Fabrication of Nanostructured Kaolinite Doped Composite Films from Silicone Rubber with Enhanced Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
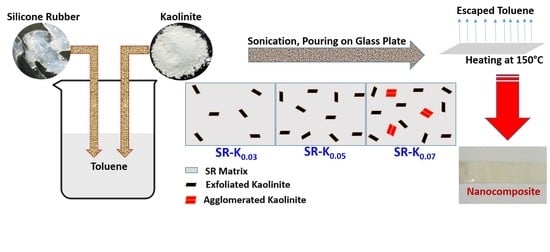
2.2.1. Preparation of Nanocomposite (NCP) Films
2.2.2. Characterization of the Fabricated Films
2.2.3. Investigation of Mechanical Performance
2.2.4. Investigation of Thermal Stability
2.2.5. Investigation of Chemical Resistance and Swelling Properties
3. Results and Discussion
3.1. FTIR Analysis
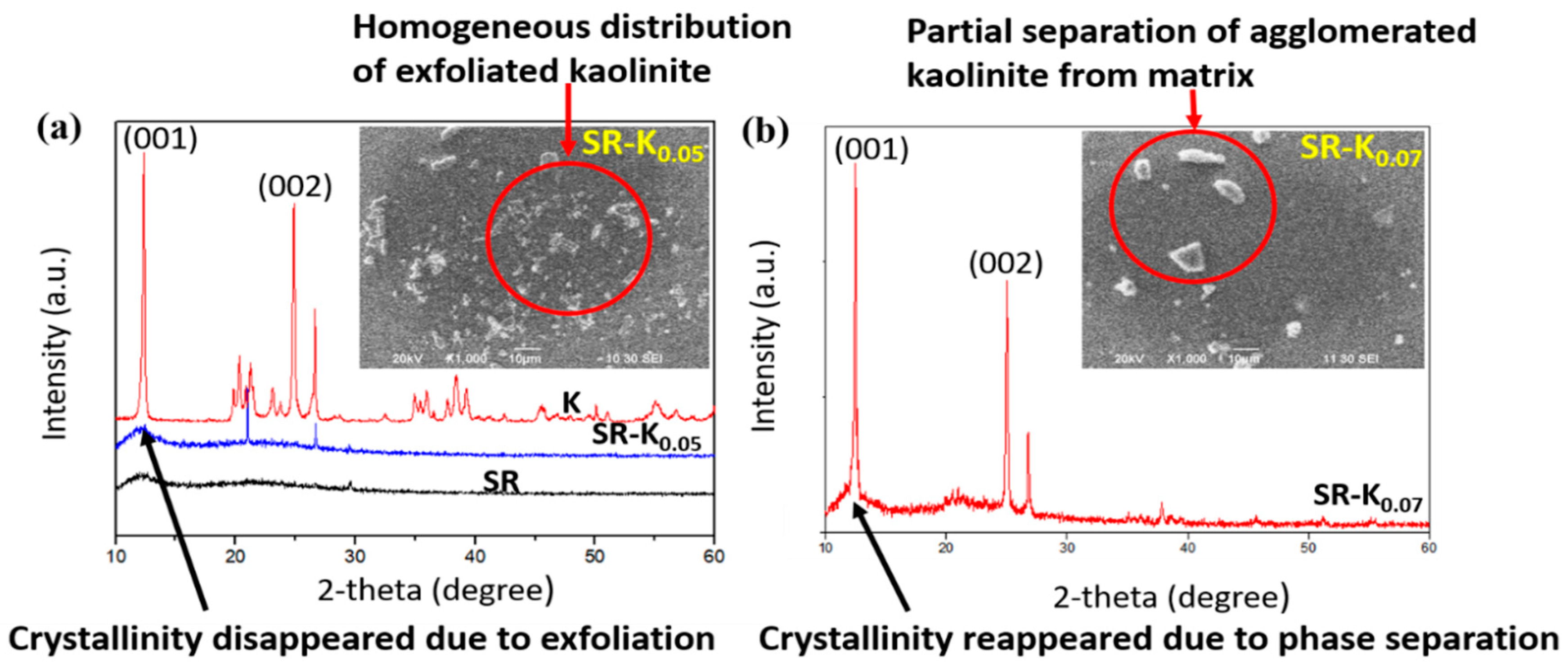
3.2. XRD Analysis
3.3. SEM Analysis
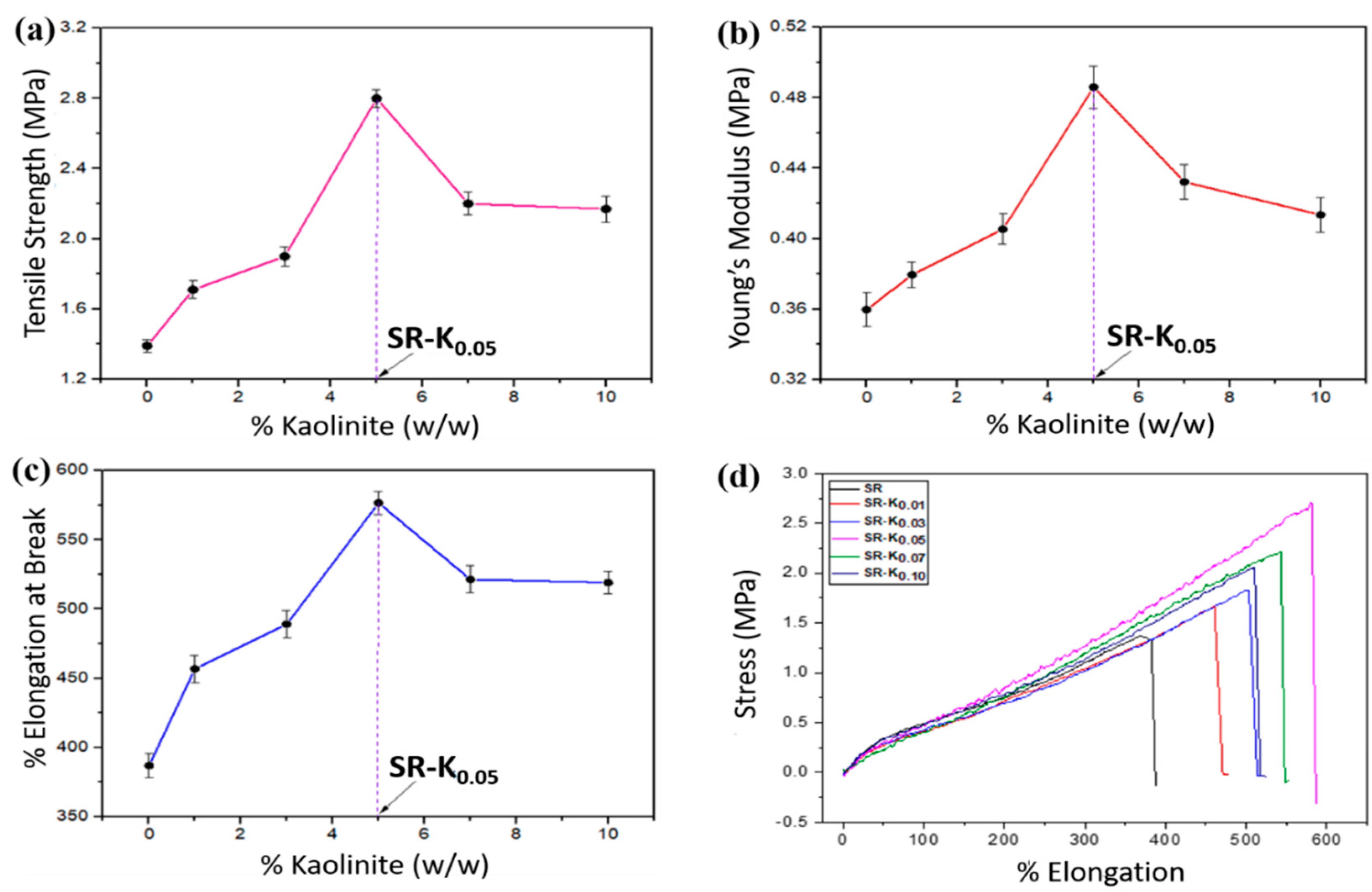
3.4. Mechanical Properties
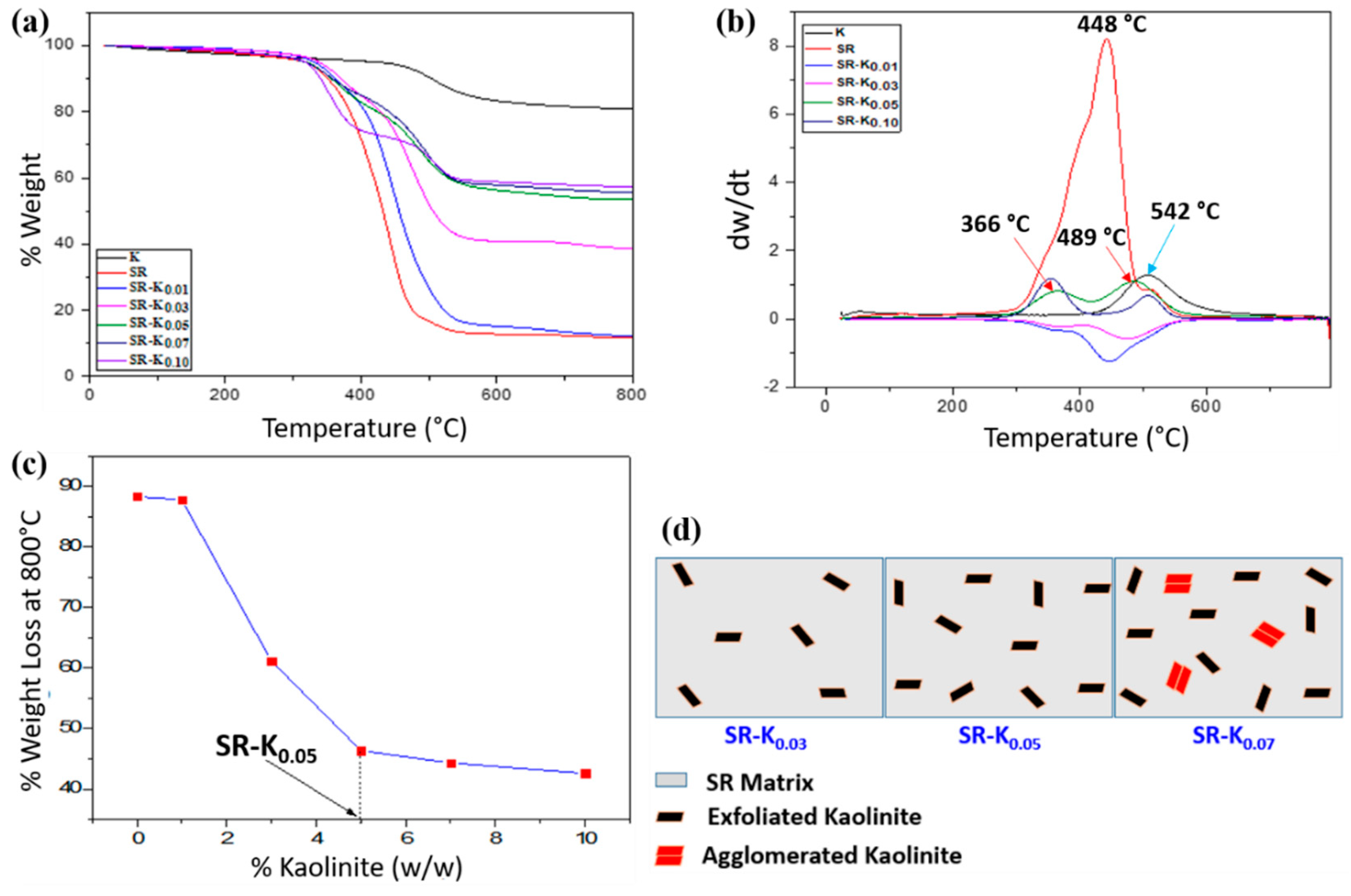
3.5. Thermal Stability
3.6. Solvent Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ismail, N.N.; Ansarifar, A.; Song, M. Preparation and characterization of high-performance exfoliated montmorillonite/silicone rubber nanocomposites with enhanced mechanical properties. Polym. Eng. Sci. 2013, 53, 2603–2614. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, R.; An, Z.; Zhang, J. Silicon rubber/hollow glass microsphere composites: Influence of broken hollow glass microsphere on mechanical and thermal insulation property. Compos. Sci. Technol. 2013, 79, 64–69. [Google Scholar] [CrossRef]
- Gonzalez-Perez, G.; Burillo, G.; Ogawa, T.; Avalos-Borja, M. Grafting of styrene and 2-vinylnaphthalene onto silicone rubber to improve radiation resistance. Polym. Degrad. Stab. 2012, 97, 1495–1503. [Google Scholar] [CrossRef]
- Fang, W.; Lai, X.; Li, H.; Chen, W.; Zeng, X.; Zhang, L.; Yang, S. Effect of urea-containing anti-tracking additive on the tracking and erosion resistance of addition-cure liquid silicone rubber. Polym. Test. 2014, 37, 19–27. [Google Scholar] [CrossRef]
