Removal of Surfactant from Nanocomposites Films Based on Thermally Reduced Graphene Oxide and Natural Rubber
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of NR/rGO and w-NR/rGO Nanocomposites
2.3. Nanocomposite Characterization
3. Results and Discussion
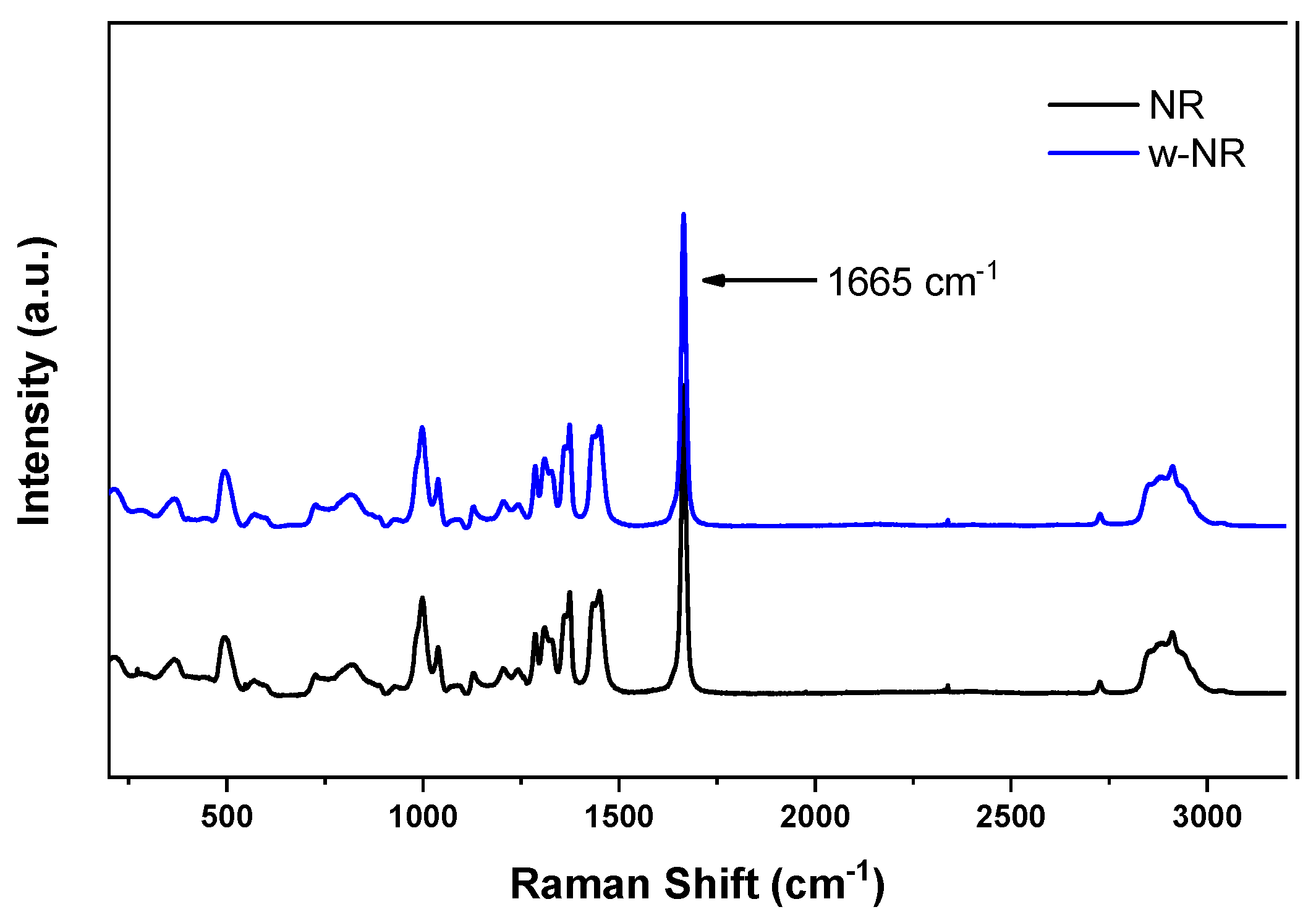
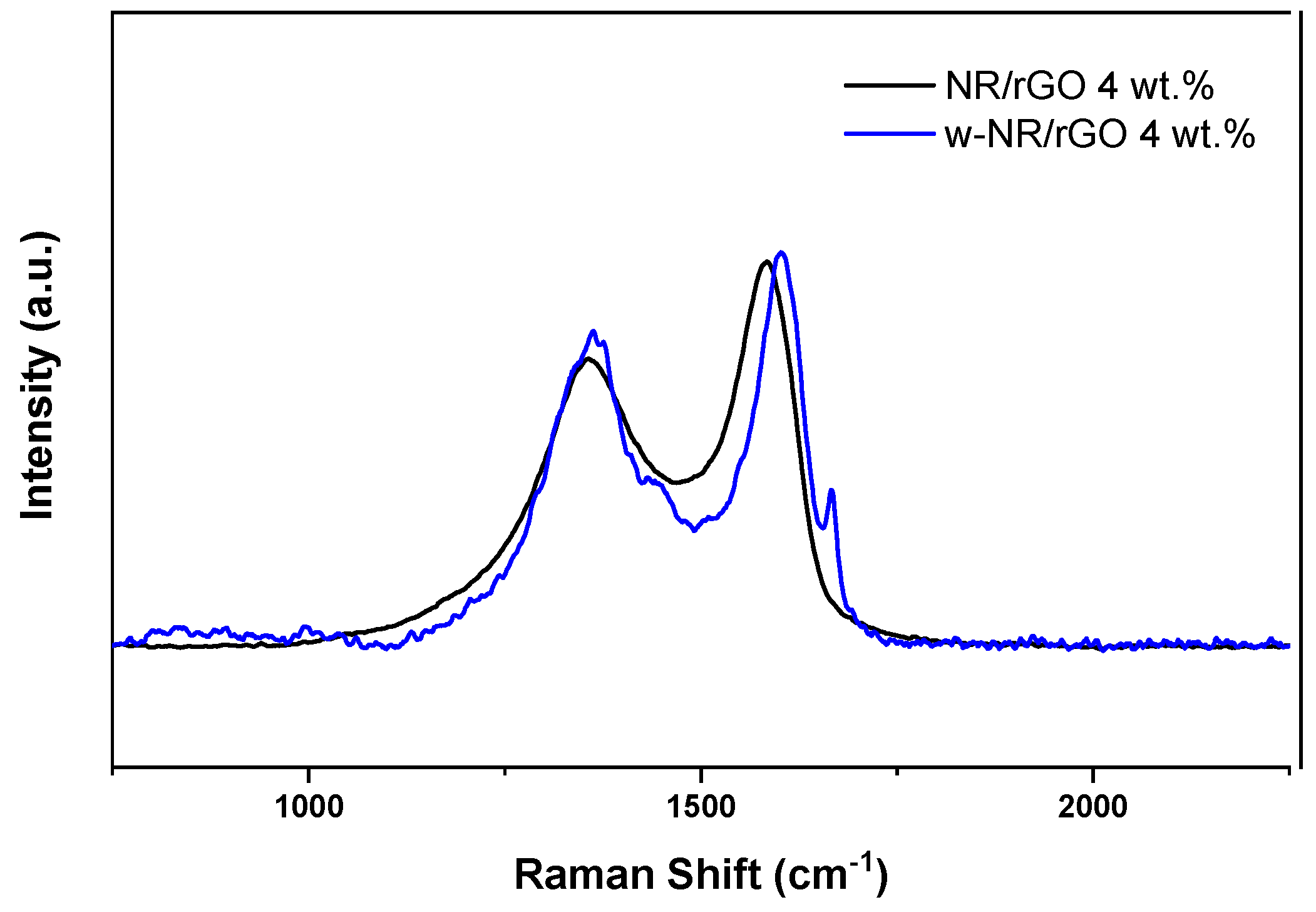
3.1. Raman Spectroscopy
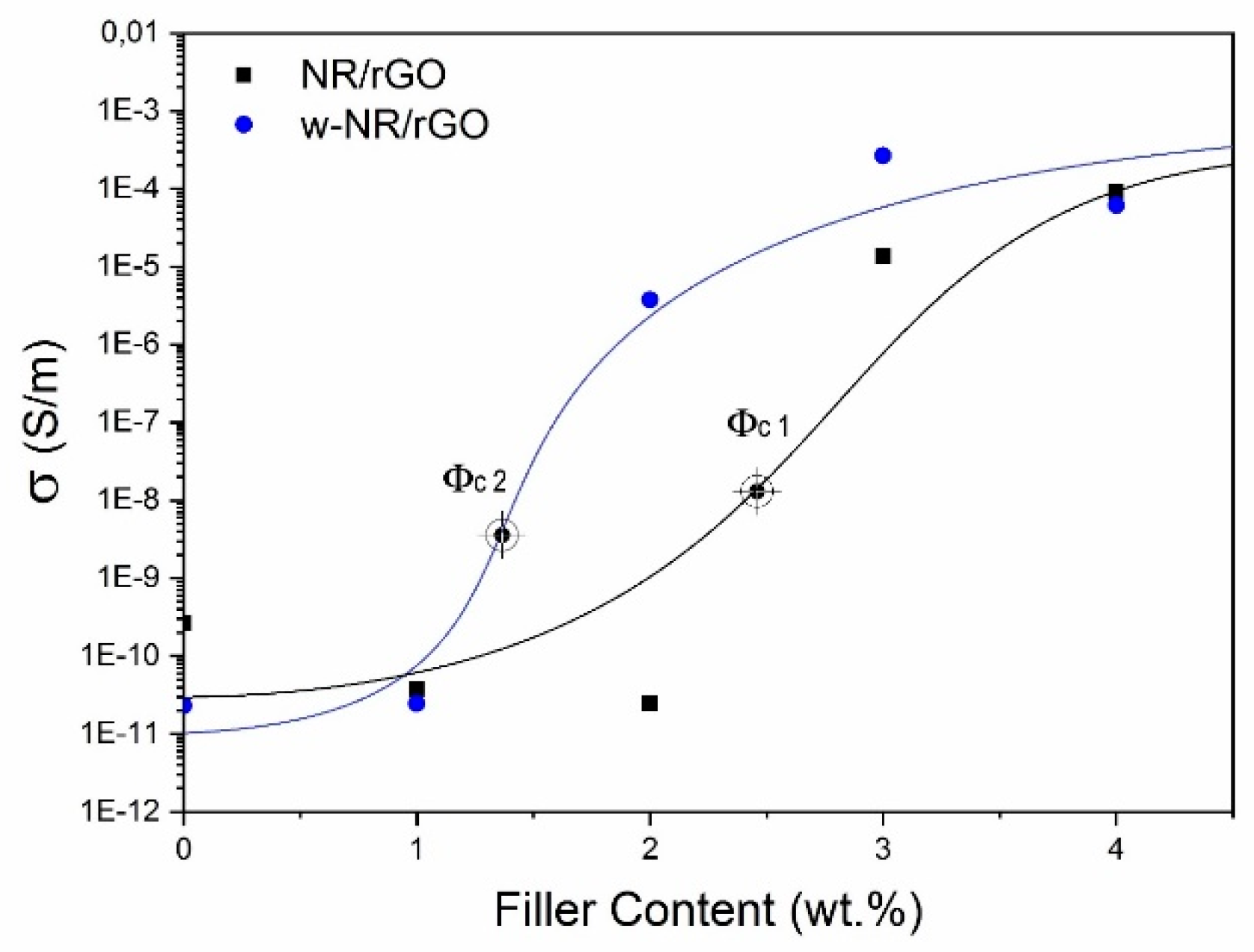
3.2. Electrical Conductivity of NR/rGO Nanocomposites
3.3. Mechanical Properties of NR/Reduced Graphene Oxide Composites
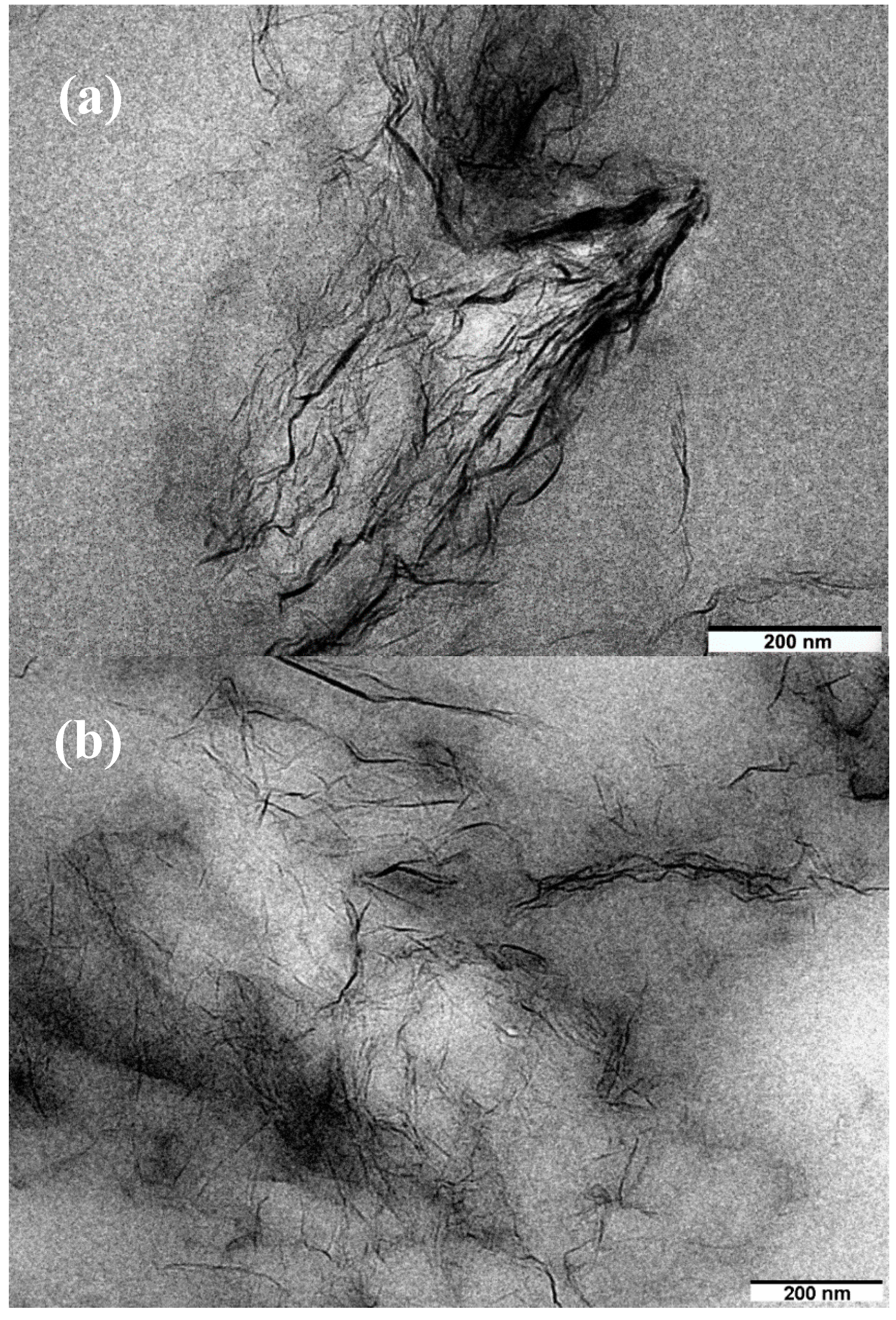
3.4. Morphology of NR/Reduced Graphene Oxide Composites
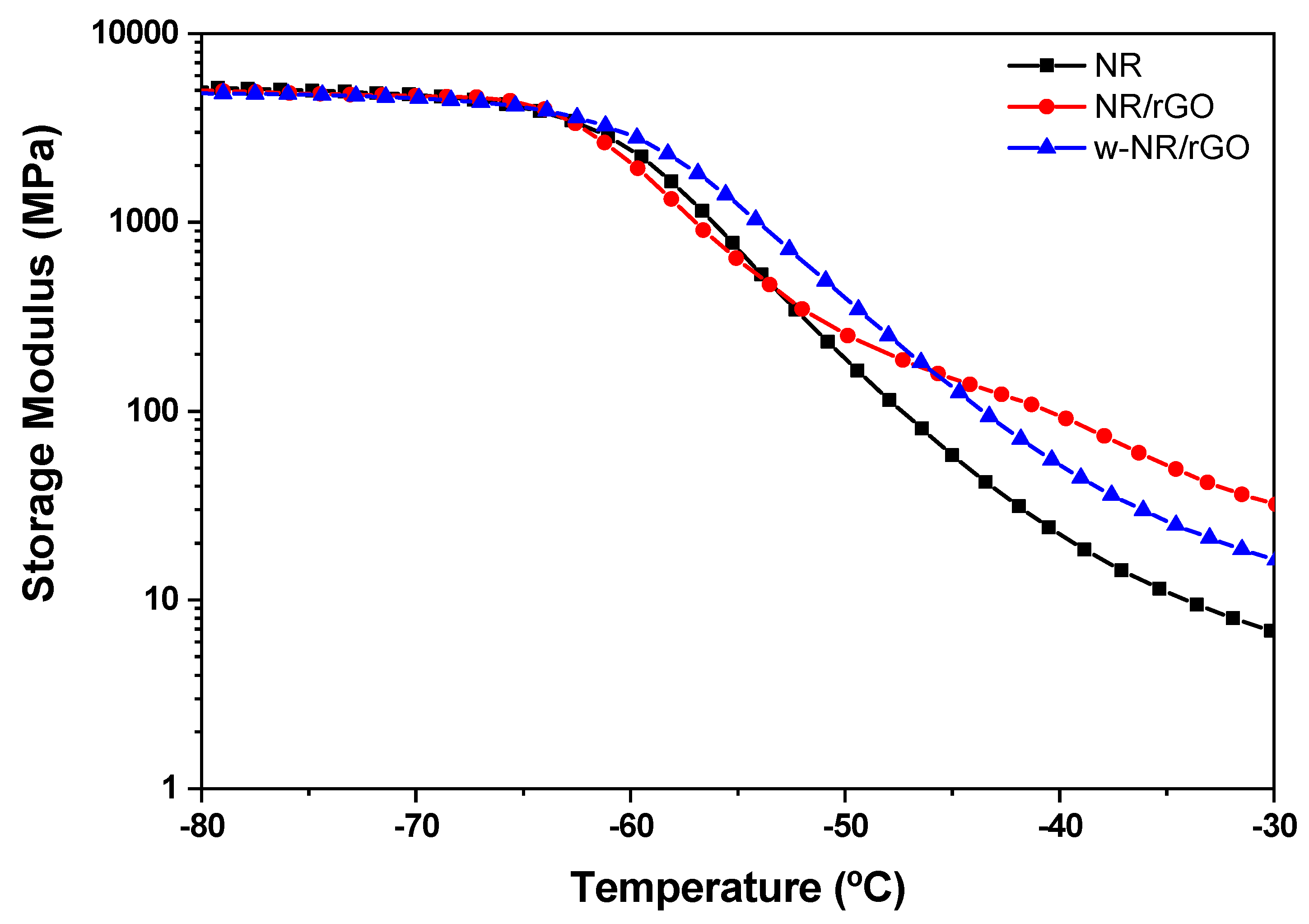
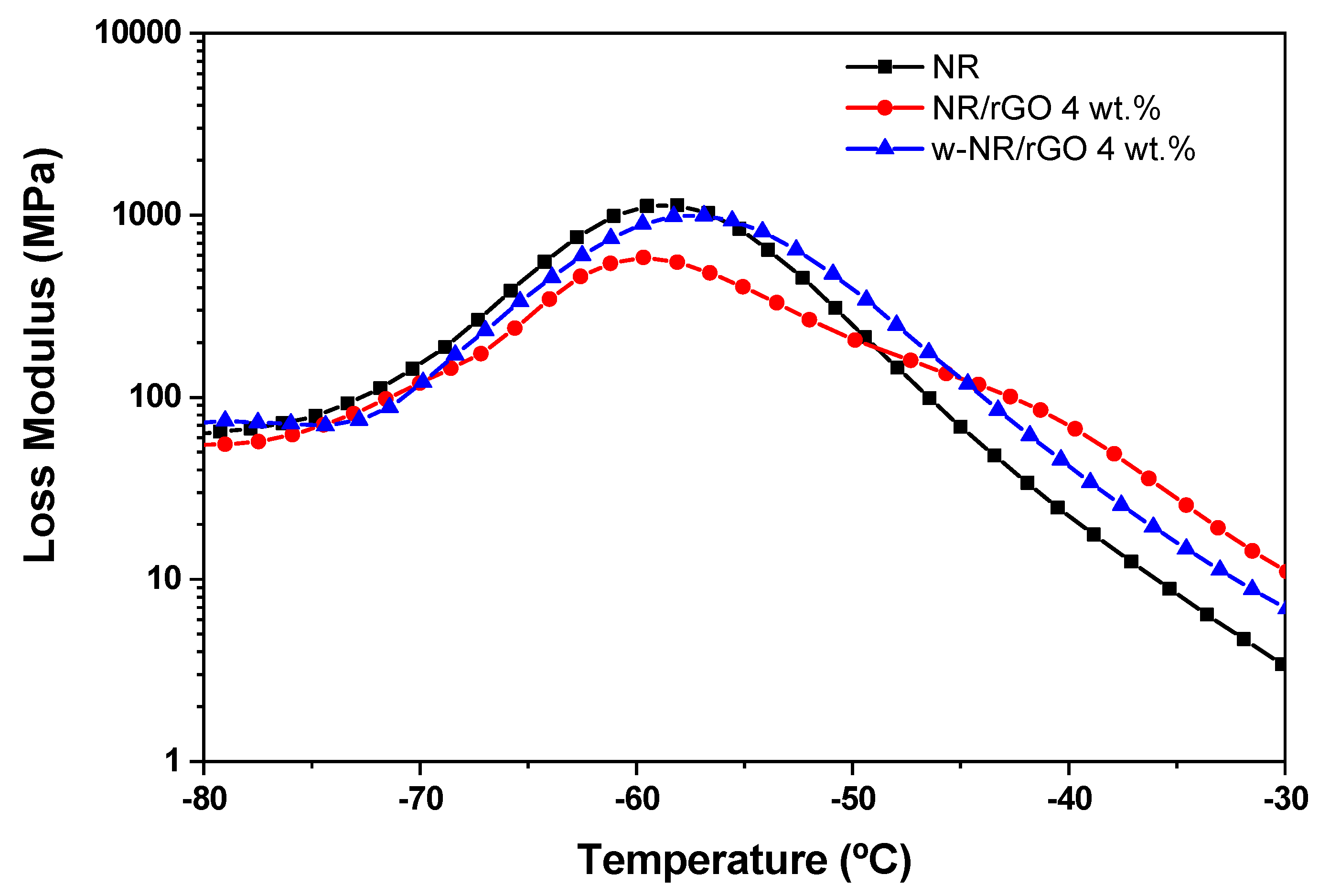
3.5. Dynamo-Mechanical of NR/Reduced Graphene Oxide Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.; Klüppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene–butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Lorenz, H.; Fritzsche, J.; Das, A.; Stöckelhuber, K.W.; Jurk, R.; Heinrich, G.; Klüppel, M. Advanced elastomer nano-composites based on CNT-hybrid filler systems. Compos. Sci. Technol. 