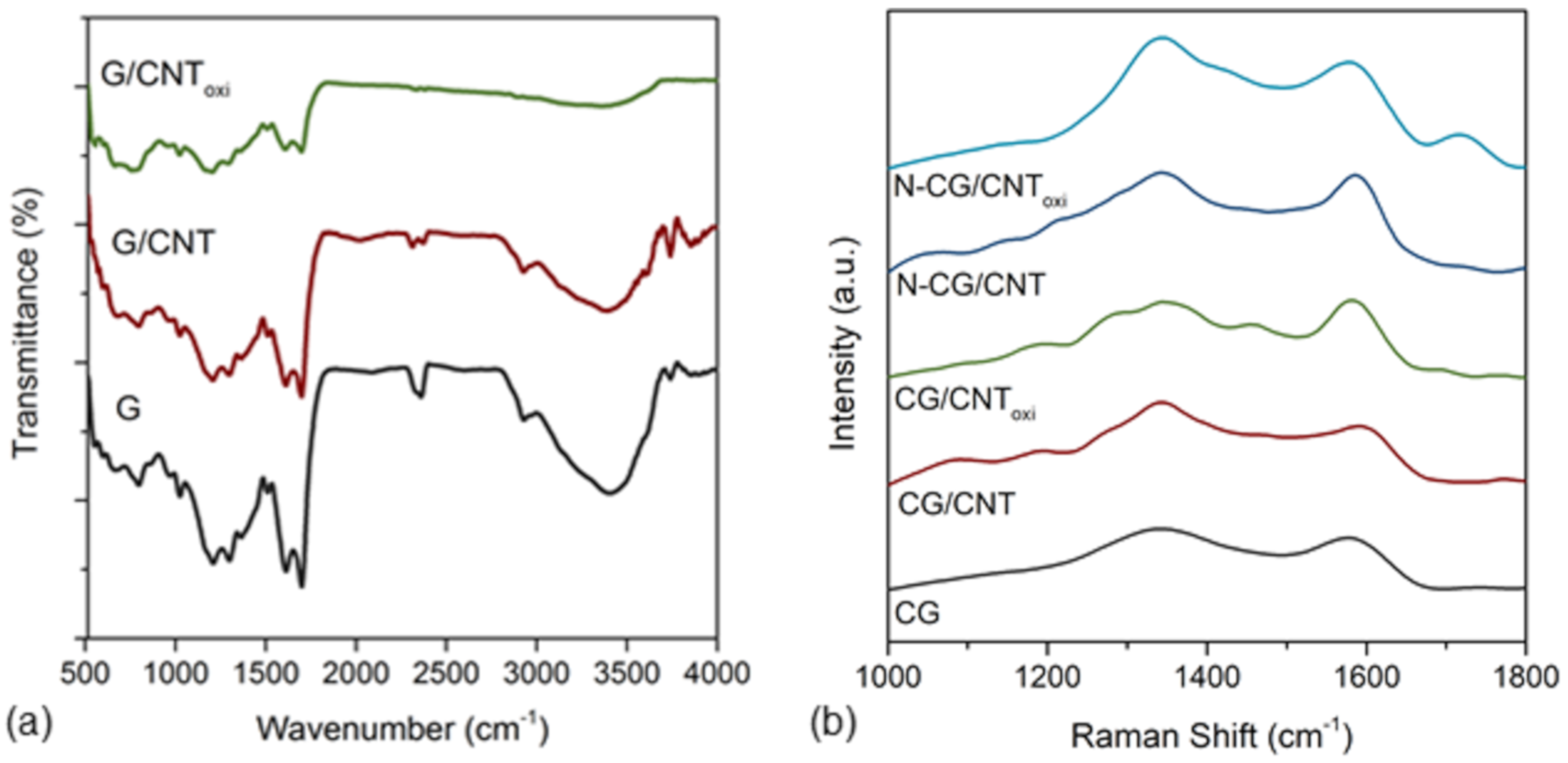
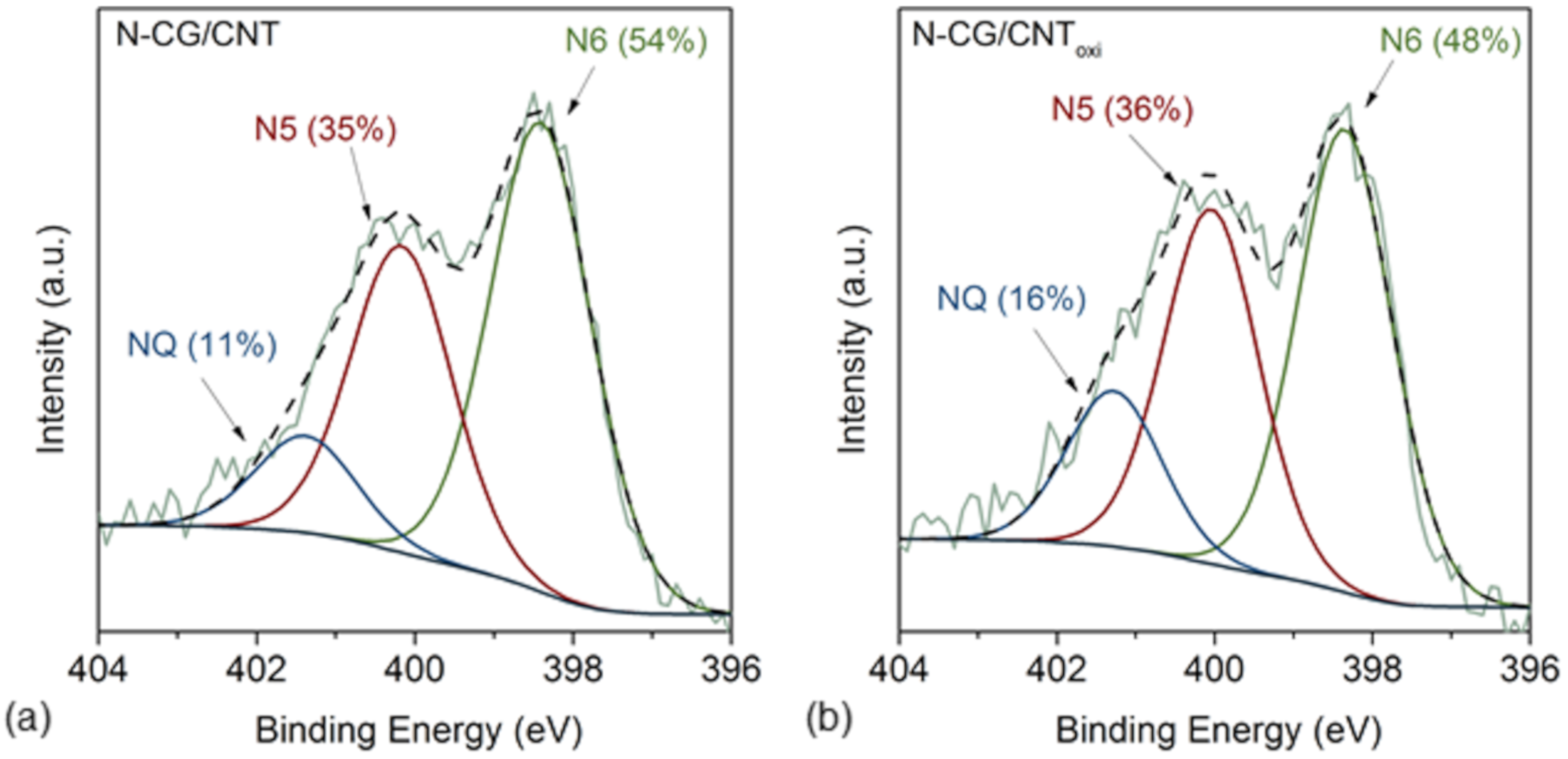
3.1. Textural, Morphological, and Chemical Properties
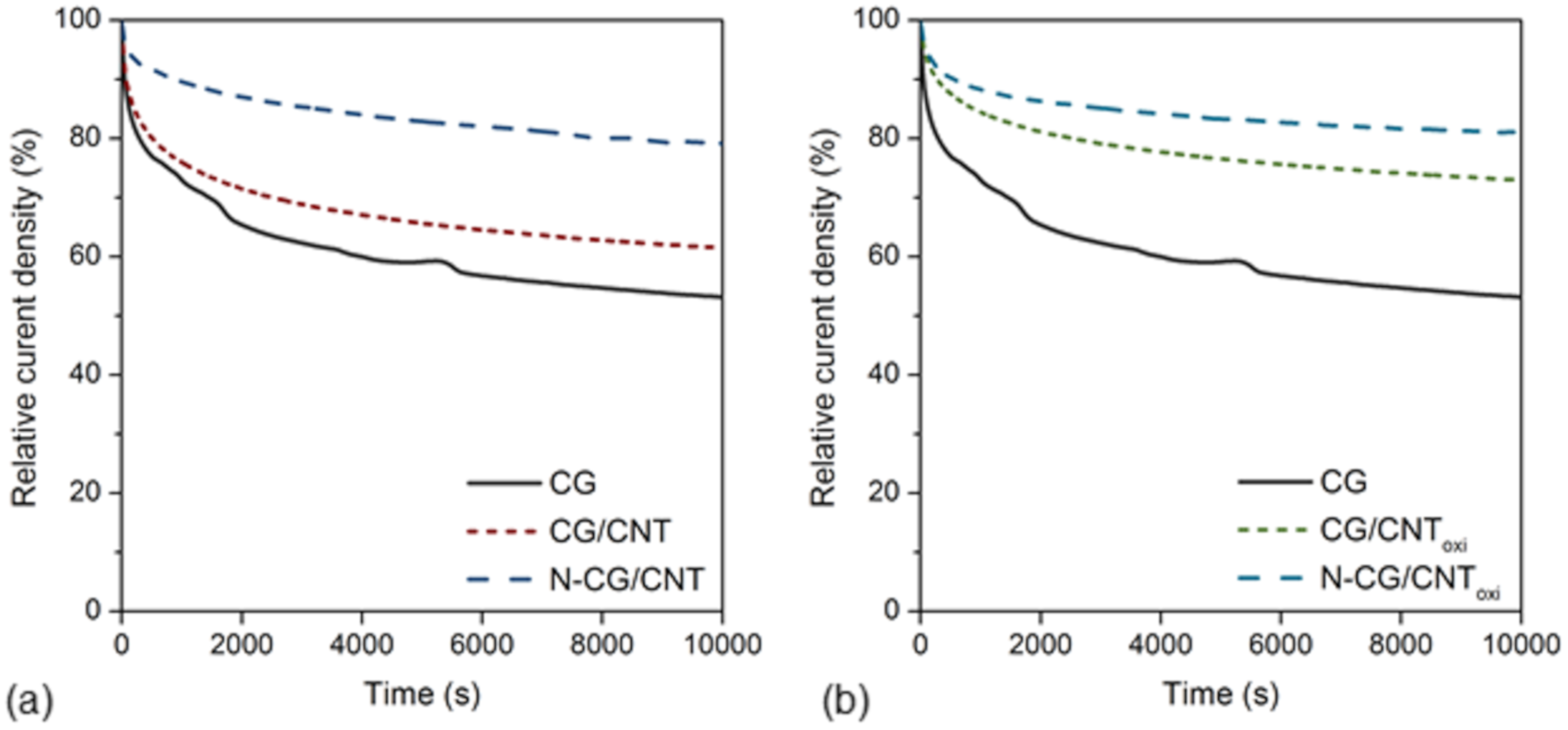
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohideen, M.M.; Liu, Y.; Ramakrishna, S. Recent progress of carbon dots and carbon nanotubes applied in oxygen reduction reaction of fuel cell for transportation. Appl. Energy 2020, 257, 114027. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Wu, Z.; Song, M.; Wang, J.; Liu, X. Recent Progress in Nitrogen-Doped Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2018, 8, 196. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.-J.; Zhang, X.; Guo, L.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Engineering self-assembled N-doped graphene–carbon nanotube composites towards efficient oxygen reduction electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13605–13609. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of oxygen reduction on heteroatom-doped nanocarbons and transition metal–nitrogen–carbon catalysts for alkaline membrane fuel cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Paul, R.; Dai, Q.; Hu, C.; Dai, L. Ten years of carbon-based metal-free electrocatalysts. Carbon Energy 2019, 1, 19–31. [Google Scholar] [CrossRef]
- Paul, R.; Du, F.; Dai, L.; Ding, Y.; Wang, Z.L.; Wei, F.; Roy, A. 3D Heteroatom-Doped Carbon Nanomaterials as Multifunctional Metal-Free Catalysts for Integrated Energy Devices. Adv. Mater. 2019, 31, 1805598. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, C.; Shi, Y.; Jing, H.; Hu, J.; Song, X.; Si, D.; Liang, S.; Hao, C. Biomass-Derived Multilayer-Graphene-Encapsulated Cobalt Nanoparticles as Efficient Electrocatalyst for Versatile Renewable Energy Applications. ACS Sustain. Chem. Eng. 2019, 7, 1137–1145. [Google Scholar] [CrossRef]
