Melt Electrowritten Biodegradable Mesh Implants with Auxetic Designs for Pelvic Organ Prolapse Repair
Abstract
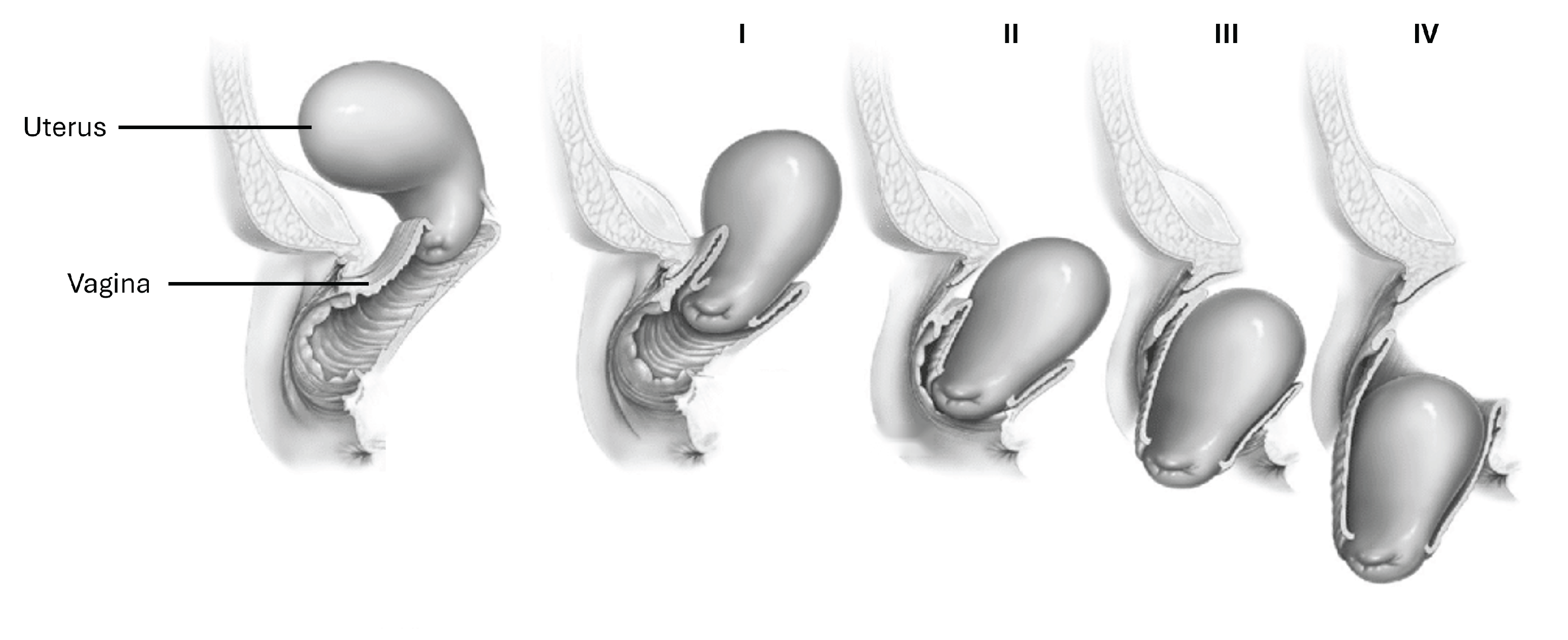
:1. Introduction
2. Materials and Methods
