The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Material Compounding and Sample Preparation
2.3. Characterizations of Blends and Nanocomposites
2.3.1. Field Emission-Scanning Electron Microscopy (FE-SEM)
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Thermogravimetric Analysis
2.3.5. Mechanical Tests
2.3.6. Rheological Measurements
2.3.7. Electrical Properties
2.3.8. Blend Biodegradability
4. Results and Discussion
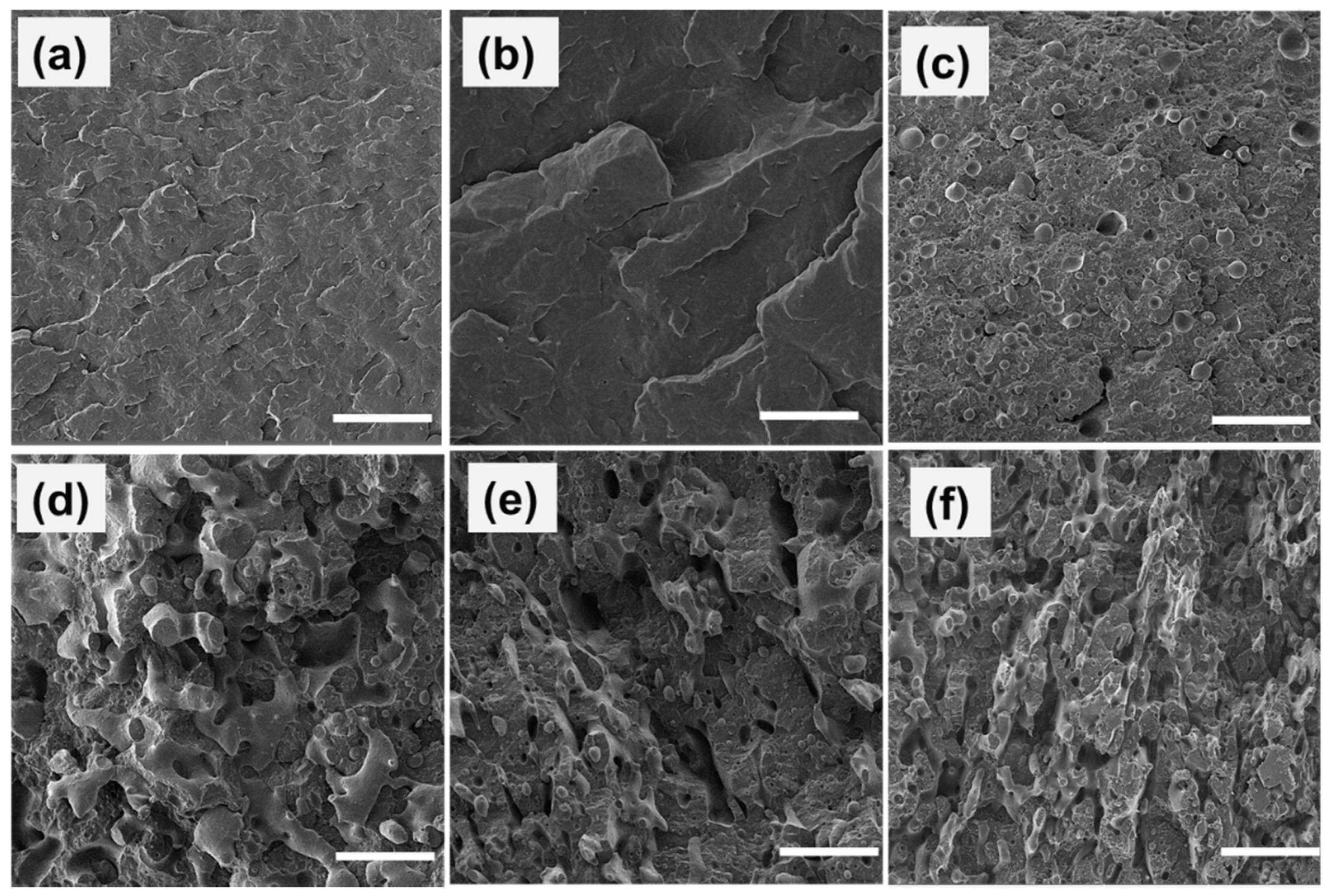
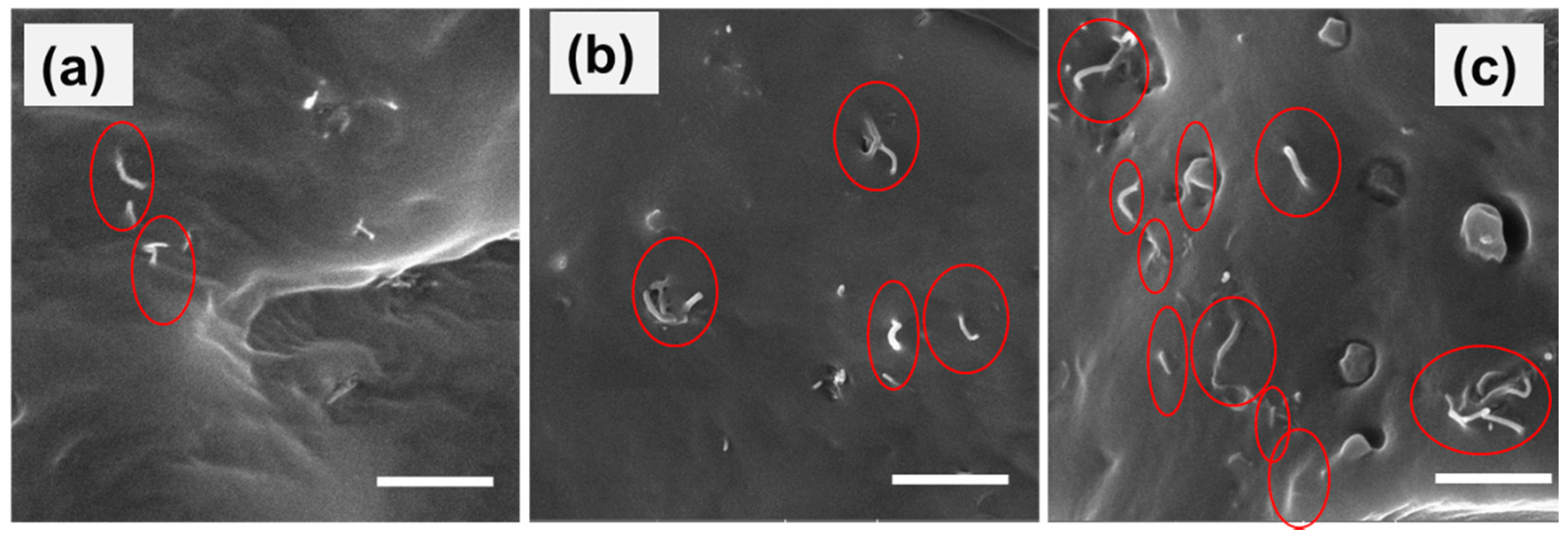
4.1. Characterization and Morphology
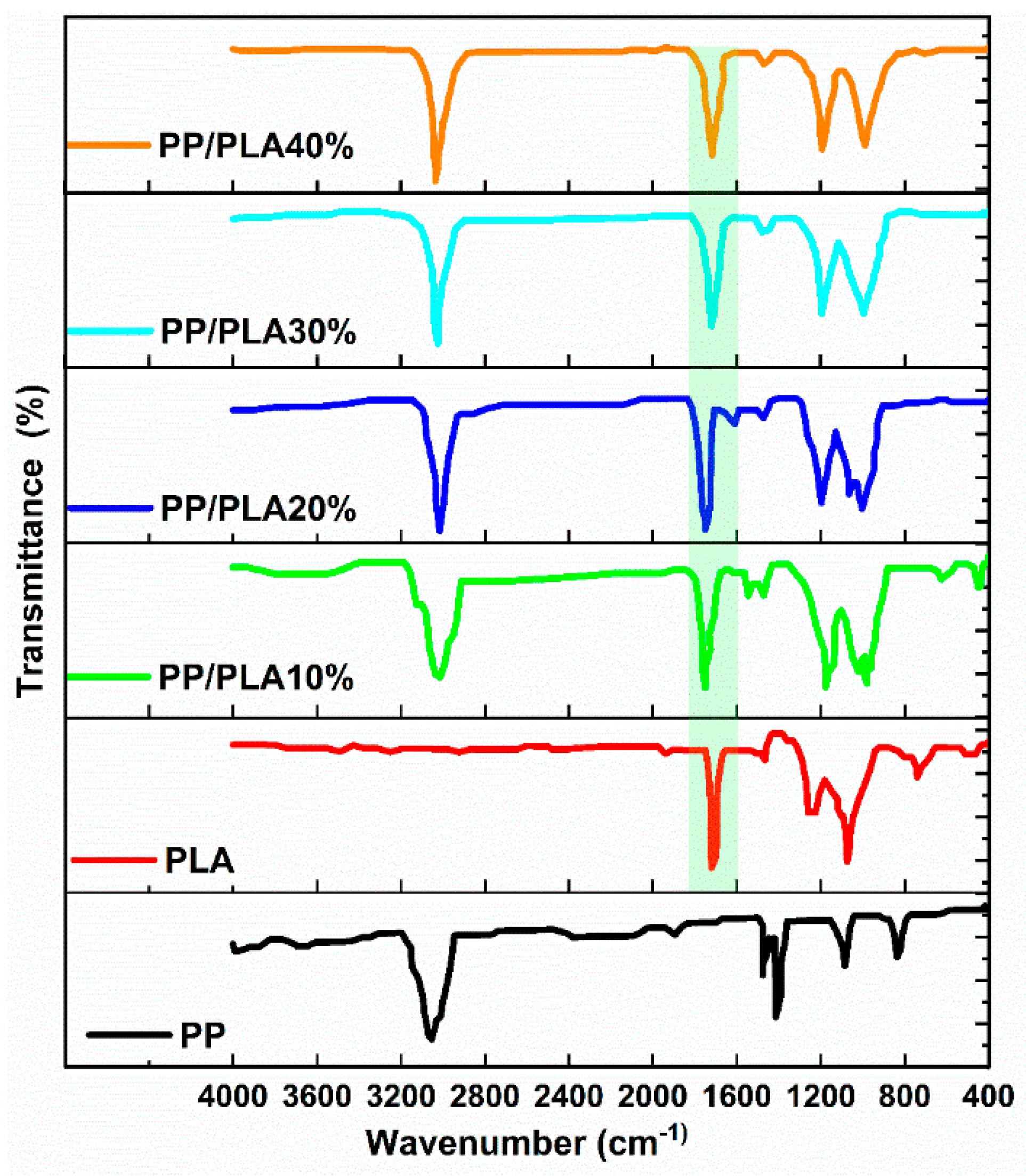
4.2. FT-IR Spectroscopy Analysis
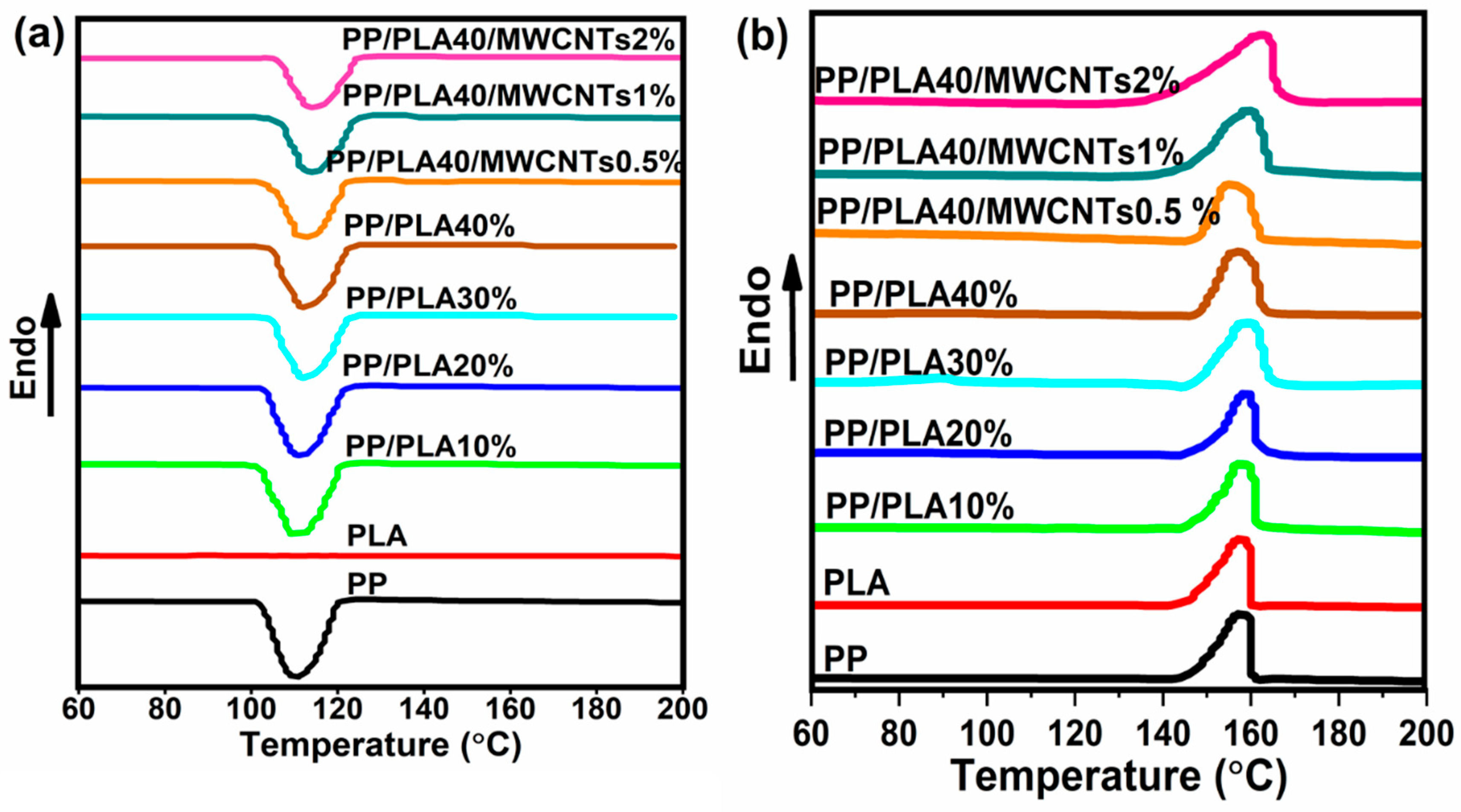
4.3. Thermal Properties
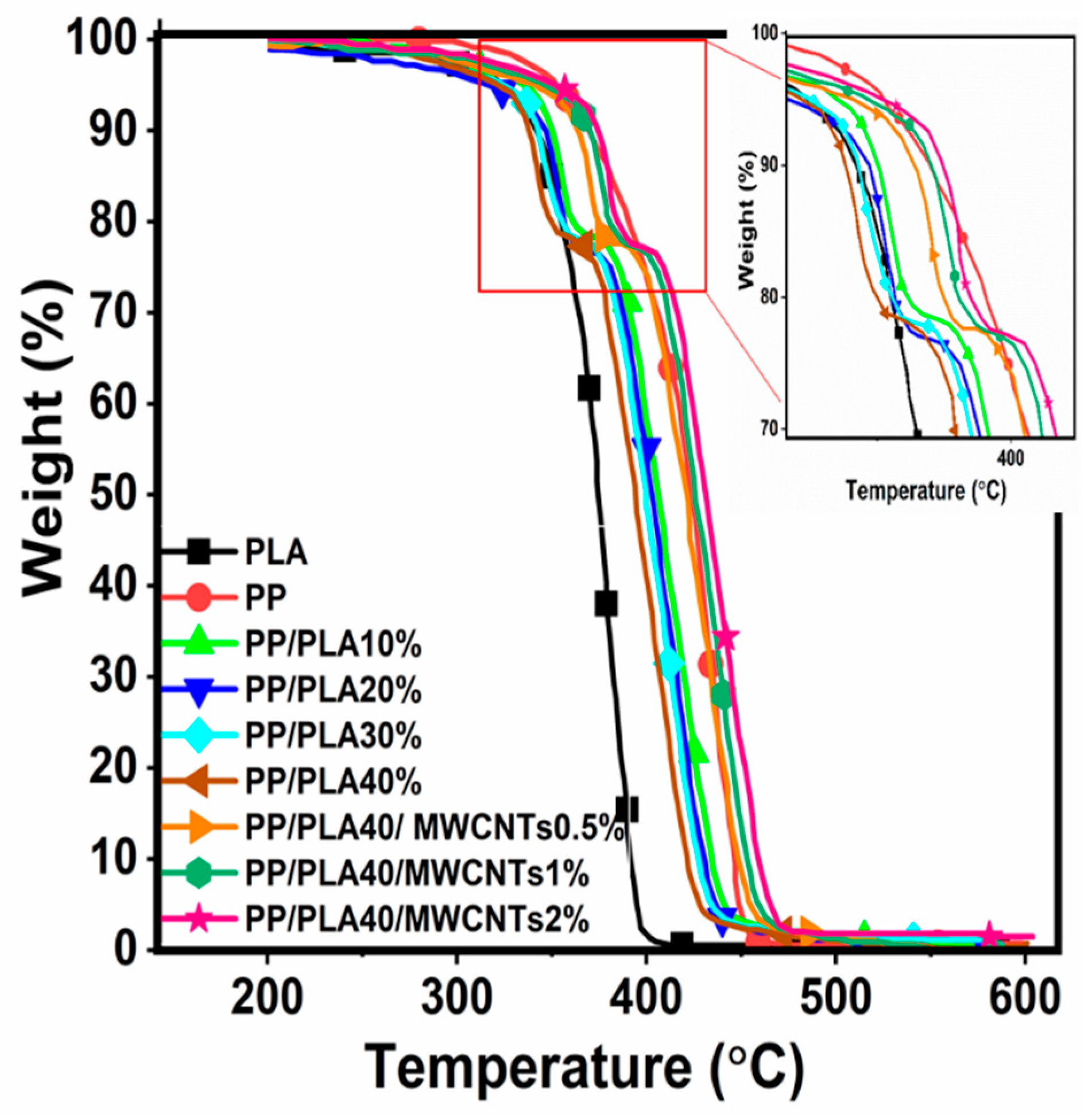
4.4. Thermogravimetric Analysis (TGA) Results
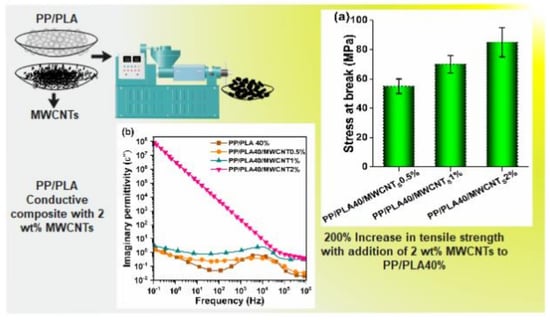
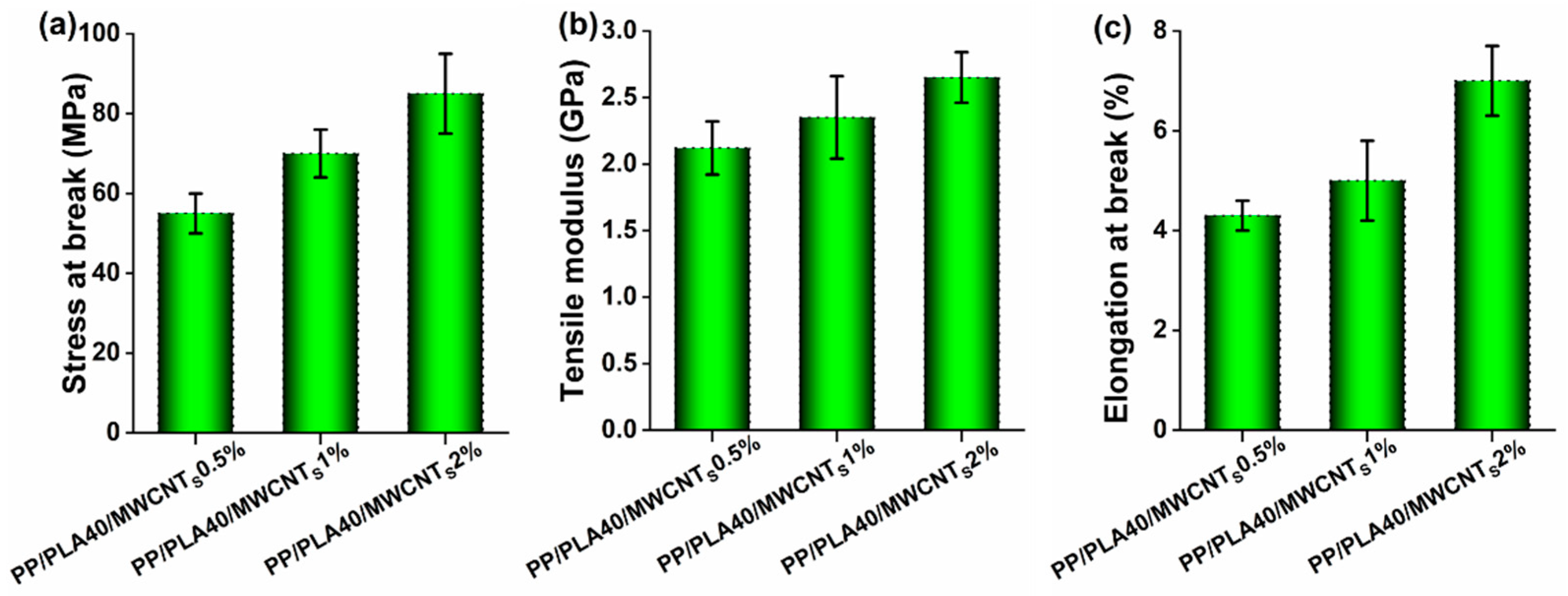
4.5. Mechanical Properties
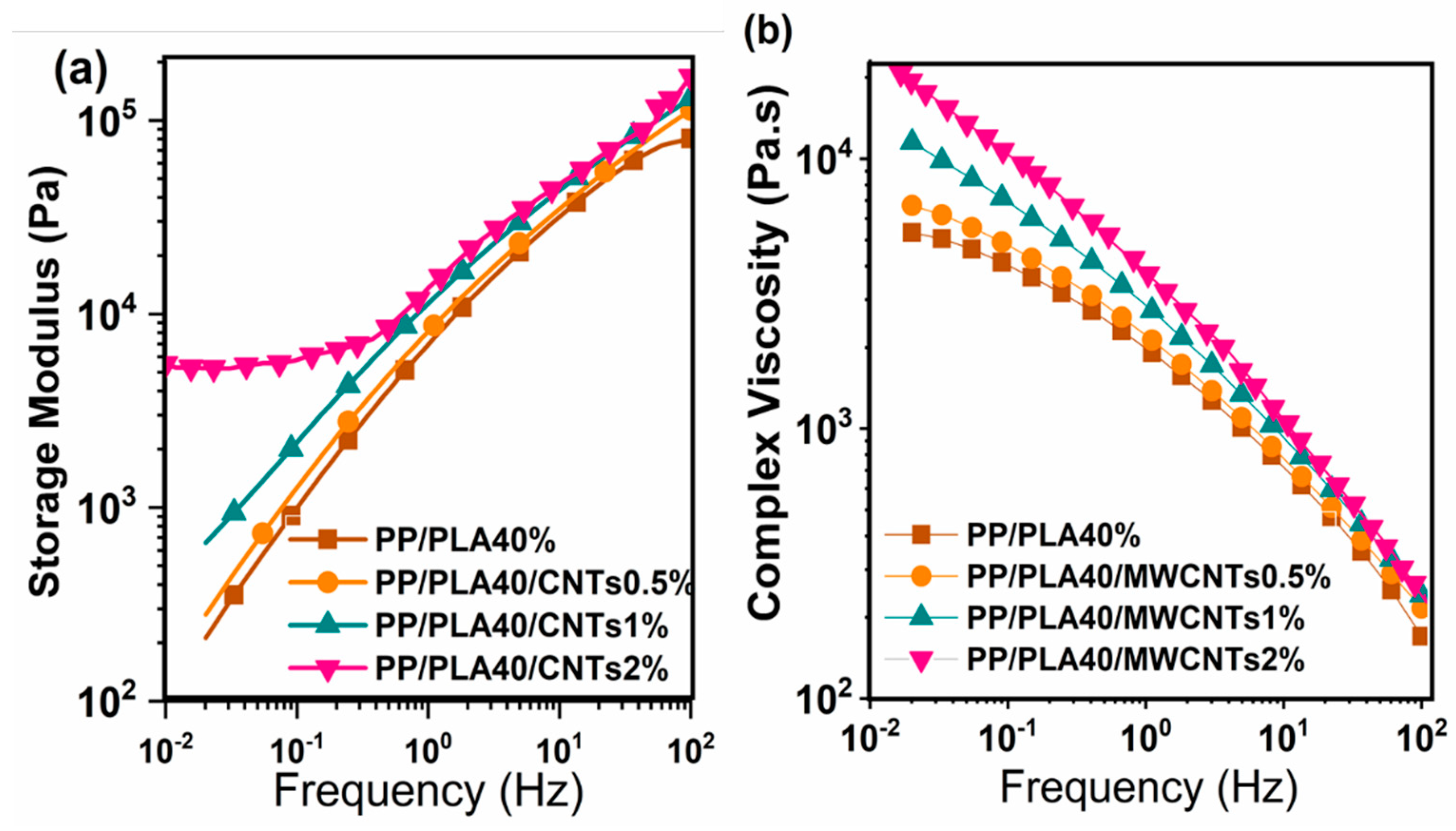
4.6. Rheology Properties
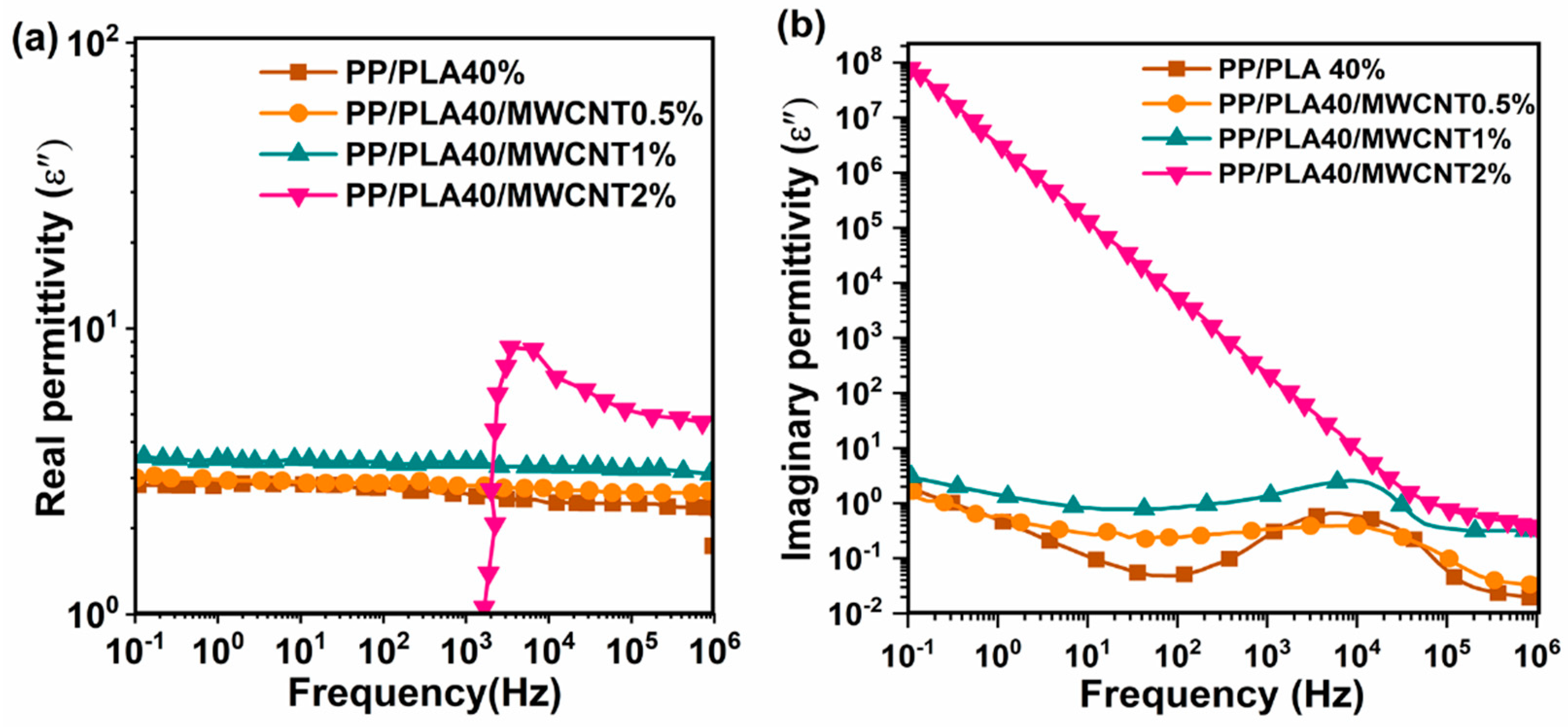
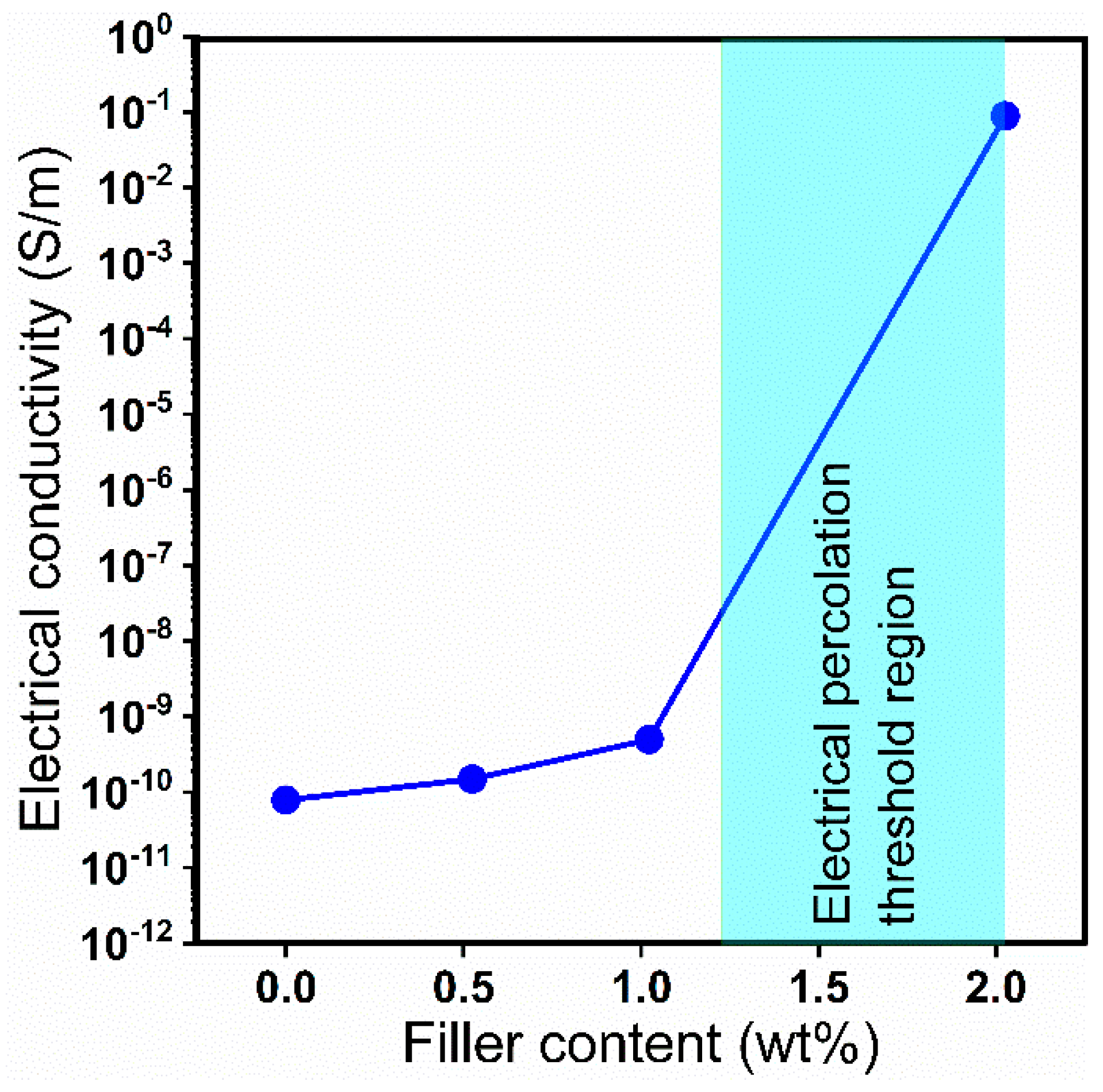
4.7. Electrical Properties
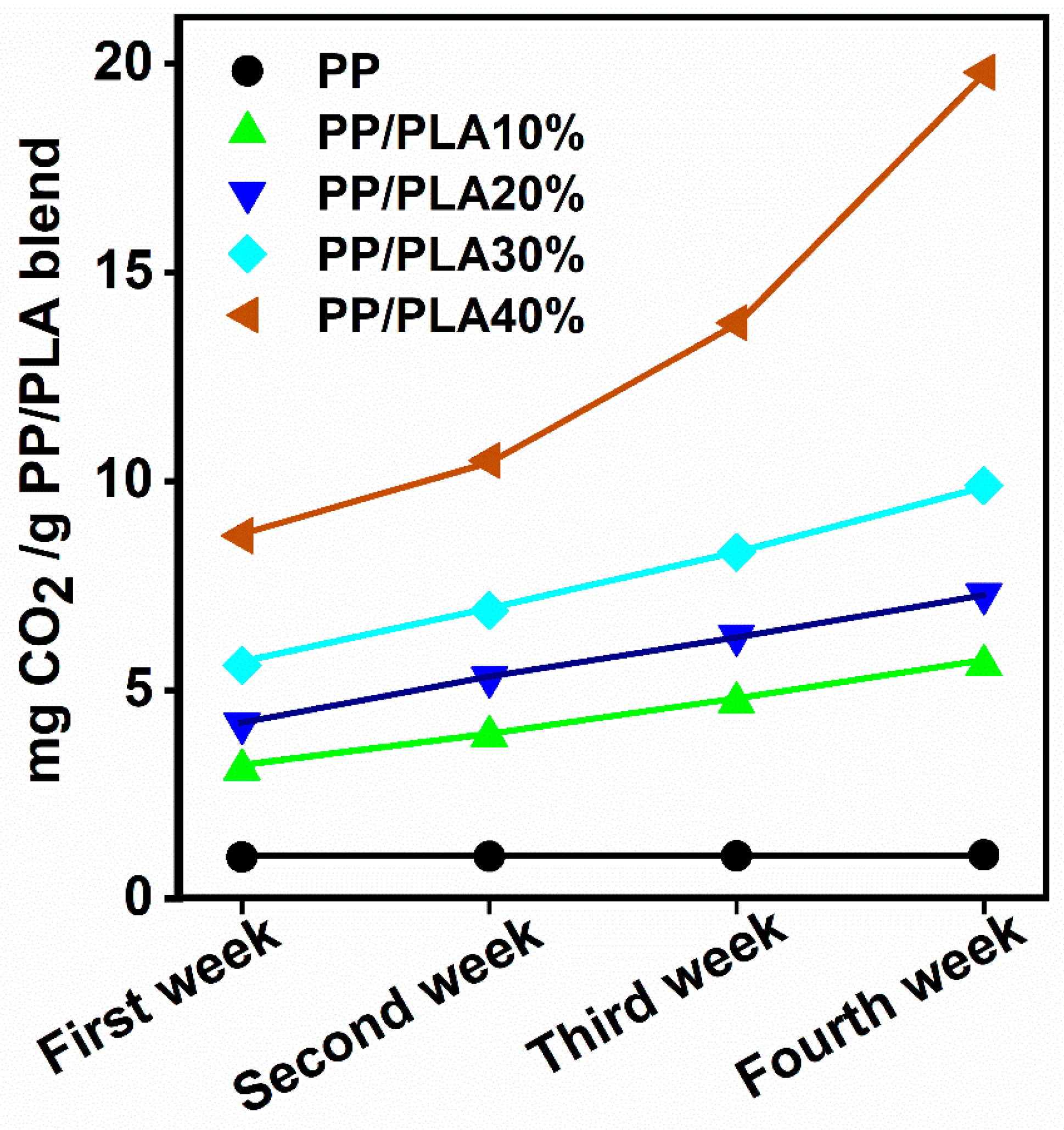
4.8. Biodegradability of Polypropylene/Polylactic Acid (PP/PLA) Blends
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamad, I.N.; Rohani, R.; Mastar, M.S.; Nor, M.T.M.; Jahim, J.M. Permeation properties of polymeric membranes for biohydrogen purification. Int. J. Hydrogen Energy 2016, 41, 4474–4488. [Google Scholar] [CrossRef]
- Azizi, M.; Ramazani, S.A.A.; Etemadi, M.H.; Shirzaei, S.E. Simulation of viscoelastic fluid flows in expansion geometry using finite volume approach. Chin. J. Polym. Sci. 2013, 31, 1599–1612. [Google Scholar] [CrossRef]
- Mousavi, S.A. CO2/H2 separation using a highly permeable polyurethane membrane: Molecular dynamics simulation. J. Mol. Struct. 2015, 1100, 401–414. [Google Scholar]