- Zheng, Y.; He, F.; Wu, J.; Ma, D.; Fan, H.; Zhu, S.; Li, X.; Lu, Y.; Liu, Q.; Hu, X. Nitrogen-Doped Carbon Nanotube-Graphene Frameworks with Encapsulated Fe/Fe3N Nanoparticles as Catalysts for Oxygen Reduction. ACS Appl. Nano Mater. 2019, 2, 3538–3547. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Zhang, S.; Lv, Z.; Yang, D.; Liu, J.; Chen, Y.; Tian, X.; Jin, H.; Song, W. Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 5740–5745. [Google Scholar] [CrossRef]
- Wütscher, A.; Eckhard, T.; Hiltrop, D.; Lotz, K.; Schuhmann, W.; Andronescu, C.; Muhler, M. Nitrogen-Doped Metal-Free Carbon Materials Derived from Cellulose as Electrocatalysts for the Oxygen Reduction Reaction. ChemElectroChem 2019, 6, 514–521. [Google Scholar] [CrossRef]
- Huang, B.; Xia, M.; Qiu, J.; Xie, Z. Biomass Derived Graphene-Like Carbons for Electrocatalytic Oxygen Reduction Reaction. ChemNanoMat 2019, 5, 682–689. [Google Scholar] [CrossRef]
- Gu, D.; Zhou, Y.; Ma, R.; Wang, F.; Liu, Q.; Wang, J. Facile Synthesis of N-Doped Graphene-Like Carbon Nanoflakes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction. Nano Micro Lett. 2018, 10, 29. [Google Scholar] [CrossRef]
- Kumar, R.; da Silva, E.T.S.G.; Singh, R.K.; Savu, R.; Alaferdov, A.V.; Fonseca, L.C.; Carossi, L.C.; Singh, A.; Khandka, S.; Kar, K.K.; et al. Microwave-assisted synthesis of palladium nanoparticles intercalated nitrogen doped reduced graphene oxide and their electrocatalytic activity for direct-ethanol fuel cells. J. Colloid Interface Sci. 2018, 515, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Smith, R.L., Jr.; Kozinski, J.A.; Minowa, T.; Arai, K. Reaction of d-glucose in water at high temperatures (410 °C) and pressures (180 MPa) for the production of dyes and nano-particles. J. Supercrit. Fluids 2011, 56, 41–47. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, R.; Zhang, H.; Huang, T.; Yu, J.; Fang, H.; Liang, D.; Guo, Z. Soybean milk derived carbon intercalated with reduced graphene oxide as high efficient electrocatalysts for oxygen reduction reaction. Int. J. Hydrog. Energy 2019, 44, 21790–21802. [Google Scholar] [CrossRef]
- Shi, M.; Ma, J.; Yao, Z.; Li, Z.; Mi, H.; Xie, Y. Iron and nitrogen co-doped porous carbon derived from soybean dregs with enhanced catalytic performance for oxygen reduction. J. Electroanal. Chem. 2019, 839, 141–148. [Google Scholar] [CrossRef]
- Titirici, M.M. Hydrothermal Carbons: Synthesis, Characterization, and Applications. In Novel Carbon Adsorbents; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 351–399. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, G.; Chen, J.; Bi, L.; Dai, L.; Wen, Z. N-doped porous carbon nanosheets as pH-universal ORR electrocatalyst in various fuel cell devices. Nano Energy 2018, 49, 393–402. [Google Scholar] [CrossRef]
- Falco, C.; Marco-Lozar, J.P.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M.; Lozano-Castelló, D. Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature. Carbon 2013, 62, 346–355. [Google Scholar] [CrossRef]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Glucose-derived carbon materials with tailored properties as electrocatalysts for the oxygen reduction reaction. Beilstein J. Nanotechnol. 