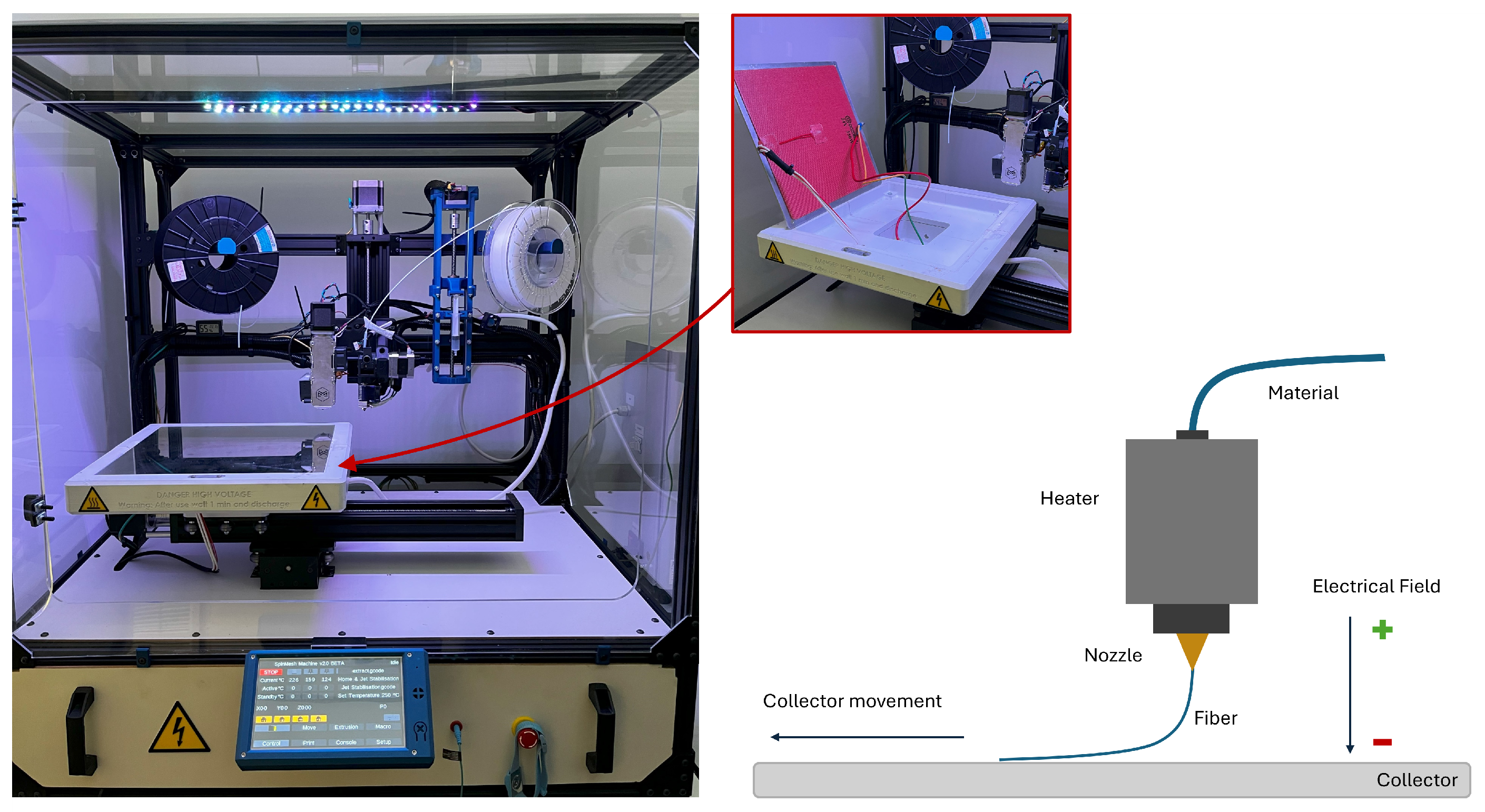
2.1. Printing Machine and PCL
2.2. 3D Printing Parameters
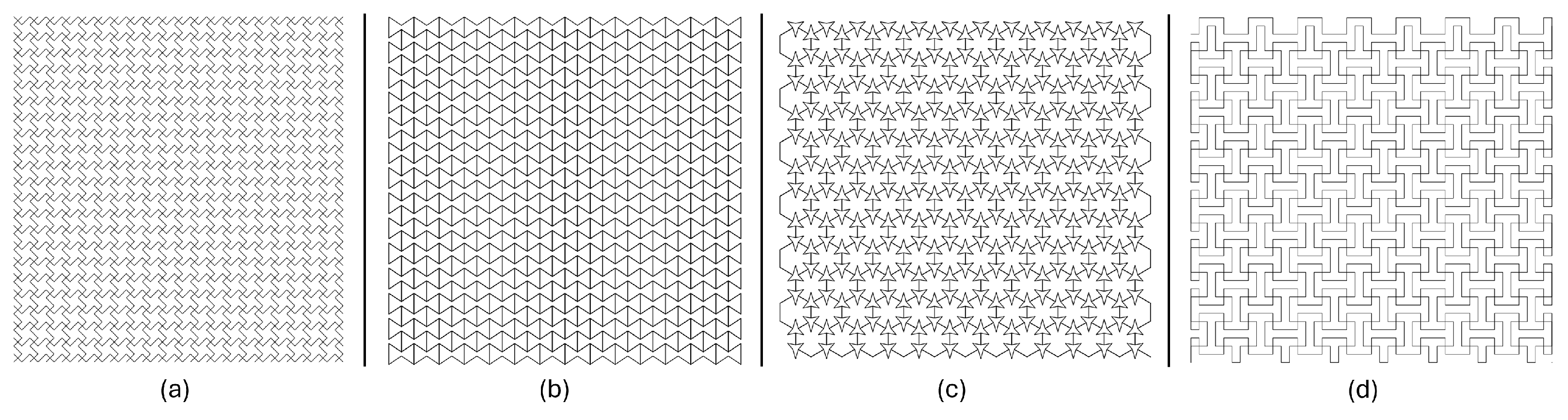
2.3. Geometry Conception
- 1.