- Wu, D.; Wu, L.; Sun, Y.; Zhang, M. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly (ε-caprolactone) composites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- Seddigh, E.; Azizi, M.; Sani, E.S.; Mohebbi-Kalhori, D. Investigation of Poly (ether-b-amide)/Nanosilica Membranes for CO2/CH4 Separation. Chin. J. Polym. Sci. 2014, 32, 402–410. [Google Scholar] [CrossRef]
- Xie, M.; Bai, W.; Bai, L.; Sun, X.; Lu, Q.; Yan, D.; Qiao, Q. Life cycle assessment of the recycling of Al-PE (a laminated foil made from polyethylene and aluminum foil) composite packaging waste. J. Clean. Prod. 2016, 112, 4430–4434. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.K.; Lim, E.; Song, Y.S. Synergistic effect of lignin/polypropylene as a compatibilizer in multiphase eco-composites. Compos. Sci. Technol. 2015, 118, 193–197. [Google Scholar] [CrossRef]
- Sudár, A.; Burgstaller, C.; Renner, K.; Móczó, J.; Pukánszky, B. Wood fiber reinforced multicomponent, multiphase PP composites: Structure, properties, failure mechanism. Compos. Sci. Technol. 2014, 103, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Arsalan, M.; Zeeshan, M. Comparison of physicochemical and electrochemical characterization of PVC incorporated ZT and ZM composite membranes and their applicability on TMS theoretical equation. J. Mol. Struct. 2015, 1098, 355–364. [Google Scholar] [CrossRef]
- Wang, K.-T.; He, Y.; Song, X.-L.; Cui, X.-M. Effects of the metakaolin-based geopolymer on high-temperature performances of geopolymer/PVC composite materials. Appl. Clay Sci. 2015, 114, 586–592. [Google Scholar] [CrossRef]
- Pajoumshariati, S.R.; Azizi, M.; Wesner, D.; Miller, P.G.; Shuler, M.L.; Abbaspourrad, A. Microfluidic-Based Cell-Embedded Microgels Using Nonfluorinated Oil as a Model for the Gastrointestinal Niche. ACS Appl. Mater. Interfaces 2018, 10, 9235–9246. [Google Scholar] [CrossRef]
- Leach, J.B.; Schmidt, C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials 2005, 26, 125–135. [Google Scholar] [CrossRef]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan–polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Chen, W.; Shen, H.; Zhu, X.; Yao, H.; Wang, W. Preparation and photochromic properties of PEG-400 assisted WO 3–TiO 2–ZnO composite films. Ceram. Int. 2015, 41, 14008–14012. [Google Scholar] [CrossRef]