2019, 10, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.S.; Qiu, S.W.; Yuan, Z.Y.; Ren, T.Z.; Bandosz, T.J. Nitrogen-containing activated carbon of improved electrochemical performance derived from cotton stalks using indirect chemical activation. J. Colloid Interface Sci. 2019, 540, 285–294. [Google Scholar] [CrossRef]
- Tran, T.N.; Song, M.Y.; Kang, T.H.; Samdani, J.; Park, H.Y.; Kim, H.; Jhung, S.H.; Yu, J.S. Iron Phosphide Incorporated into Iron-Treated Heteroatoms-Doped Porous Bio-Carbon as Efficient Electrocatalyst for the Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 1944–1953. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.E.; Kim, S.; Lim, M.S.; Jin, A.; Kim, O.H.; Kim, M.J.; Lee, K.S.; Kim, J.; Kim, S.S.; et al. Biomass-Derived Air Cathode Materials: Pore-Controlled S,N-Co-doped Carbon for Fuel Cells and Metal-Air Batteries. ACS Catal. 2019, 9, 3389–3398. [Google Scholar] [CrossRef]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Quílez Bermejo, J.; Melle Franco, M.; San Fabián, E.; Morallon, E.; Cazorla-Amorós, D. Towards understanding of the active sites for ORR in N-doped carbon materials through a fine-tuning of nitrogen functionalities: An experimental and computational approach. J. Mater. Chem. A 2019, 7, 24239–24250. [Google Scholar] [CrossRef]
- Ratso, S.; Käärik, M.; Kook, M.; Paiste, P.; Aruväli, J.; Vlassov, S.; Kisand, V.; Leis, J.; Kannan, A.M.; Tammeveski, K. High performance catalysts based on Fe/N co-doped carbide-derived carbon and carbon nanotube composites for oxygen reduction reaction in acid media. Int. J. Hydrog. Energy 2019, 44, 12636–12648. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Enterría, M.; Martins, J.I.; Pereira, M.F.R.; Figueiredo, J.L. Influence of Multiwalled Carbon Nanotubes as Additives in Biomass-Derived Carbons for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2019, 11, 6066–6077. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Z.; Chai, L.; Xu, L.; Zhu, Z.; Hu, Y.; Qian, J.; Huang, S. MOF derived N-doped carbon coated CoP particle/carbon nanotube composite for efficient oxygen evolution reaction. Carbon 2019, 141, 643–651. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Iron and nitrogen co-doped carbon nanotube@hollow carbon fibers derived from plant biomass as efficient catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9658–9667. [Google Scholar] [CrossRef]
- Chung, H.T.; Won, J.H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Barroso Bogeat, A. Understanding and Tuning the Electrical Conductivity of Activated Carbon: A State-of-the-Art Review. Crit. Rev. Solid State Mater. Sci. 2019, 1–37. [Google Scholar] [CrossRef]
- Nunes, M.; Rocha, I.M.; Fernandes, D.M.; Mestre, A.S.; Moura, C.N.; Carvalho, A.P.; Pereira, M.F.R.; Freire, C. Sucrose-derived activated carbons: Electron transfer properties and application as oxygen reduction electrocatalysts. RSC Adv. 2015, 5, 102919–102931. [Google Scholar] [CrossRef]


| Sample | SBET (m2 g−1) | Vmicro (cm3 g−1) | Sext (m2 g−1) |
|---|---|---|---|
| CG | 574 | 0.22 | 15 |
| CG/CNT | 545 | 0.21 | 21 |
| CG/CNToxi | 489 | 0.20 | 37 |
| N-CG/CNT | 387 | 0.16 | 44 |
| N-CG/CNToxi | 451 | 0.19 | 57 |
| Sample | C (wt.%) | H (wt.%) | O (wt.%) | N (wt.%) | K (S m−1) |
|---|---|---|---|---|---|
| CG | 93.9 | 2.0 | 4.1 | 0.0 | 11 |
| CG/CNT | 94.3 | 2.1 | 3.6 | 0.0 | 150 |
| CG/CNToxi | 94.6 | 2.1 | 3.3 | 0.0 | 400 |