- Bortz, D.R.; Merino, C.; Martin-Gullon, I. Carbon nanofibers enhance the fracture toughness and fatigue performance of a structural epoxy system. Compos. Sci. Technol. 2011, 71, 31–38. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zheng, J. Effect and mechanism of iron oxide modified carbon nanotubes on thermal oxidative stability of silicone rubber. Compos. Sci. Technol. 2014, 99, 1–7. [Google Scholar] [CrossRef]
- Van den Ende, D.A.; Gubbels, G.H.M. Fracture toughness of hydroxide catalysis bonds between silicon carbide and zerodur low thermal expansion glass-ceramic. Mater. Chem. Phys. 2014, 143, 1236–1242. [Google Scholar] [CrossRef]
- Pramanik, M.; Srivastava, S.K.; Samantaray, B.K.; Bhowmick, A.K. Rubber–clay nanocomposite by solution blending. J. Appl. Polym. Sci. 2003, 87, 2216–2220. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Pinnavaia, T.J. Clay nanolayer reinforcement of a silicone elastomer. Chem. Mater. 2001, 13, 3760–3765. [Google Scholar] [CrossRef]
- Burnside, S.D.; Giannelis, E.P. Nanostructure and properties of polysiloxane-layered silicate nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1595–1604. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Zhang, Y. Improving the mechanical and electrical properties of ceramizable silicone rubber/halloysite composites and their ceramic residues by incorporation of different borates. Polymers 2018, 10, 388. [Google Scholar] [CrossRef]
- Karami, Z.; Jazani, O.M.; Navarchian, A.H.; Karrabi, M.; Vahabi, H.; Saeb, M.R. Well-cured silicone/halloysite nanotubes nanocomposite coatings. Prog. Org. Coat. 2019, 129, 357–365. [Google Scholar] [CrossRef]
