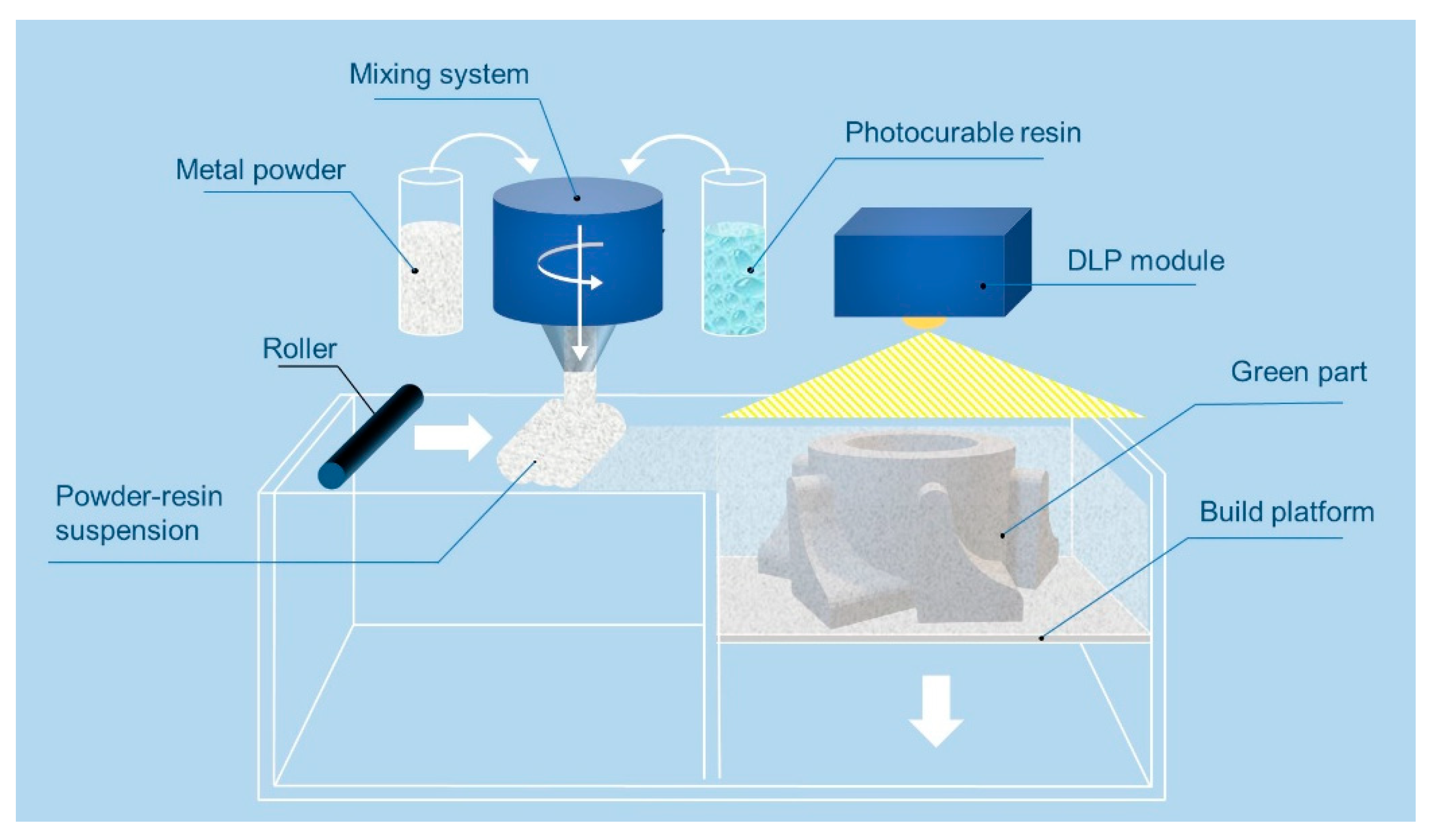
2. Materials and Methods
It has been demonstrated in prior studies that the SEAM process can be utilized to print SS420, resulting in the attainment of a relative density that exceeds 99.7%, while maintaining the integrity of the material and the absence of any deformation or cracking. However, this outcome was highly dependent on the two-step printing strategy, which renders the precured suspensions non-reusable, thereby increasing material expenditures and wasting overtime. In the absence of a precuring process, the quality of the printed samples is significantly compromised due to the occurrence of segregation issues. This phenomenon can result in the failure of the printing process. As shown in
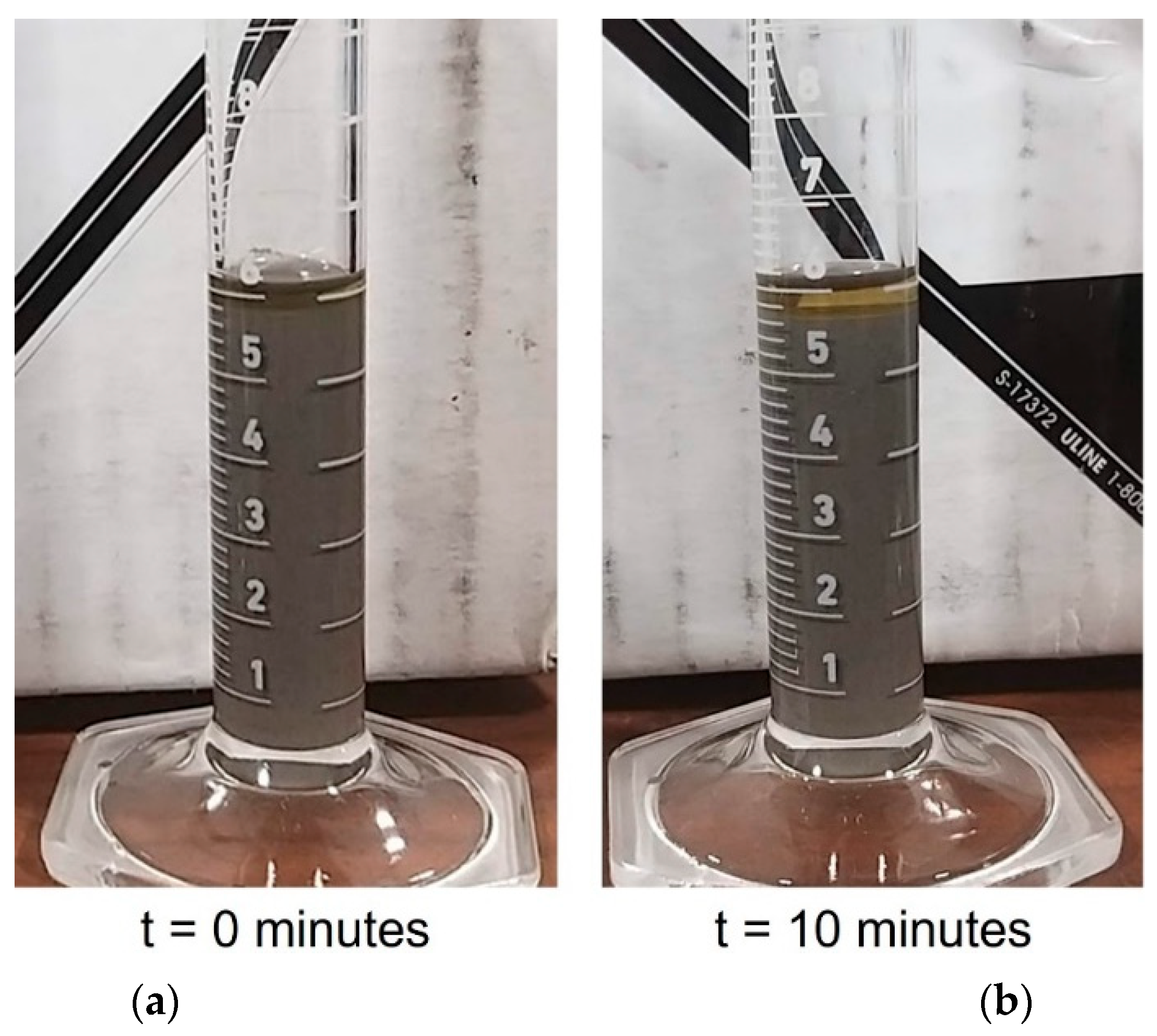
Figure 4, the mixing of 90 wt% SS420 powder (−125/+45 μm) (Oerlikon Metco—Plymouth, MI, USA) with 10 wt% CPS 3010 UV-curable resin (Colorado Photopolymer Solution—Boulder, CO, USA) using a benchtop high-speed mixer (DAC 150.1 FVZ, FlackTek SpeedMixer—Landrum, SC, USA) operating at 1500 rpm for 90 s corresponds to a powder loading of approximately 57 vol%. It was observed that after 10 min of rest, a substantial sedimentation phenomenon occurred. This observation indicates that, in the absence of precuring, the sedimentation of the uncured suspension commences within 10 min of printing, a process which may potentially result in print failure.
To address the issue of sedimentation [
9], the initial approach was to increase the powder loading. This study hypothesizes that each metal powder particle absorbs a certain amount of resin when the resin is mixed with metal powder. The adsorption capacity of metal powder is subjected to a saturation limit; if the resin content does not exceed this limit, sedimentation will not occur. This phenomenon occurs because all the resin is completely absorbed onto the surface of the metal powder particles, resulting in no excess resin remaining in the suspension. To test this hypothesis, an experiment was conducted in which the metal powder content was increased continuously while the resin content was reduced until the resin percentage fell below the saturation threshold. It was determined that, upon reaching a metal powder content of 92.65 wt%, sedimentation was fully eradicated. As shown in
Figure 5, even after a duration of 30 min, no sedimentation was observed. This finding suggests that, given the disparity in density between the metal powder and the resin, provided that the resin content remains below 7.35 wt%, sedimentation does not occur. Consequently, the issue of sedimentation was effectively addressed. However, the reduced resin content led to a substantial decrease in the suspension’s flowability. Given the SEAM process’s requirement of a highly flowable suspension to ensure even distribution by the roller onto the print platform, enhancing the suspension’s flowability emerged as the subsequent focal point of our research endeavors.