2009, 69, 2135–2143. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Garlof, S.; Fukuda, T.; Mecklenburg, M.; Smazna, D.; Mishra, Y.K.; Adelung, R.; Schulte, K.; Fiedler, B. Electro-mechanical piezoresistive properties of three dimensionally interconnected carbon aerogel (Aerographite)-epoxy composites. Compos. Sci. Technol. 2016, 134, 226–233. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Brasero, J.; Lopez-Manchado, M.A.; Yazdani-Pedram, M. High performance natural rubber/thermally reduced graphite oxide nanocomposites by latex technology. Compos. Part B Eng. 2014, 67, 449–454. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Contreras-Cid, A.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Flores, M.; Fuentealba, P.; Neira-Carrillo, A.; Verdejo, R.; López-Manchado, M.A. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J. Colloid Interface Sci. 2018, 524, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and Scalable One-Step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195 (Suppl. C), 145–154. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation–reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Hernández, M.; Bernal, M.; Verdejo, R.; Ezquerra, T.; López-Manchado, M. Overall performance of natural rubber/graphene nanocomposites. Compos. Sci. Technol. 2012, 73, 40–46. [Google Scholar] [CrossRef]
- Li, C.; Feng, C.; Peng, Z.; Gong, W.; Kong, L. Ammonium-assisted green fabrication of graphene/natural rubber latex composite. Polym. Compos. 2013, 34, 88–95. [Google Scholar] [CrossRef]
- Wu, J.; Xing, W.; Huang, G.; Li, H.; Tang, M.; Wu, S.; Liu, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- Xing, W.; Wu, J.; Huang, G.; Li, H.; Tang, M.; Fu, X. Enhanced mechanical properties of graphene/natural rubber nanocomposites at low content. Polym. Int. 2014, 63, 1674–1681. [Google Scholar] [CrossRef]