- Mishra, R.M.; Rai, J.S.P. Compatibilizing effect of halloysite nanotubes on polyetherimide/silicone rubber blend-based nanocomposites. Polym.-Plast. Technol. Mater. 2019, 58, 341–347. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, R.; Blanco, C.; Menendez, R.; Verdejo, R.; Lopez-Manchado, M.A. Multifunctional silicone rubber nanocomposites by controlling the structure and morphology of graphene material. Polymers 2019, 11, 449. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced graphene oxide embedded with MQ silicone resin nano-aggregates for silicone rubber composites with enhanced thermal conductivity and mechanical performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef]
- Chen, K.; Kang, M.; Lu, A.; Chen, L.; Song, L.; Sun, R. Visualization of silica dispersion states in silicone rubber by fluorescent labeling. J. Mater. Sci. 2019, 54, 5149–5159. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater. Des. 2019, 165, 107580. [Google Scholar] [CrossRef]
- Tamore, M.S.; Ratna, D.; Mishra, S.; Shimpi, N.G. Effect of functionalized multi-walled carbon nanotubes on physicomechanical properties of silicone rubber nanocomposites. J. Compos. Mater. 2019. [Google Scholar] [CrossRef]
- Vaimakis-Tsogkas, D.T.; Bekas, D.G.; Giannakopoulou, T.; Todorova, N.; Paipetis, A.S.; Barkoula, N.M. Effect of TiO2 addition/coating on the performance of polydimethylsiloxane-based silicone elastomers for outdoor applications. Mater. Chem. Phys. 2019, 223, 366–373. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Jia, Z.; Wang, J.; Chen, C. Nonlinear electrical properties and field dependency of BST and nano-ZnO-doped silicone rubber composites. Molecules 2018, 23, 3153. [Google Scholar] [CrossRef]
- Han, R.; Wang, Z.; Zhang, Y.; Niu, K. Thermal stability of CeO2/graphene/phenyl silicone rubber composites. Polym. Test. 2019, 75, 277–283. [Google Scholar] [CrossRef]
- Hafiz, M.; Fairus, M.; Mariatti, M.; Kamarol, M. A Comparative study on electrical tree growth in silicone rubber containing nanoalumina and halloysite nanoclay. IEEE Access 2019, 7, 24452–24462. [Google Scholar] [CrossRef]