- Maier, C.; Calafut, T. Polypropylene: the Definitive User’s Guide and Databook; William Andrew: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Risite, H.; Oualid, H.A.; Mabrouk, K.E. Effects of Vinyltriethoxysilane and Maleic Anhydride Grafted Polypropylenes on the Morphological, Thermal, Rheological, and Mechanical Properties of Polypropylene/Clay Nanocomposites. Proceedings 2018, 3, 5500. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly (butylene adipate-co-terephthalate)(PBAT), poly (lactic acid)(PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Salehiyan, R.; Yussuf, A.; Hanani, N.F.; Hassan, A.; Akbari, A. Polylactic acid/polycaprolactone nanocomposite: Influence of montmorillonite and impact modifier on mechanical, thermal, and morphological properties. J. Elastom. Plast. 2015, 47, 69–87. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Stoehr, N.; Baudrit, B.; Haberstroh, E.; Nase, M.; Heidemeyer, P.; Bastian, M. Properties and weldability of plasticized polylactic acid films. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zare, Y.; Garmabi, H.; Rhee, K.Y. Prediction of complex modulus in phase-separated poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes nanocomposites. Polym. Test. 2018, 66, 189–194. [Google Scholar] [CrossRef]
- You, J.; Lou, L.; Yu, W.; Zhou, C. The preparation and crystallization of long chain branching polylactide made by melt radicals reaction. J. Appl. Polym. Sci. 2013, 129, 1959–1970. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.-W. Nanocomposite solid polymer electrolytes based on poly (ethylene oxide)/POSS-PEG (n= 13.3) hybrid nanoparticles for lithium ion batteries. J. Ind. Eng. Chem. 2015, 31, 323–329. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A. Thermal, mechanical and electroactive shape memory properties of polyurethane (PU)/poly (lactic acid)(PLA)/CNT nanocomposites. Eur. Polym. J. 2013, 49, 3492–3500. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Photo-oxidative stabilization of carbon nanotubes on polylactic acid. Polym. Degrad. Stab. 2013, 98, 963–971. [Google Scholar] [CrossRef]