| N-CG/CNT | 85.1 | 1.7 | 6.4 | 6.8 | 280 |
| N-CG/CNToxi | 86.5 | 1.7 | 6.2 | 5.6 | 714 |
| CNT | 99.5 | 0.1 | 0.4 | 0.0 | 2500 |
| CNToxi | 95.3 | 0.9 | 3.8 | 0.0 | 1900 |
| Sample | Eonset (V) 1 | JL (mA cm−2) | H2O2 (%) 2 | Jk (mA cm−2) 3 |
|---|---|---|---|---|
| CG | 0.66 | 1.7 | 75 | 1.3 |
| CG/CNT | 0.68 | 1.8 | 67 | 2.0 |
| CG/CNToxi | 0.70 | 2.1 | 67 | 3.1 |
| N-CG/CNT | 0.73 | 2.0 | 43 | 2.3 |
| N-CG/CNToxi | 0.79 | 2.5 | 36 | 5.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, R.G.; Rey-Raap, N.; Costa, R.S.; Pereira, C.; Guedes, A.; Figueiredo, J.L.; Pereira, M.F.R. Hydrothermal Carbon/Carbon Nanotube Composites as Electrocatalysts for the Oxygen Reduction Reaction. J. Compos. Sci. 2020, 4, 20. https://doi.org/10.3390/jcs4010020
Morais RG, Rey-Raap N, Costa RS, Pereira C, Guedes A, Figueiredo JL, Pereira MFR. Hydrothermal Carbon/Carbon Nanotube Composites as Electrocatalysts for the Oxygen Reduction Reaction. Journal of Composites Science. 2020; 4(1):20. https://doi.org/10.3390/jcs4010020
Chicago/Turabian StyleMorais, Rafael G., Natalia Rey-Raap, Rui S. Costa, Clara Pereira, Alexandra Guedes, José L. Figueiredo, and M. Fernando R. Pereira. 2020. "Hydrothermal Carbon/Carbon Nanotube Composites as Electrocatalysts for the Oxygen Reduction Reaction" Journal of Composites Science 4, no. 1: 20. https://doi.org/10.3390/jcs4010020
APA StyleMorais, R. G., Rey-Raap, N., Costa, R. S., Pereira, C., Guedes, A., Figueiredo, J. L., & Pereira, M. F. R. (2020). Hydrothermal Carbon/Carbon Nanotube Composites as Electrocatalysts for the Oxygen Reduction Reaction. Journal of Composites Science, 4(1), 20. https://doi.org/10.3390/jcs4010020