- Pinnavaia, T.J. Intercalated clay catalysts. Science 1983, 220, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Shimpi, N.G.; Mali, A.D. Surface modification of montmorillonite (MMT) using column chromatography technique and its application in silicone rubber nanocomposites. Macromol. Res. 2012, 20, 44–50. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, E.J.; Lee, T.H.; Yoon, J.S. Clay modification and its effect on the physical properties of silicone rubber/clay composites. J. Appl. Polym. Sci. 2012, 125, E298–E304. [Google Scholar] [CrossRef]
- Kong, Q.; Hu, Y.; Song, L.; Wang, Y.; Chen, Z.; Fan, W. Influence of Fe-MMT on crosslinking and thermal degradation in silicone rubber/clay nanocomposites. Polym. Adv. Technol. 2006, 17, 463–467. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. Mechanism of clay tactoid exfoliation in epoxy-clay nanocomposites. Chem. Mater. 1995, 7, 2144–2150. [Google Scholar] [CrossRef]
- Yoon, J.T.; Jo, W.H.; Lee, M.S.; Ko, M.B. Effects of comonomers and shear on the melt intercalation of styrenics/clay nanocomposites. Polymer 2001, 42, 329–336. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.Z.; Kuan, H.C.; Dasari, A.; Mai, Y.W. A new strategy to exfoliate silicone rubber/clay nanocomposites. Macromol. Rapid Commun. 2005, 26, 830–833. [Google Scholar] [CrossRef]
- EN ISO 1421:1998, Method 1-Strip Test (Tensile Strength and Elongation at Break). Available online: https://www.mecmesin.com/bs-en-iso-1421-1998-rubber-or-plastic-coated-fabrics-determination-of-tensile-strength-and-elongation-at-break (accessed on 10 April 2019).
- Dengke, C.; Xishan, W.; Lei, L. Research on characterization of RTV silicone rubber/LS (layered silicate) electrical insulation nanocomposites. In Proceedings of the 8th International Conference on Solid Dielectrics, Toulouse, France, 5–9 July 2004; Volume 2, pp. 796–799. [Google Scholar]