- Hassan, A.; Balakrishnan, H.; Akbari, A. Polylactic Acid Based Blends, Composites and Nanocomposites, Advances in Natural Polymers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 361–396. [Google Scholar]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Effect of PEG on PLA/PEG blend and its nanocomposites: A study of thermo-mechanical and morphological characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Choudhary, P.; Mohanty, S.; Nayak, S.K.; Unnikrishnan, L. Poly(L-lactide)/polypropylene blends: Evaluation of mechanical, thermal, and morphological characteristics. J. Appl. Polym. Sci. 2011, 121, 3223–3237. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Zhang, X.; Xu, K.; Bian, J.; Dong, L. Poly (L-lactide)/Poly (D-lactide)/clay nanocomposites: Enhanced dispersion, crystallization, mechanical properties, and hydrolytic degradation. Polym. Eng. Sci. 2014, 54, 914–924. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T. A new biodegradable blends prepared from polylactide and hyaluronic acid. Polymer 2005, 46, 10017–10026. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Lumyong, S.; Leejarkpai, T.; Punyodom, W. Biodegradable nanocomposite blown films based on poly (lactic acid) containing silver-loaded kaolinite: A route to controlling moisture barrier property and silver ion release with a prediction of extended shelf life of dried longan. Polymer 2014, 55, 6776–6788. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the degradation of polyolefins through additives and blending. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ploypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of polypropylene/poly (lactic acid) for medical packaging application: physicochemical, thermal, mechanical, and barrier properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef]