- Matos, C.; Galembeck, F.; Zarbin, A. Multifunctional and environmentally friendly nanocomposites between natural rubber and graphene or graphene oxide. Carbon 2014, 78, 469–479. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Brasero, J.; Lopez-Manchado, M. Influence of the Surfactant Nature on the Occurrence of Self-Assembly between Rubber Particles and Thermally Reduced Graphite Oxide during the Preparation of Natural Rubber Nanocomposites. J. Nanomater. 2015, 16, 311. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Lopez-Manchado, M.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites. Compos. Part B Eng. 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Contreras-Cid, A.; López-Manchado, M.; May-Pat, A.; Avilés, A. Influence of the morphology of carbon nanostructures on the piezoresistivity of hybrid natural rubber nanocomposites. Compos. Part B Eng. 2017, 109, 147–154. [Google Scholar] [CrossRef]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Huczko, A.; Lange, H.; Gemming, T.; Büchner, B.; Rümmeli, M.H. Dispersion and diameter separation of multi-wall carbon nanotubes in aqueous solutions. J. Colloid Interface Sci. 2010, 345, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants. New J. Phys. 2010, 12, 125008. [Google Scholar] [CrossRef]
- Scher, H.; Zallen, R. Critical Density in Percolation Processes. J. Chem. Phys. 1970, 53, 3759–3761. [Google Scholar] [CrossRef]
- Liang, G.D.; Tjong, S.C. Electrical properties of percolative polystyrene/carbon nanofiber composites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 214–220. [Google Scholar] [CrossRef]
- Lu, W.; Lin, H.; Wu, D.; Chen, G. Unsaturated polyester resin/graphite nanosheet conducting composites with a low percolation threshold. Polymer 2006, 47, 4440–4444. [Google Scholar] [CrossRef]
- Mamunya, E.P.; Davidenko, V.V.; Lebedev, E.V. Percolation conductivity of polymer composites filled with dispersed conductive filler. Polym. Compos. 1995, 16, 319–324. [Google Scholar] [CrossRef]