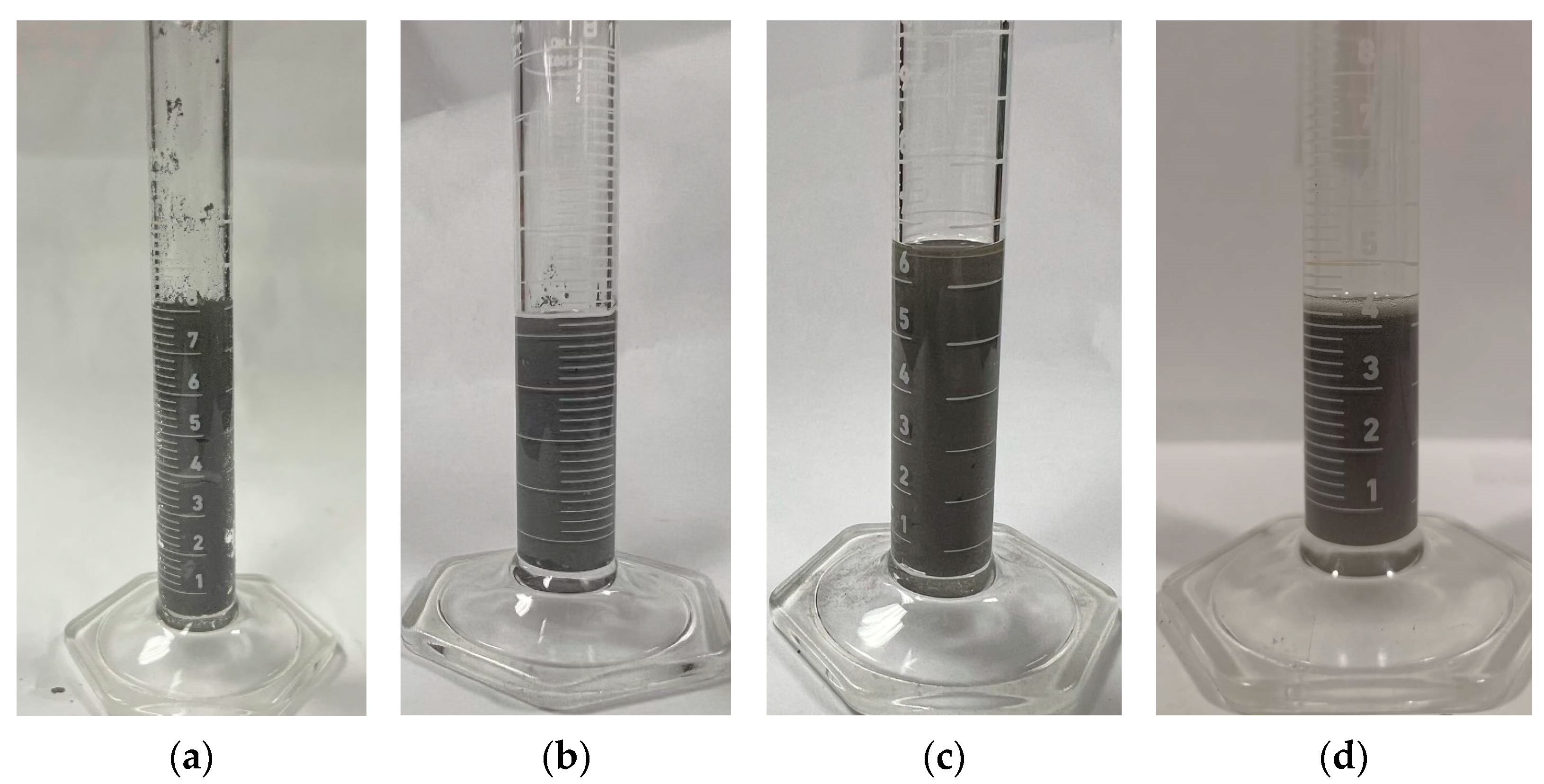
To enhance the flowability of the suspension, and to address the issue of particle sedimentation, dispersants were investigated as a potential solution. Several types of dispersants were evaluated by mixing them into SS420 suspensions at identical weight ratios. A total of 92.65 g of SS420 powder was mixed with 7.35 g of CPS3010 resin to prepare the suspension. Then, 2 g of each dispersant—BYK3560, BYK111, BYK2013, and BYK2155 (BYK-Gardner GmbH, Geretsried, Germany)—was individually added into 100 g of the prepared suspension (2 wt% of dispersant). Each mixture was subsequently homogenized using a high-speed mixer at 1500 rpm for 90 s to ensure uniform particle distribution. The flowability and sedimentation behavior of the suspensions were assessed followed by mixing. After 10 min of static observation, as illustrated in
Figure 6, samples (a), (b), and (c) exhibited varying degrees of sedimentation. A distinct layer of resin could be seen at the top of the graduated cylinders, while the SS420 powders had settled to the bottom. In contrast, sample (d), which contained BYK2155, showed a significantly reduced level of sedimentation under the same conditions. Based on this observation, subsequent experiments focused on optimizing the amount of BYK2155 by varying its concentration to identify the optimal mixing ratio.
BYK 2155 (BYK-Gardner GmbH, Geretsried, Germany) was incorporated. In order to facilitate the experimental process, the proportions of SS420 powder and CPS3010 resin were established with the previous ratio at 92.65 g and 7.35 g, respectively, while varying quantities of BYK 2155 were incorporated. The composition of each suspension, including the content of different materials, is listed in
Table 1. Each sample was subjected to a high-speed mixer at 1500 rpm for 90 s again to ensure the uniform distribution of particles within the suspension. The flowability of the suspension was investigated while monitoring its sedimentation.