- Tjong, S.C.; Xu, S.A.; Mai, Y.W. Impact-specific essential work of fracture of maleic anhydride-compatibilized polypropylene/elastomer blends and their composites. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1881–1892. [Google Scholar] [CrossRef]
- Xu, S.A.; Tjong, S.C. Effect of compatibilizer content on the tensile properties and fracture toughness of high density polyethylene/polystyrene blends. Polym. J. 2000, 32, 208. [Google Scholar] [CrossRef]
- Ebadi-Dehaghani, H.; Khonakdar, H.A.; Barikani, M.; Jafari, S.H. Experimental and theoretical analyses of mechanical properties of PP/PLA/clay nanocomposites. Compos. Part B Eng. 2015, 69, 133–144. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.; Chen, C.; Ma, J.; Wang, B. Mechanical properties and morphology of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Des. (1980–2015) 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Frackowiak, S.; Ludwiczak, J.; Leluk, K.; Orzechowski, K.; Kozlowski, M. Foamed poly (lactic acid) composites with carbonaceous fillers for electromagnetic shielding. Mater. Des. (1980–2015) 2015, 65, 749–756. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; la Mantia, F.; Malatesta, V. Photo-oxidation behaviour of polyethylene/multi-wall carbon nanotube composite films. Polym. Degrad. Stab. 2009, 94, 162–170. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chang, Y.-H.; Liang, M. Poly (2, 6-dimethyl-1, 4-phenylene oxide)(PPO) multi-bonded carbon nanotube (CNT): Preparation and formation of PPO/CNT nanocomposites. Polymer 2008, 49, 5405–5409. [Google Scholar] [CrossRef]
- Martone, A.; Faiella, G.; Antonucci, V.; Giordano, M.; Zarrelli, M. The effect of the aspect ratio of carbon nanotubes on their effective reinforcement modulus in an epoxy matrix. Compos. Sci. Technol. 2011, 71, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Cho, J.W.; Kim, K.H. Crystallization, orientation, and mechanical properties of laser-heated photothermally drawn polypropylene/multi-walled carbon nanotube fibers. Eur. Polym. J. 2017, 91, 70–80. [Google Scholar] [CrossRef]