- Li, C.; Thostenson, E.T.; Chou, T.-W. Dominant role of tunneling resistance in the electrical conductivity of carbon nanotube–based composites. Appl. Phys. Lett. 2007, 91, 223114. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. Low percolation threshold of graphene/polymer composites prepared by solvothermal reduction of graphene oxide in the polymer solution. Nanoscale Res. Lett. 2013, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Prasertsri, S.; Lagarde, F.; Rattanasom, N.; Sirisinha, C.; Daniel, P. Raman spectroscopy and thermal analysis of gum and silica-filled NR/SBR blends prepared from latex system. Polym. Test. 2013, 32, 852–861. [Google Scholar] [CrossRef]
- Pavlovsky, S.; Siegmann, A. Chemical sensing materials II: Electrically conductive peroxide crosslinked SEBS copolymers systems. J. Appl. Polym. Sci. 2009, 114, 1390–1396. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Structural and Electrochemical Properties of Nanocomposite Polymer Electrolyte for Electrochemical Devices. Ind. Eng. Chem. Res. 2014, 53, 14993–15001. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef]
| Sample | Modulus E100 (MPa) | Modulus E300 (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| NR | 0.80 | 1.53 | 19.5 | 754 |
| NR/rGO 1 wt.% | 1.07 | 2.74 | 25.2 | 674 |
| NR/rGO 2 wt.% | 1.52 | 4.37 | 22.1 | 596 |
| NR/rGO 3 wt.% | 2.14 | 6.07 | 24.1 | 602 |
| NR/rGO 4 wt.% | 1.93 | 5.49 | 15.9 | 495 |
| w-NR/rGO 1 wt.% | 0.96 | 2.44 | 24.9 | 691 |
| w-NR/rGO 2 wt.% | 1.52 | 4.70 | 24.8 | 620 |
| w-NR/rGO 3 wt.% | 1.95 | 6.17 | 19.4 | 526 |
| w-NR/rGO 4 wt.% | 2.48 | 7.44 | 19.3 | 423 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Bolados, H.; Contreras-Cid, A.; Neira-Carrillo, A.; Lopez-Manchado, M.; Yazdani-Pedram, M. Removal of Surfactant from Nanocomposites Films Based on Thermally Reduced Graphene Oxide and Natural Rubber. J. Compos. Sci. 2019, 3, 31. https://doi.org/10.3390/jcs3020031
Aguilar-Bolados H, Contreras-Cid A, Neira-Carrillo A, Lopez-Manchado M, Yazdani-Pedram M. Removal of Surfactant from Nanocomposites Films Based on Thermally Reduced Graphene Oxide and Natural Rubber. Journal of Composites Science. 2019; 3(2):31. https://doi.org/10.3390/jcs3020031
Chicago/Turabian StyleAguilar-Bolados, Hector, Ahirton Contreras-Cid, Andronico Neira-Carrillo, Miguel Lopez-Manchado, and Mehrdad Yazdani-Pedram. 2019. "Removal of Surfactant from Nanocomposites Films Based on Thermally Reduced Graphene Oxide and Natural Rubber" Journal of Composites Science 3, no. 2: 31. https://doi.org/10.3390/jcs3020031
APA StyleAguilar-Bolados, H., Contreras-Cid, A., Neira-Carrillo, A., Lopez-Manchado, M., & Yazdani-Pedram, M. (2019). Removal of Surfactant from Nanocomposites Films Based on Thermally Reduced Graphene Oxide and Natural Rubber. Journal of Composites Science, 3(2), 31. https://doi.org/10.3390/jcs3020031