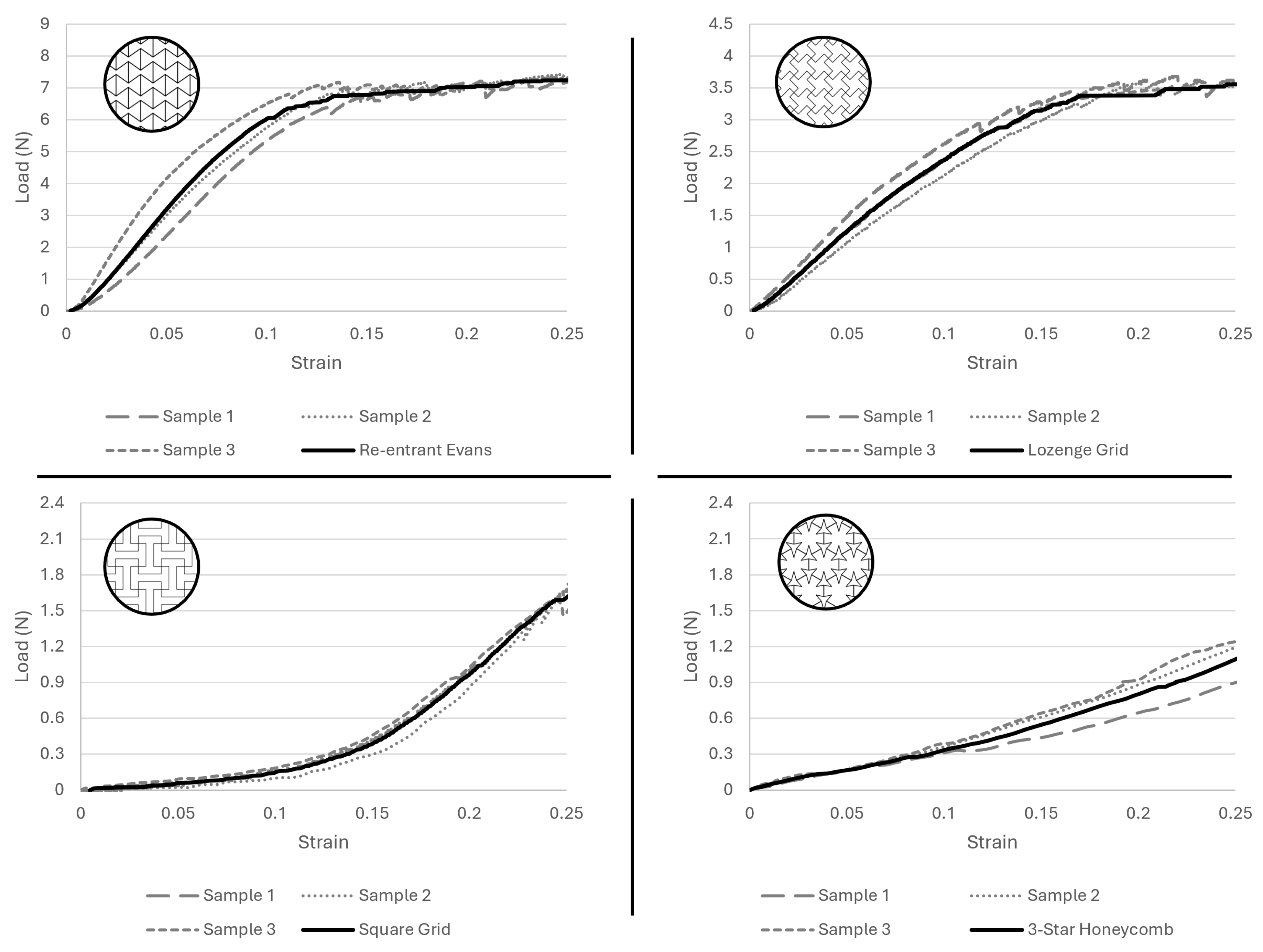
- Lozenge grid, Figure 3a:This configuration modifies the traditional hexagonal honeycomb by incorporating re-entrant (inward-buckling) cell walls. This design allows the structure to contract laterally under compression and to expand laterally when stretched. The angles of the re-entrant walls and the thickness can be adjusted to refine both the auxetic properties and mechanical performance of the material [26].
- 2.