3. Results
As demonstrated in
Figure 7, suspensions (a), (b), (c), and (d) correspond to the addition of 0 g, 0.3 g, 0.5 g, and 1 g of BYK 2155, respectively, following a 10 min setting period. After resting for 10 min, suspensions (a), (b), (c) or (d) did not reveal the phenomenon of sedimentation. However, upon further observation, it was found that although sample (a) did not show sedimentation, its flowability was significantly inadequate due to the absence of a dispersant. In contrast, the flowability of samples (b), (c) and (d) was notably enhanced by the addition of a dispersant. Among the samples examined, two of them which contained the higher amount of dispersant (sample c and sample d) exhibited optimal flowability. However, the superiority of sample (c) and (d) in terms of flowability does not guarantee its supremacy in this regard. Given that the printed samples are to undergo a debinding process, the presence of excessive dispersant content poses several issues. Dispersants, on the other hand, tend to be less volatile during debinding and are prone to carbonization at high temperatures, which can negatively impact the mechanical properties of the samples during the subsequent sintering process. Additionally, the utilization of excessive dispersant quantities has been observed to diminish powder loading, thereby impeding the attainment of high-density samples. Furthermore, the experimental findings demonstrated that the presence of dispersants diminished the curability of the suspension, leading to an increased amount of curing time for each layer and ultimately prolonging the printing process. As a result, sample (c) and (d) show poor curability during the experiment compared to (b). After a thorough evaluation of the available factors, it was determined that sample (b) would be the focus of subsequent experimentation. The composition of the substance under consideration contains a relatively small amount of dispersant, the purpose of which is to minimize potential issues during the debinding process. This is achieved while still significantly enhancing flowability compared to sample (a).
The experimental findings indicated a decline in the curability of suspension with an increase in dispersant content. Despite the minimal addition of 0.3 wt% of dispersant, this adjustment yielded a substantial effect on the suspension’s curability. After resolving the issues of flowability and sedimentation, the UV curing depth of sample (b) was evaluated. Curing depth is a critical parameter in the printing process, as it is indicative of the extent to which UV light can penetrate through the suspension. This, in turn, determines the maximum thickness of each layer that can be cured. If the curing depth is inadequate, the UV light may not fully penetrate the bottom layer, which can result in a weakening of the adhesion between each layer. This inadequate bonding hinders the upper cured suspension from adequately adhering to the lower cured layer, thereby compromising the structural integrity of the printed part. The issue is exacerbated during the initial layer printing process due to inadequate adhesion to the build platform, which can result in the misalignment or detachment of the specimen during printing, ultimately leading to print failure. Furthermore, an excessive curing depth increases the thickness of each cured layer, thereby reducing the final resolution of the printed sample. Consequently, ascertaining the optimal curing depth is a crucial step in the printing process.
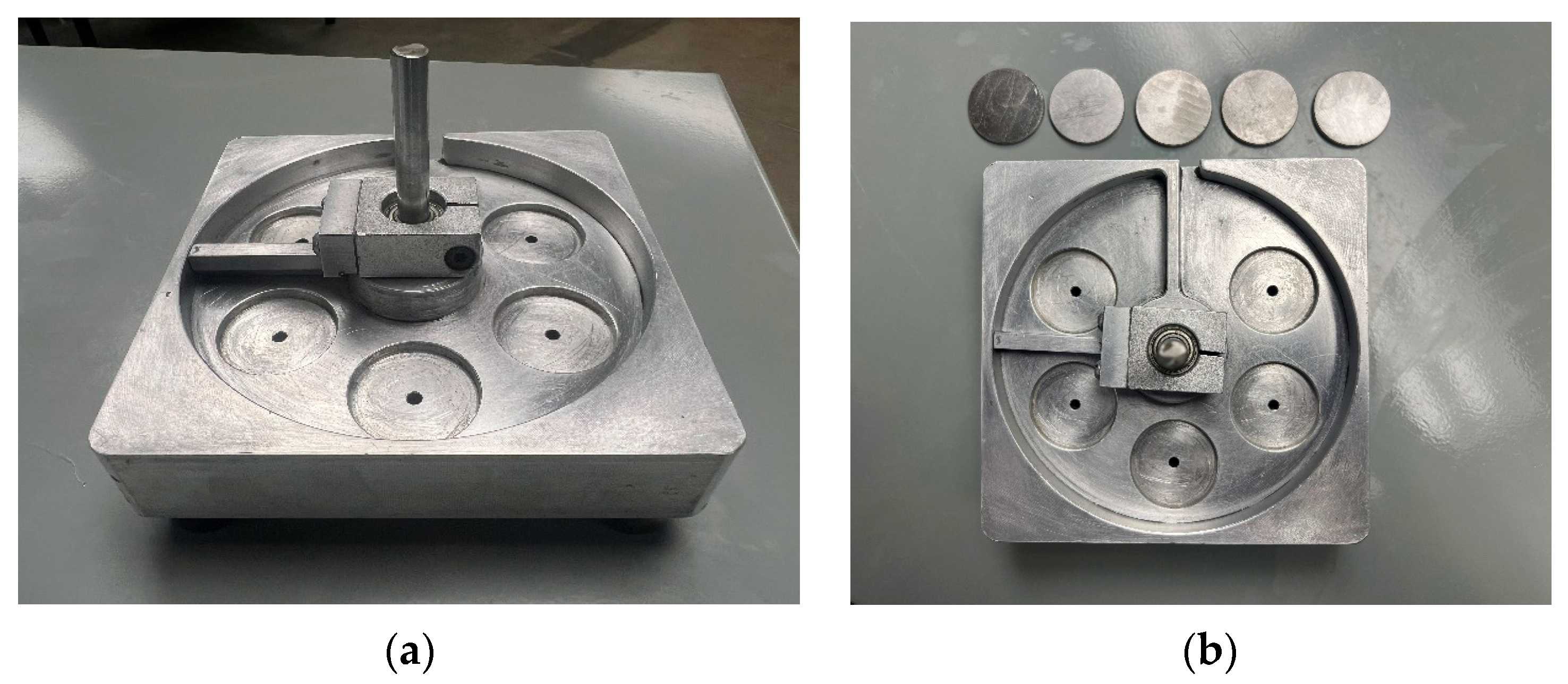
As previously indicated, the critical importance of curing depth necessitates the implementation of curability tests whenever there is a change in the suspension’s properties. Such alterations may occur in response to the use of diverse powder types or resins, or adjustments to powder loading. In this instance, the addition of the dispersant has resulted in alternations to the suspension’s curability, necessitating the execution of curability tests to ascertain the most appropriate curing depth. To accurately assess curing depth, a special device was designed, as shown in
Figure 8. The device under consideration features five circular pockets, each with a uniform depth of 300 µm. Additionally, to establish a range of curing depths, a series of five disks with thicknesses of 150 µm, 175 µm, 200 µm, 225 µm, and 250 µm were designed and fabricated. When positioned within the pre-designed 300 µm pockets, these disks generate gaps with dimensions of 50 µm, 75 µm, 100 µm, 125 µm, and 150 µm, respectively.