- Bourmaud, A.; Pimbert, S. Investigations on mechanical properties of poly (propylene) and poly (lactic acid) reinforced by miscanthus fibers. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1444–1454. [Google Scholar] [CrossRef]
- Bao, S.; Tjong, S.C. Mechanical behaviors of polypropylene/carbon nanotube nanocomposites: The effects of loading rate and temperature. Mater. Sci. Eng. A 2008, 485, 508–516. [Google Scholar] [CrossRef]
- Castellani, F.; Esposito, A.; Stanzione, V.; Altieri, R. Measuring the biodegradability of plastic polymers in olive-mill waste compost with an experimental apparatus. Adv. Mater. Sci. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Roy, S.; Srivastava, S.K.; Pionteck, J.; Mittal, V. Mechanically and Thermally Enhanced Multiwalled Carbon Nanotube–Graphene Hybrid filled Thermoplastic Polyurethane Nanocomposites. Macromol. Mater. Eng. 2015, 300, 346–357. [Google Scholar] [CrossRef]
- Boussaboun, Z.; Azizi, S.; Ouellet-Plamondon, C. Conductive clay containing graphene layers. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1065–1069. [Google Scholar]
- Ren, F.; Li, Z.; Xu, L.; Sun, Z.; Ren, P.; Yan, D.; Li, Z. Large-scale preparation of segregated PLA/carbon nanotube composite with high efficient electromagnetic interference shielding and favourable mechanical properties. Compos. Part B Eng. 2018. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Helal, E.; Moghimian, N.; David, E.; Demarquette, N. The Role of Selectively Located Commercial Graphene Nanoplatelets in the Electrical Properties, Morphology, and Stability of EVA/LLDPE Blends. Macromol. Mater. Eng. 2018, 1800187. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.W.; Kim, S.H.; Youn, J.R. Rheological and electrical properties of polypropylene/MWCNT composites prepared with MWCNT masterbatch chips. Eur. Polym. J. 2008, 44, 1620–1630. [Google Scholar] [CrossRef]