- Re-entrant Evans, Figure 3b:The re-entrant honeycomb structure features unit cells with angles that protrude inward, forming an inverted or concave polygon shape. This arrangement enables the material to demonstrate a negative Poisson’s ratio, thus exhibiting auxetic behavior. The degree of auxeticity can be modified by altering the cell angles and the structure’s relative density [27,28,29].
- 3.
- Three-star honeycomb, Figure 3c:This configuration features unit cells shaped like three-pointed stars, with arms extending from a central point. When mechanical forces are applied, the arms of the stars flex and rotate, resulting in a negative Poisson’s ratio [30].
- 4.
- Square grid, Figure 3d:Lozenge grids consist of diamond-shaped units arranged in a grid pattern. When subjected to tensile or compressive forces, the lozenge shapes can rotate and deform in a manner that leads to auxetic behavior. The extent of this behavior is influenced by the geometry of the lozenges and their connectivity [30].
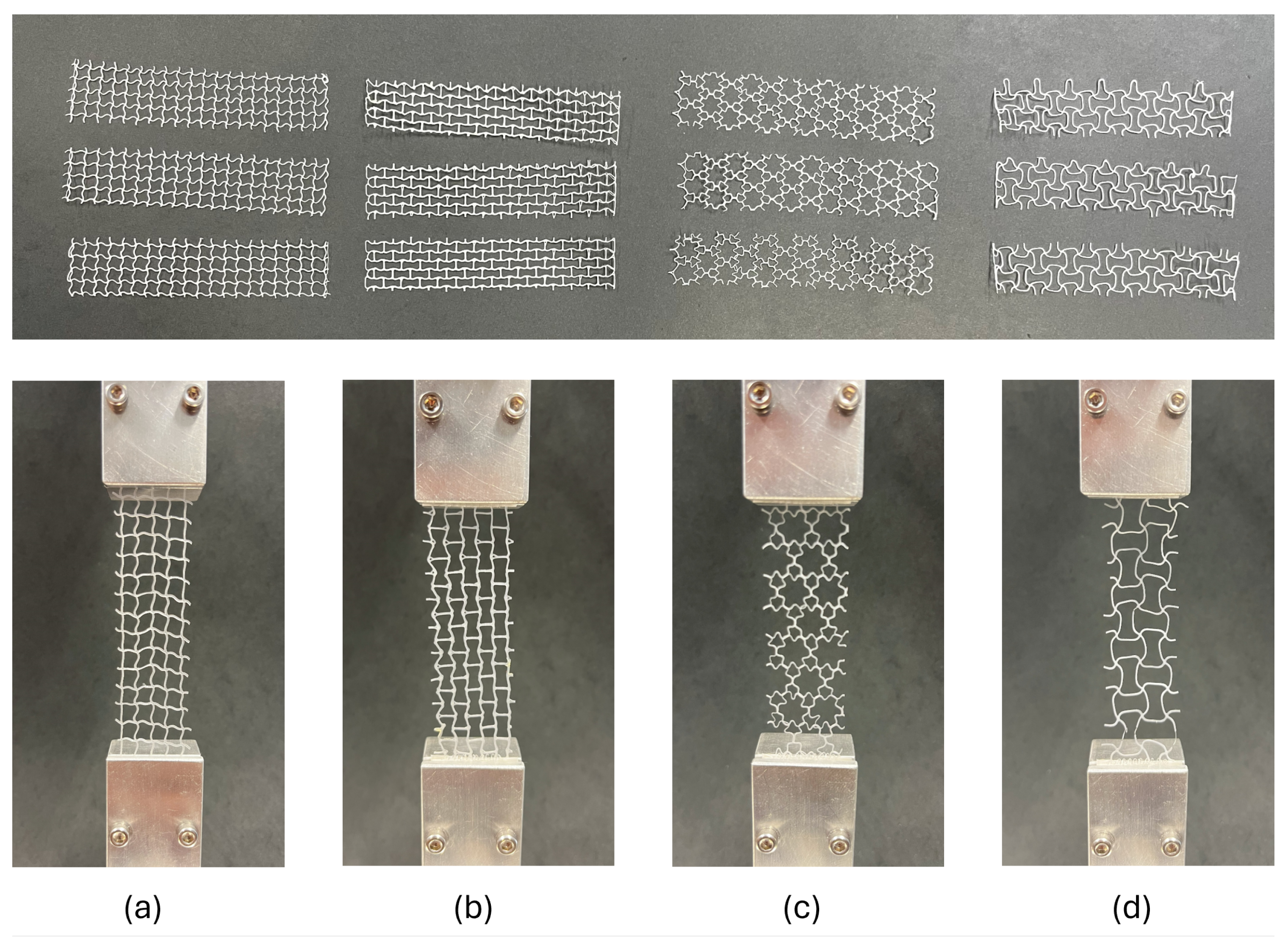
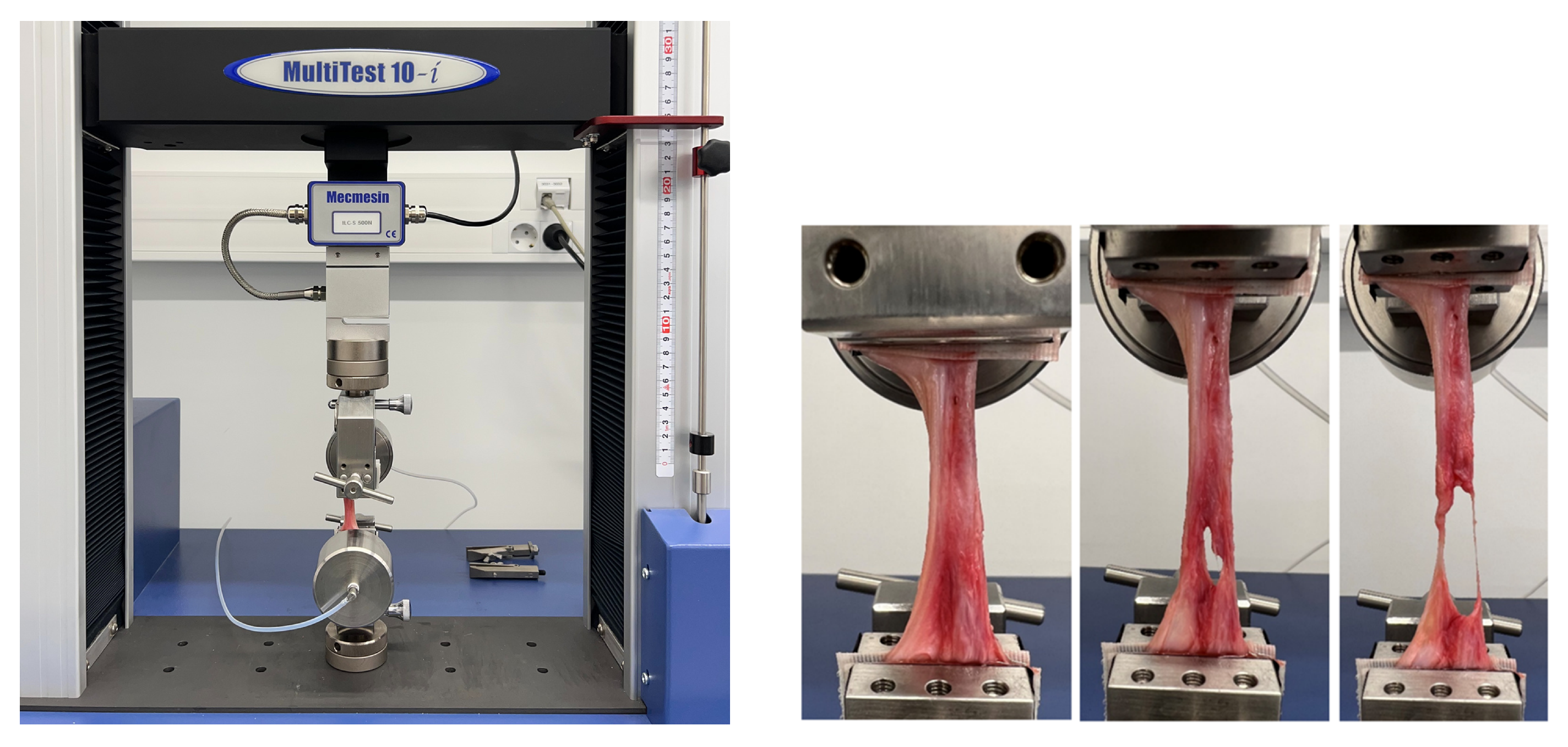
2.4. Mechanical Testing
2.4.1. Auxetic Meshes
2.4.2. Sow Tissue
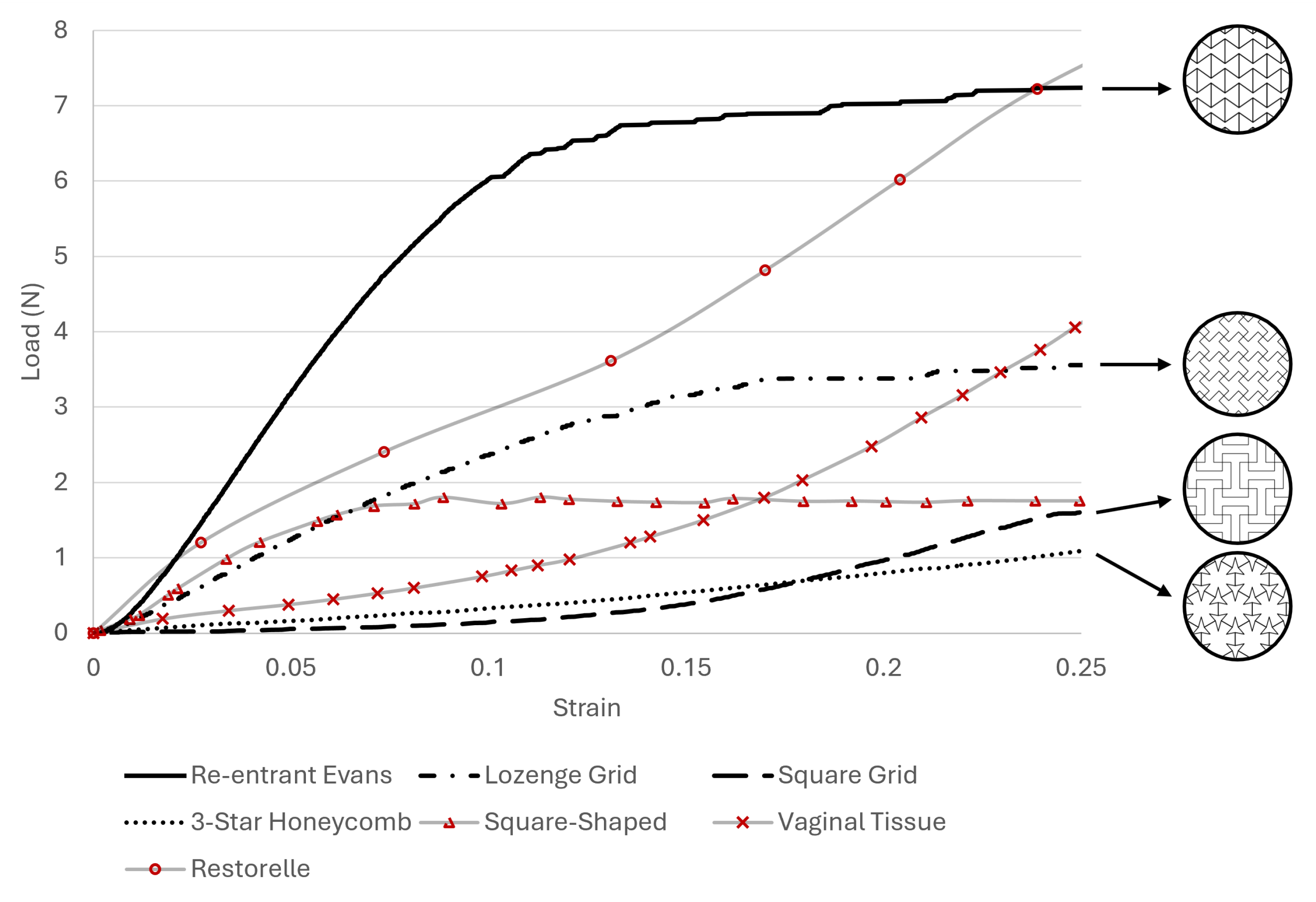
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef]