- Letaief, S.; Leclercq, J.; Liu, Y.; Detellier, C. Single kaolinite nanometer layers prepared by an in-situ polymerization–exfoliation process in the presence of ionic liquids. Langmuir 2011, 27, 15248–15254. [Google Scholar] [CrossRef]
- Kaneko, M.L.Q.A.; Romero, R.B.; Gonçalves, M.D.C.; Yoshida, I.V.P. High molar mass silicone rubber reinforced with montmorillonite clay masterbatches: Morphology and mechanical properties. Eur. Polym. J. 2010, 46, 881–890. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Kandola, B.; Milnes, G.J.; Sitpalan, A.; Hadimani, R.L. The potential for ultrasound to improve nanoparticle dispersion and increase flame resistance in fibre-forming polymers. Polym. Degrad. Stab. 2012, 97, 2511–2523. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B.; Blanchet, P. Ultrasonication technique: A method for dispersing nanoclay in wood adhesives. J. Nanometer. 2013, 2013, 341897. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Hanif, A.; Hao, W.; Li, Z.; Sun, G. Comparative evaluation on the dispersion and stability of graphene oxide in water and cement pore solution by incorporating silica fume. Cem. Concr. Compos. 2018, 94, 33–42. [Google Scholar] [CrossRef]
- Lu, Z.; Hanif, A.; Ning, C.; Shao, H.; Yin, R.; Li, Z. Steric stabilization of graphene oxide in alkaline cementitious solutions: Mechanical enhancement of cement composite. Mater. Des. 2017, 127, 154–161. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Kloczkowski, A.; Mark, J.E. Dynamic mechanical losses in filled poly (dimethylsiloxane) networks. Rubber Chem. Technol. 1995, 68, 601–608. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the biopolymer charge and the nanoclay morphology on nanocomposite materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Wang, S.; Long, C.; Wang, X.; Li, Q.; Qi, Z. Synthesis and properties of silicone rubber/organomont- morillonite hybrid nanocomposites. J. Appl. Polym. Sci. 1998, 69, 1557–1561. [Google Scholar] [CrossRef]