- Mijović, J.; Sy, J.-W. Molecular dynamics during crystallization of poly (L-lactic acid) as studied by broad-band dielectric relaxation spectroscopy. Macromolecules 2002, 35, 6370–6376. [Google Scholar] [CrossRef]
- Xu, P.; Luo, X.; Zhou, Y.; Yang, Y.; Ding, Y. Enhanced cold crystallization and dielectric polarization of PLA composites induced by P [MPEGMA-IL] and graphene. Thermochim. Acta 2017, 657, 156–162. [Google Scholar] [CrossRef]
- Mai, F.; Deng, H.; Tu, W.; Chankajorn, S.; Fu, Q.; Bilotti, E.; Peijs, T. Oriented poly (lactic acid)/carbon nanotube composite tapes with high electrical conductivity and mechanical properties. Macromol. Mater. Eng. 2015, 300, 1257–1267. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C. Electrical and thermal conductivity of ethylene vinyl acetate composite with graphene and carbon black filler. Polym. Test. 2018. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal phenomena in low-density polyethylene/carbon black composites near the percolation threshold. J. Appl. Polym. Sci. 2018, 47043. [Google Scholar] [CrossRef]
- Azizi, S.; Ouellet-Plamondon, C.; David, E.; Fréchette, M. Electrical and thermal properties of low-density polyethylene/graphene-like composite. In Proceedings of the 2017 IEEE Conference on Electrical Insulation and Dielectric Phenomenon (CEIDP), Fort Worth, TX, USA, 22–25 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 517–520. [Google Scholar]
- Song, Z.; Xiao, H.; Zhao, Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr. Polym. 2014, 111, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hakkarainen, M. Recycling PLA to multifunctional oligomeric compatibilizers for PLA/starch composites. Eur. Polym. J. 2015, 64, 126–137. [Google Scholar] [CrossRef]
- De L. Freitas, A.L.P.; Filho, L.R.T.; Calvão, P.S.; de Souza, A.M.C. Effect of montmorillonite and chain extender on rheological, morphological and biodegradation behavior of PLA/PBAT blends. Polym. Test. 2017, 62, 189–195. [Google Scholar]