- MacCraith, E.; Cunnane, E.M.; Joyce, M.; Forde, J.C.; O’Brien, F.J.; Davis, N.F. Comparison of synthetic mesh erosion and chronic pain rates after surgery for pelvic organ prolapse and stress urinary incontinence: A systematic review. Int. Urogynecol. J. 2021, 32, 573–580. [Google Scholar] [CrossRef]
- Guler, Z.; Roovers, J.P. Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse. Biomolecules 2022, 12, 94. [Google Scholar] [CrossRef]
- FDA. FDA Takes Action to Protect Women’s Health, Orders Manufacturers of Surgical Mesh Intended for Transvaginal Repair of Pelvic Organ Prolapse to Stop Selling All Devices; FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. npj Regen. Med. 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sterk, S.; Silva, M.E.; Fernandes, A.A.; Huß, A.; Wittek, A. Development of new surgical mesh geometries with different mechanical properties using the design freedom of 3D printing. J. Appl. Polym. Sci. 2023, 140, e54687. [Google Scholar] [CrossRef]
- Vaz, M.F.; Martins, J.A.P.; Pinheiro, F.; Ferreira, N.M.; Brandão, S.; Alves, J.L.; Fernandes, A.A.; Parente, M.P.L.; Silva, M.E. Optimizing melt electrowriting prototypes for printing non-medical and medical grade polycaprolactone meshes in prolapse repair. J. Appl. Polym. Sci. 2024, 142, e56408. [Google Scholar] [CrossRef]
- Da Cunha, M.N.B.; Rynkevic, R.; da Silva, M.E.T.; da Silva Brandão, A.F.M.; Alves, J.L.; Fernandes, A.A. Melt Electrospinning Writing of Mesh Implants for Pelvic Organ Prolapse Repair. 3D Print. Addit. Manuf. 2022, 9, 389–398. [Google Scholar] [CrossRef]