After determining the gap above each disk, the prepared sample (b) suspension was poured into one side of the device. The suspension was then spread across the device toward the other side using a spreading blade. Consequently, layers of suspension with thicknesses of 50 µm, 75 µm, 100 µm, 125 µm, and 150 µm were obtained, and distributed above each disk. Subsequently, as shown in
Figure 9a, a DLP (3DLP9000, Digital Light Innovations—Austin, TX, USA) was used to project UV light onto the five disks for 55 s. After the exposure, each disk surface was thoroughly rinsed with water. If the curing depth proved to be inadequate, that is, in instances where the UV light failed to fully penetrate the bottom of the suspension layer, the cured sample was susceptible to detachment when subjected to water pressure during the rinsing process. Conversely, if the sample remained intact after rinsing, it indicated that the UV light successfully penetrated the entire thickness, confirming sufficient curability at that thickness. As shown in
Figure 9b, following 55 s of UV exposure, all specimens maintained their integrity during the rinsing procedure, suggesting that sample (b) demonstrated sufficient curability for layer thicknesses ranging from 50 µm to 150 µm. To ensure the maintenance of high printing speed, a curing depth of 150 µm was selected, which was then designated as the layer thickness for the printing process.
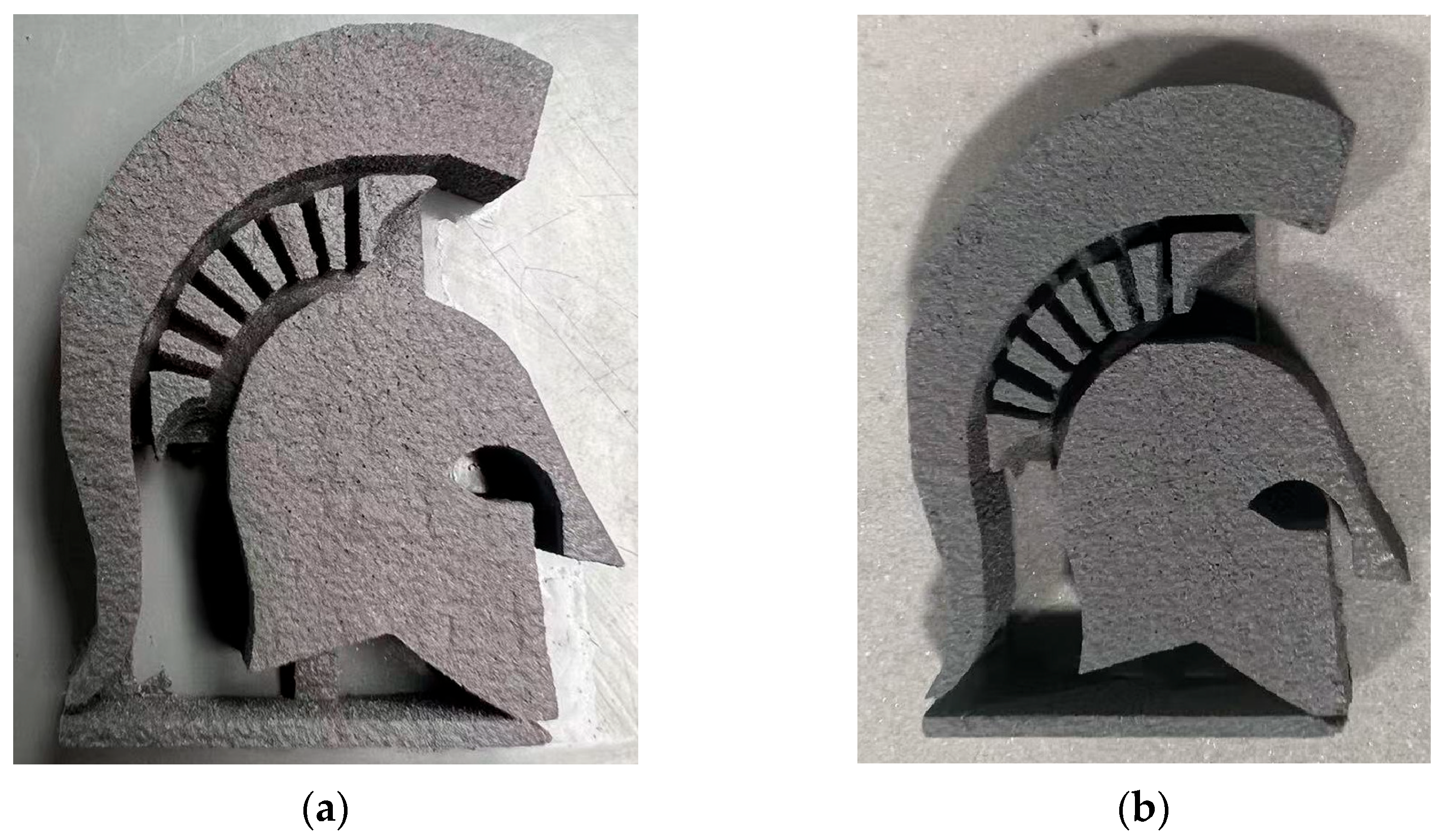