- Bokobza, L. Elastomeric composites. I. Silicone composites. J. Appl. Polym. Sci. 2004, 93, 2095–2104. [Google Scholar] [CrossRef]
- Grassie, N.; Macfarlane, I.G. The thermal degradation of polysiloxanes—I. Poly (dimethylsiloxane). Eur. Polym. J. 1978, 14, 875–884. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zang, C.G.; Wen, Y.Q.; Jiao, Q.J. Thermal and mechanical properties of liquid silicone rubber composites filled with functionalized graphene oxide. J. Appl. Polym. Sci. 2015, 132, 42582. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Chen, S.; Qi, Z. FTIR spectra, thermal properties, and dispersibility of a polystyrene/montmorillonite nanocomposite. Macromol. Chem. Phys. 2001, 202, 1189–1193. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bio-nanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes sandwiched between chitosan layers: Novel bio-nanocomposites with multilayer structures. New J. Chem. 2018, 42, 8384–8390. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; Volume 213. [Google Scholar]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and thermal properties of room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesquioxane (POSS) as a cross linking agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
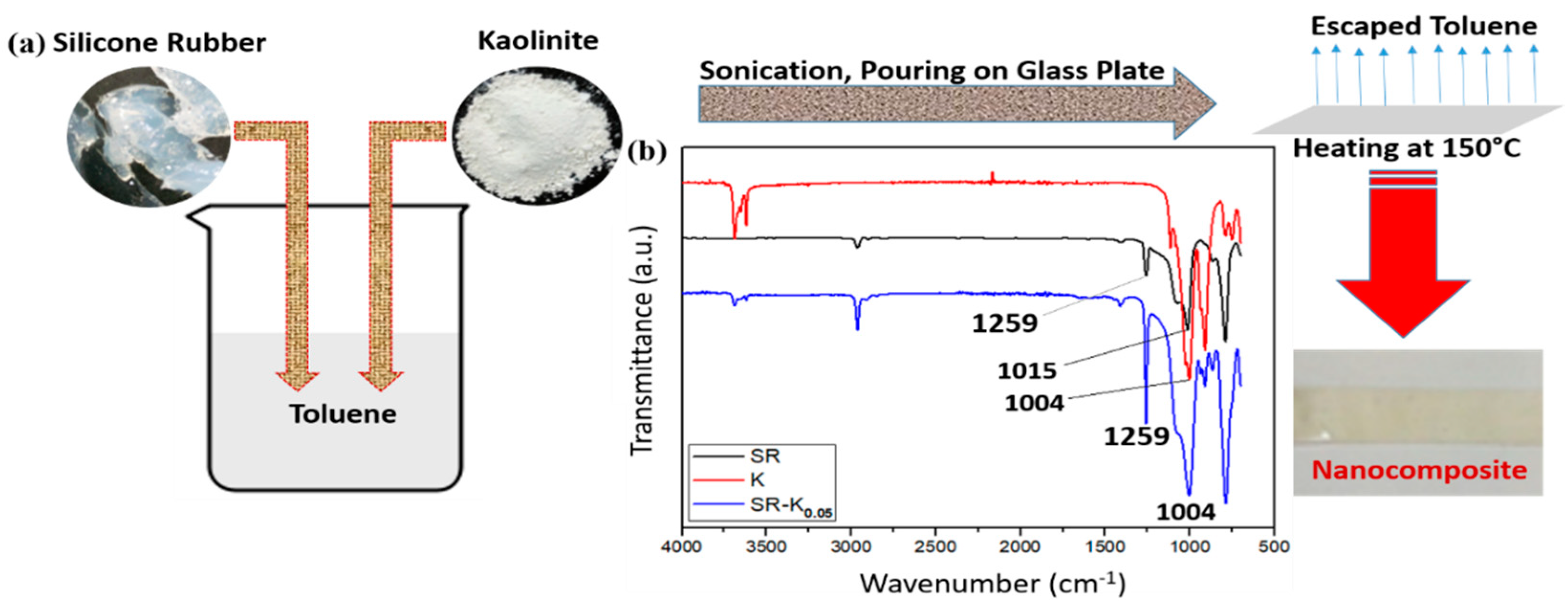

| Film Code | % Silicone Rubber (w/w) | % Kaolinite (w/w) |
|---|---|---|
| SR | 100 | 0 |
| SR-K0.01 | 99 | 1 |
| SR-K0.03 | 97 | 3 |
| SR-K0.05 | 95 | 5 |
| SR-K0.07 | 93 | 7 |
| SR-K0.10 | 90 | 10 |