- Rynkevic, R.; Silva, M.; Martins, P.; Mascarenhas, T.; Alves, J.; Fernandes, A. Characterisation of polycaprolactone scaffolds made by melt electrospinning writing for pelvic organ prolapse correction - a pilot study. Mater. Today Commun. 2022, 32, 104101. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Baylon, K.; Rodriguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Gwak, S.J.; Cho, Y.S. Fabrication of polycaprolactone/nano hydroxyapatite (Pcl/nha) 3d scaffold with enhanced in vitro cell response via design for additive manufacturing (dfam). Polymers 2021, 13, 1394. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Cahill, P.A.; McGuinness, G.B. Solvent system effects on the physical and mechanical properties of electrospun Poly(-caprolactone) scaffolds for in vitro lung models. J. Mech. Behav. Biomed. Mater. 2022, 136, 105493. [Google Scholar] [CrossRef]
- Kim, Y.; Son, K.H.; Lee, J.W.; Kim, C.; Son, Y.; Lee, K.H. Auxetic Structures for Tissue Engineering Scaffolds and Biomedical Devices. Materials 2021, 14, 6821. [Google Scholar] [CrossRef]
- Chen, C.C.G.; Ridgeway, B.; Paraiso, M.F.R. Biologic grafts and synthetic meshes in pelvic reconstructive surgery. Clin. Obstet. Gynecol. 2007, 50, 383–411. [Google Scholar] [CrossRef] [PubMed]