After conducting various tests on sample (b) suspension, the optimal parameters were determined: a curing time of 55 s and a layer thickness of 150 µm. According to the findings of previous studies, the enhancement of packing density was achieved through the implementation of a mathematical model in conjunction with a simulation technique, which consequently led to the selection of a bimodal powder mixture. The SS420 powders were characterized by particle size distributions of −125/+45 µm and −45/+15 µm and were blended in a 67:34 ratio. Moreover, prior research has demonstrated that adding boron nitride as a sintering additive effectively reduces the eutectic temperature of SS420, thereby enhancing the sintering density. Utilizing the aforementioned parameters, the Green Part is fabricated through the SEAM process as illustrated in
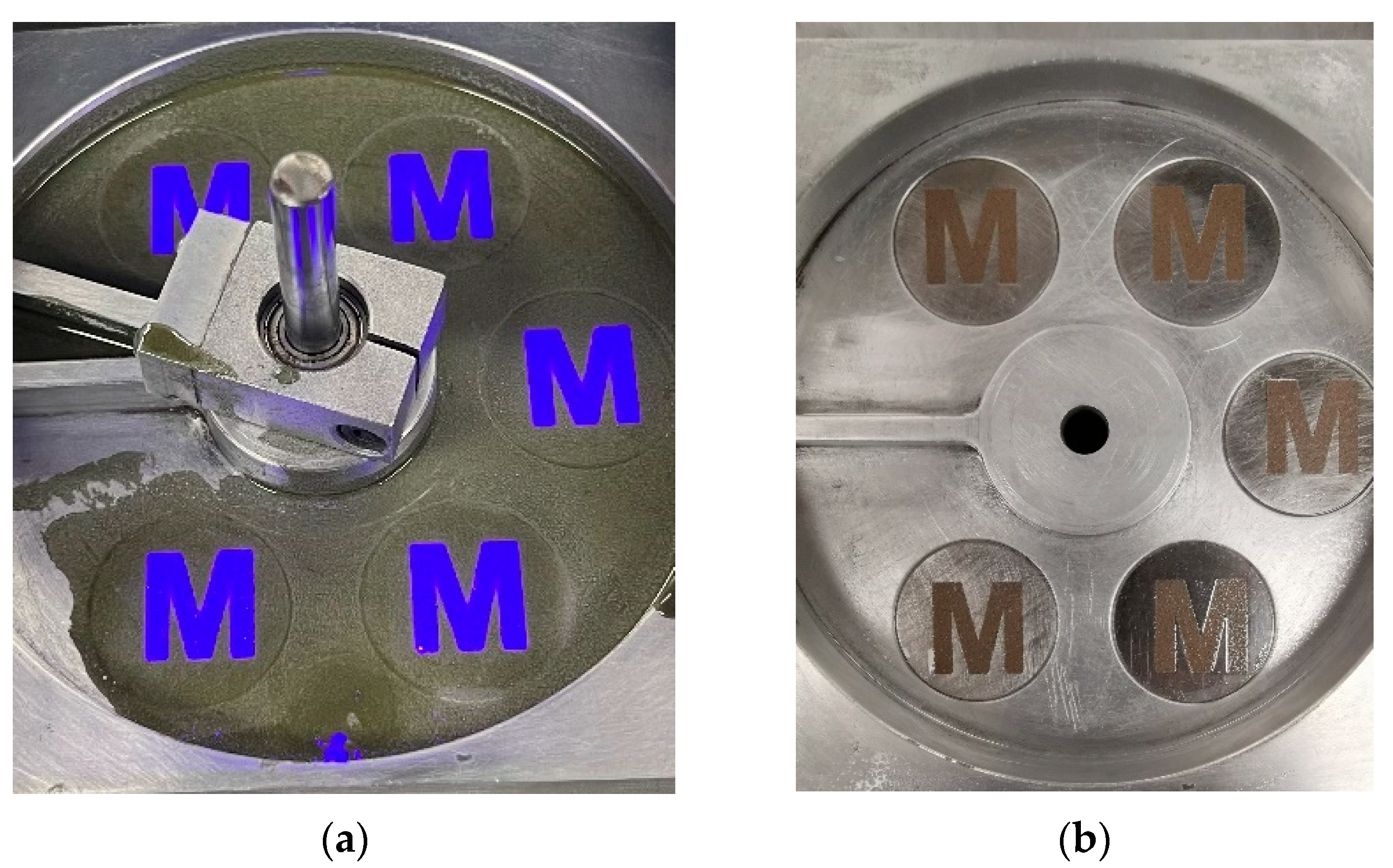
Figure 10a. Before the implementation of the dispersant, the two-step printing strategy was employed to address the segregation issue. While this method effectively mitigated segregation, the short-time precuring process caused the resin to enter a semi-cured state, rendering it non-recyclable. However, in this printing process, the introduction of a dispersant served to address the issue of segregation, thereby eliminating the necessity for the two-step printing strategy, as shown in
Figure 3. As a result, the uncured portion of the suspension remains recyclable, thereby significantly reducing additional powder consumption and printing costs. To evaluate the quality of the recycled suspension, its curability and potential sedimentation behavior were examined. After recycling the suspension, the printing process was repeated using the same parameters as in the first printing process. As shown in
Figure 10b, another “Spartan helmet” was successfully fabricated using the recycled suspension under identical printing conditions.