| Film Code | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| SR | 1.39 ± 0.034 | 0.36 ± 0.009 | 386.89 ± 8.862 |
| SR-K0.01 | 1.71 ± 0.049 | 0.38 ± 0.007 | 456.81 ± 9.792 |
| SR-K0.03 | 1.99 ± 0.054 | 0.41 ± 0.008 | 489.07 ± 10.098 |
| SR-K0.05 | 2.80 ± 0.051 | 0.49 ± 0.011 | 576.67 ± 8.244 |
| SR-K0.07 | 2.20 ± 0.065 | 0.43 ± 0.009 | 521.44 ± 9.977 |
| SR-K0.10 | 2.17 ± 0.074 | 0.41 ± 0.009 | 519.11 ± 8.318 |
| Film Code | T10 (°C) | T50 (°C) | R800 (°C) |
|---|---|---|---|
| SR | 351 | 431 | 11.72 |
| SR-K0.01 | 355 | 456 | 12.22 |
| SR-K0.03 | 360 | 506 | 38.84 |
| SR-K0.05 | 362 | - | 53.65 |
| SR-K0.07 | 367 | - | 55.66 |
| SR-K0.10 | 376 | - | 57.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, A.M.; Dey, S.C.; Rahman, M.M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. Fabrication of Nanostructured Kaolinite Doped Composite Films from Silicone Rubber with Enhanced Properties. J. Compos. Sci. 2019, 3, 50. https://doi.org/10.3390/jcs3020050
Zakaria AM, Dey SC, Rahman MM, Sarker M, Ashaduzzaman M, Shamsuddin SM. Fabrication of Nanostructured Kaolinite Doped Composite Films from Silicone Rubber with Enhanced Properties. Journal of Composites Science. 2019; 3(2):50. https://doi.org/10.3390/jcs3020050
Chicago/Turabian StyleZakaria, Abdullah Muhammad, Shaikat Chandra Dey, Muhammad Mominur Rahman, Mithun Sarker, Md. Ashaduzzaman, and Sayed Md. Shamsuddin. 2019. "Fabrication of Nanostructured Kaolinite Doped Composite Films from Silicone Rubber with Enhanced Properties" Journal of Composites Science 3, no. 2: 50. https://doi.org/10.3390/jcs3020050
APA StyleZakaria, A. M., Dey, S. C., Rahman, M. M., Sarker, M., Ashaduzzaman, M., & Shamsuddin, S. M. (2019). Fabrication of Nanostructured Kaolinite Doped Composite Films from Silicone Rubber with Enhanced Properties. Journal of Composites Science, 3(2), 50. https://doi.org/10.3390/jcs3020050