- Vashaghian, M.; Zaat, S.J.; Smit, T.H.; Roovers, J.P. Biomimetic implants for pelvic floor repair. Neurourol. Urodyn. 2018, 37, 566–580. [Google Scholar] [CrossRef]
- Saxena, K.K.; Das, R.; Calius, E.P. Three Decades of Auxetics Research - Materials with Negative Poisson’s Ratio: A Review. Adv. Eng. Mater. 2016, 18, 1847–1870. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.H.; Lee, J.H.; Park, K.Y.; Son, K.H.; Lee, J.W. Development of Multi-layer Tubular Vascular Scaffold to Enhance Compliance by Exhibiting a Negative Poisson’s Ratio. Int. J. Precis. Eng. Manuf. Green Technol. 2021, 8, 841–853. [Google Scholar] [CrossRef]
- Kapnisi, M.; Mansfield, C.; Marijon, C.; Guex, A.G.; Perbellini, F.; Bardi, I.; Humphrey, E.J.; Puetzer, J.L.; Mawad, D.; Koutsogeorgis, D.C.; et al. Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater. 2018, 28, 1800618. [Google Scholar] [CrossRef]
- Mardling, P.; Alderson, A.; Jordan-Mahy, N.; Maitre, C.L.L. The use of auxetic materials in tissue engineering. Biomater. Sci. 2020, 8, 2074–2083. [Google Scholar] [CrossRef]
- Raggio, J.I.C.; Arancibia, C.T.; Millán, C.; Ploeg, H.L.; Aiyangar, A.; Vivanco, J.F. Height-to-Diameter Ratio and Porosity Strongly Influence Bulk Compressive Mechanical Properties of 3D-Printed Polymer Scaffolds. Polymers 2022, 14, 5017. [Google Scholar] [CrossRef]
- 3D4Makers. Facilan™ PCL 100 Filament|3D Printing.