After fabricating the Green Part, a debinding process is carried out to remove all the binder used to hold the particles together. The optimal debinding parameters, determined in a previous study [
7,
10], are as follows: initial heating at a rate of 10 °C/min to 150 °C, followed by heating at 1 °C/min to 430 °C, and concluding with a 6 h hold at 430 °C in an air environment. This process ensures that the majority of the binder evaporates while allowing necking among particles [
6,
8] to form. This, in turn, helps maintain structural integrity and prevents collapse after binder removal. Following the debinding process, the samples are subjected to a sintering procedure at elevated temperatures under vacuum conditions. The present study sought to evaluate the impact of sintering temperature on the quality of the final product. To this end, two distinct temperatures, 1250 °C and 1300 °C, were selected for testing. The results of this study will provide a comparative analysis of the temperature–quality relationship, thereby offering valuable insights into the optimal sintering conditions for achieving superior product quality.
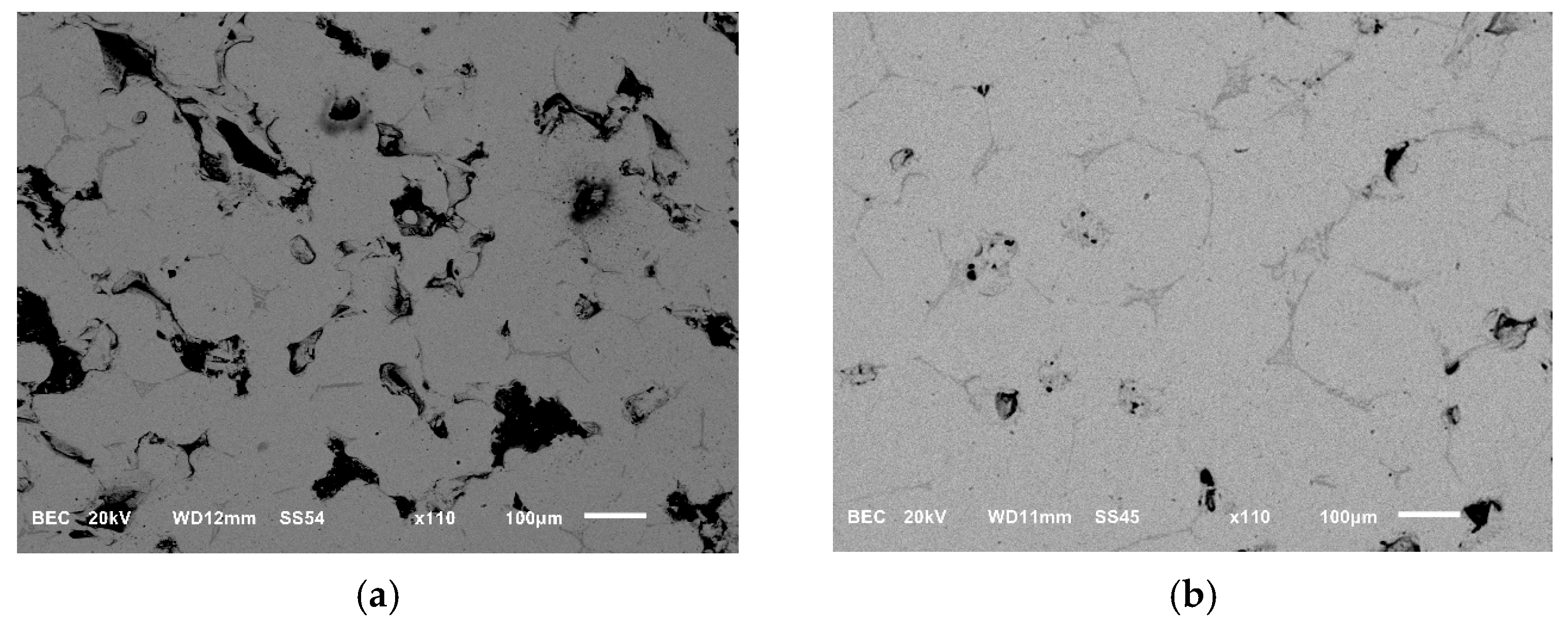
The sintered samples were initially subjected to a cutting and polishing procedure before undergoing cross-sectional analysis using a scanning electron microscope (SEM). Subsequently, the relative density of the samples was calculated using the imaging analysis software, ImageJ (version 1.54g, Java 1.8.0_345. 64-bit). As shown in
Figure 11, sample (a) was sintered under a vacuum atmosphere at a temperature of 1300 °C, while sample (b) was sintered under vacuum conditions at a temperature of 1250 °C. The relative density of samples (a) and (b) was calculated using ImageJ, yielding values of 99.33% and 92.15%, respectively. This finding suggests that a 50 °C increase in sintering temperature results in a substantial enhancement in relative density, evidenced by a substantial increase of 7.18%.
As previously stated, a two-step printing strategy was employed to prevent segregation during the printing process before the addition of a dispersant. The primary cause of suspension segregation is the significant density difference between SS420 powder and CPS3010 resin, leading to the sedimentation problem of SS420 powder. Despite employing a high-speed mixer to stir the suspension, it was still challenging to attain a complete and uniform coating of each SS420 powder with resin. When the interstitial distance between particles is sufficiently small, they tend to aggregate, thereby impeding the resin’s capacity to effectively encompass the particles and consequently reducing the flowability of the suspension. This phenomenon is attributable to the interplay of repulsive and attractive forces between particles. The repulsive force is attributed to surface charges on the particles, while the attractive force is predominantly attributable to Van der Waals interactions. In the case of particles that are close to one another, the Van der Waals attraction dominates over the repulsive force, thereby resulting in the aggregation of particles. As a result, the flowability of the suspension diminishes, and segregation intensifies. This explains why, before the addition of dispersants, the suspension demonstrated inadequate flowability and encountered more manifest segregation challenges.