- Seligra, P.G.; Lamanna, M.; Famá, L. Promising PLA-functionalized MWCNT composites to use in nanotechnology. Polym. Compos. 2016, 37, 3066–3072. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]

| Labeled Sample | PP (wt%) | PLA (wt%) | PP-g-MA (wt% of PLA) | MWCNTs (wt% of Total Sample) |
|---|---|---|---|---|
| PP | 100 | 0 | 0 | 0 |
| PLA | 0 | 100 | 0 | 0 |
| PP/PLA10% | 90 | 10 | 1 | 0 |
| PP/PLA20% | 80 | 20 | 2 | 0 |
| PP/PLA30% | 70 | 30 | 3 | 0 |
| PP/PLA40% | 60 | 40 | 4 | 0 |
| PP/PLA40/MWCNTs 0.5% | 60 | 40 | 4 | 0.5 |
| PP/PLA40/MWCNTs 1% | 60 | 40 | 4 | 1 |
| PP/PLA40/MWCNTs 2% | 60 | 40 | 4 | 2 |
| Sample | DSC | TGA | ||||
|---|---|---|---|---|---|---|
| Tc (°C) | Tm (°C) | Xc (%) | T5% (°C) | T50% (°C) | Ash Percent at 500 (°C) | |
| PP | 115 | 159 | 50.5 | 340 | 450 | 0 |
| PLA | - | 158 | - | 290 | 390 | 0 |
| PP/PLA10% | 117 | 159 | 50.1 | 293 | 420 | 0 |
| PP/PLA20% | 117 | 159 | 49.8 | 295 | 423 | 0 |
| PP/PLA30% | 117 | 158 | 49.6 | 296 | 426 | 0 |
| PP/PLA40% | 117 | 158 | 49.7 | 350 | 428 | 0 |
| PP/PLA40/MWCNTs 0.5% | 118 | 161 | 49.8 | 352 | 445 | 0.4 |
| PP/PLA40/MWCNTs 1% | 118 | 162 | 50.3 | 353 | 447 | 0.9 |
| PP/PLA40/MWCNTs 2% | 118 | 163 | 50.5 | 355 | 448 | 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, S.; Azizi, M.; Sabetzadeh, M. The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites. J. Compos. Sci. 2019, 3, 64. https://doi.org/10.3390/jcs3030064
Azizi S, Azizi M, Sabetzadeh M. The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites. Journal of Composites Science. 2019; 3(3):64. https://doi.org/10.3390/jcs3030064
Chicago/Turabian StyleAzizi, Sohrab, Morteza Azizi, and Maryam Sabetzadeh. 2019. "The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites" Journal of Composites Science 3, no. 3: 64. https://doi.org/10.3390/jcs3030064
APA StyleAzizi, S., Azizi, M., & Sabetzadeh, M. (2019). The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites. Journal of Composites Science, 3(3), 64. https://doi.org/10.3390/jcs3030064