- Gleadall, A. FullControl GCode Designer: Open-source software for unconstrained design in additive manufacturing. Addit. Manuf. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Vaz, M.F.R.; Martins, J.A.P.; Pinheiro, F.; Ferreira, N.M.; Brandão, S.; Alves, J.L.; Fernandes, A.A.; Parente, M.P.L.; Silva, M.E.T. Medical- and Non-Medical-Grade Polycaprolactone Mesh Printing for Prolapse Repair: Establishment of Melt Electrowriting Prototype Parameters. Appl. Sci. 2024, 14, 9670. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, D. Mechanical Properties of Auxetic Cellular Material Consisting of Re-Entrant Hexagonal Honeycombs. Materials 2016, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Mustahsan, F.; Khan, S.Z.; Zaidi, A.A.; Alahmadi, Y.H.; Mahmoud, E.R.I.; Almohamadi, H.; Zhang, J.; Liu, L.; Wang, Y.; Gao, X.; et al. Re-Entrant Honeycomb Auxetic Structure with Enhanced Directional Properties. Materials 2022, 15, 8022. [Google Scholar] [CrossRef]
- Masters, I.G.; Evans, K.E. Models for the elastic deformation of honeycombs. Compos. Struct. 1996, 35, 403–422. [Google Scholar] [CrossRef]
- Smith, C.W.; Grima, J.N.; Evans, K.E. A novel mechanism for generating auxetic beha in reticulated foams: Missing rib foam model. Acta Mater. 2000, 48, 4349–4356. [Google Scholar] [CrossRef]
- Kolken, H.M.; Zadpoor, A.A. Auxetic mechanical metamaterials. RSC Adv. 2017, 7, 5111–5129. [Google Scholar] [CrossRef]
- Squier, C.A.; Mantz, M.J.; Schlievert, P.M.; Davis, C.C. Porcine vagina Ex Vivo as a model for studying permeability and pathogenesis in mucosa. J. Pharm. Sci. 2008, 97, 9–21. [Google Scholar] [CrossRef]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet. Res. 2015, 46, 116. [Google Scholar] [CrossRef]
- Soares, C.; Martins, P.; Silva, E.; Hympanova, L.; Rynkevic, R. Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept. Surgeries 2022, 3, 101–110. [Google Scholar] [CrossRef]
- Vaz, M.F.R.R.; Silva, M.E.; Parente, M.; Brandão, S.; Fernandes, A.A. 3D printing and development of computational models of biodegradable meshes for pelvic organ prolapse. Eng. Comput. 2024, 41, 1399–1423. [Google Scholar] [CrossRef]
- Hedayati, R.; Yousefi, A.; Dezaki, M.L.; Bodaghi, M. Analytical relationships for 2D Re-entrant auxetic metamaterials: An application to 3D printing flexible implants. J. Mech. Behav. Biomed. Mater. 2023, 143, 105938. [Google Scholar] [CrossRef] [PubMed]
- Gharehbaghi, H.; Jamshidi, M.; Almomani, A. Experimental and numerical investigation of energy absorption in honeycomb structures based on lozenge grid unit cells under various loading angles. Compos. Part Open Access 2024, 15, 100546. [Google Scholar] [CrossRef]
- Pinheiro, D. Structure Design Optimisation Of Biodegradable Implants For Melt Electrowriting. Master’s Thesis, Faculty of Engineering of Porto, Porto, Portugal, 2022. Available online: https://repositorio-aberto.up.pt/bitstream/10216/143649/2/575022.pdf (accessed on 14 October 2024).
- Li, X.; Li, Z.; Guo, Z.; Zhou, Y.; Han, S.; Mo, Z.; Li, J. A Parametric Study on Sandwich Structures with Star-Shaped Honeycomb Core Under Blast Loads; IOP Publishing: Bristol, UK, 2024; p. 42016. [Google Scholar] [CrossRef]
- Charkaoui, A.; Hassan, N.M.; Bahroun, Z. Enhancing Mechanical Properties of Cellular Core Sandwich Panels: A Review of Topological Parameters and Design Improvements. Mater. Res. Express 2023, 10, 102001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, N.M.; Antoniadi, E.; Silva, A.T.; Silva, A.; Parente, M.; Fernandes, A.; Silva, E. Melt Electrowritten Biodegradable Mesh Implants with Auxetic Designs for Pelvic Organ Prolapse Repair. J. Manuf. Mater. Process. 2025, 9, 111. https://doi.org/10.3390/jmmp9040111
Ferreira NM, Antoniadi E, Silva AT, Silva A, Parente M, Fernandes A, Silva E. Melt Electrowritten Biodegradable Mesh Implants with Auxetic Designs for Pelvic Organ Prolapse Repair. Journal of Manufacturing and Materials Processing. 2025; 9(4):111. https://doi.org/10.3390/jmmp9040111
Chicago/Turabian StyleFerreira, Nuno Miguel, Evangelia Antoniadi, Ana Telma Silva, António Silva, Marco Parente, António Fernandes, and Elisabete Silva. 2025. "Melt Electrowritten Biodegradable Mesh Implants with Auxetic Designs for Pelvic Organ Prolapse Repair" Journal of Manufacturing and Materials Processing 9, no. 4: 111. https://doi.org/10.3390/jmmp9040111
APA StyleFerreira, N. M., Antoniadi, E., Silva, A. T., Silva, A., Parente, M., Fernandes, A., & Silva, E. (2025). Melt Electrowritten Biodegradable Mesh Implants with Auxetic Designs for Pelvic Organ Prolapse Repair. Journal of Manufacturing and Materials Processing, 9(4), 111. https://doi.org/10.3390/jmmp9040111