After adding the dispersant, not only was the segregation issue effectively addressed [
11,
12], but the overall flowability of the suspension was notably enhanced, thereby ensuring a more stable and uniform distribution of particles within the suspension. This improvement can be primarily attributed to the increased spacing between individual particles, which allows the resin to completely and uniformly encapsulate each particle, thereby preventing direct contact between them. Improved encapsulation has been shown to yield a more homogeneous suspension, thereby mitigating the potential for adverse effects such as uneven distribution, sedimentation, or localized concentration differences that could negatively impact the printing process. Additionally, the larger gaps between particles weaken the Van der Waals attraction [
13,
14,
15,
16,
17,
18], thereby considerably hindering the propensity of particles to clump together or form aggregates. In the absence of these aggregates, the suspension’s flowability is unaffected, and the print quality remains uncompromised. As a result, the probability of particle aggregation is significantly diminished, leading to enhanced dispersion stability, enhanced suspension rheology, and superior flow properties.
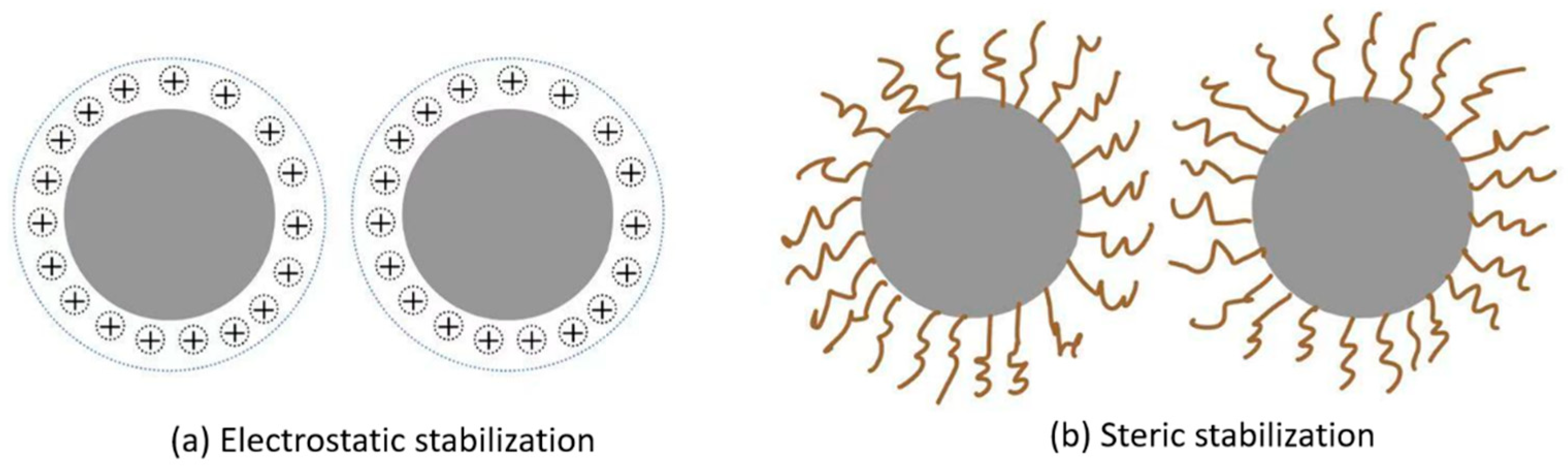
Farrokhpay [
11] discussed the stabilization of pigments, as shown in
Figure 12, which is a phenomenon of electrosteric stabilization [
19,
20,
21,
22,
23], which itself is a combination of electrostatic stabilization [
9,
24] and steric stabilization [
25]. Electrostatic stabilization is a critical component in ensuring the uniform and stable dispersion of particles in a suspension. The dispersant contains ionic polymers that strongly adsorb onto the surfaces of the particles, forming a protective charged layer around each particle. This charged layer introduces electrostatic repulsion, as charges inherently repel each other, thereby creating a repulsive force that counteracts the Van der Waals attraction force between particles, leading to their attraction and subsequent clustering. Due to this repulsion, the particles are forced to maintain a certain interstitial distance from one another, preventing them from coming close to each other and forming aggregates. The steric stabilization phenomenon exerts a similar function in repelling particles from one another, yet it does so via distinct mechanisms. Steric stabilization provides an additional mechanism to hinder particle aggregation by introducing steric hindrance—physical barriers that keep the particles separated. This occurs when polymers or surfactants adsorb onto particle surfaces, forming a protective layer of polymer chains that extend into the surrounding medium. As two particles approach one another, there is an overlap of their polymer chains, which generates osmotic pressure that exerts a force of separation between them. Concurrently, entropic repulsion manifests, impeding the process of aggregation.
This electrosteric stabilization ensures that the particles remain evenly dispersed throughout the suspension, effectively minimizing the risk of segregation, promoting better uniformity, and maintaining consistent material properties throughout the entire printing process. By improving the dispersion state, the suspension exhibits enhanced rheological behavior, ensuring more uniform and more predictable flow characteristics, which ultimately contribute to higher print quality, better structural integrity, and more efficient material utilization. This enhancement in printing process reliability and precision leads to more cost-effective utilization of materials and greater production efficiency.