Hindering Effect of Solid-Solutioning on Intermetallic Growth in Aluminum–Matrix Composite Reinforced with Mechanically Alloyed Ni-Cu Particles
Abstract
1. Introduction
2. Materials
3. Results
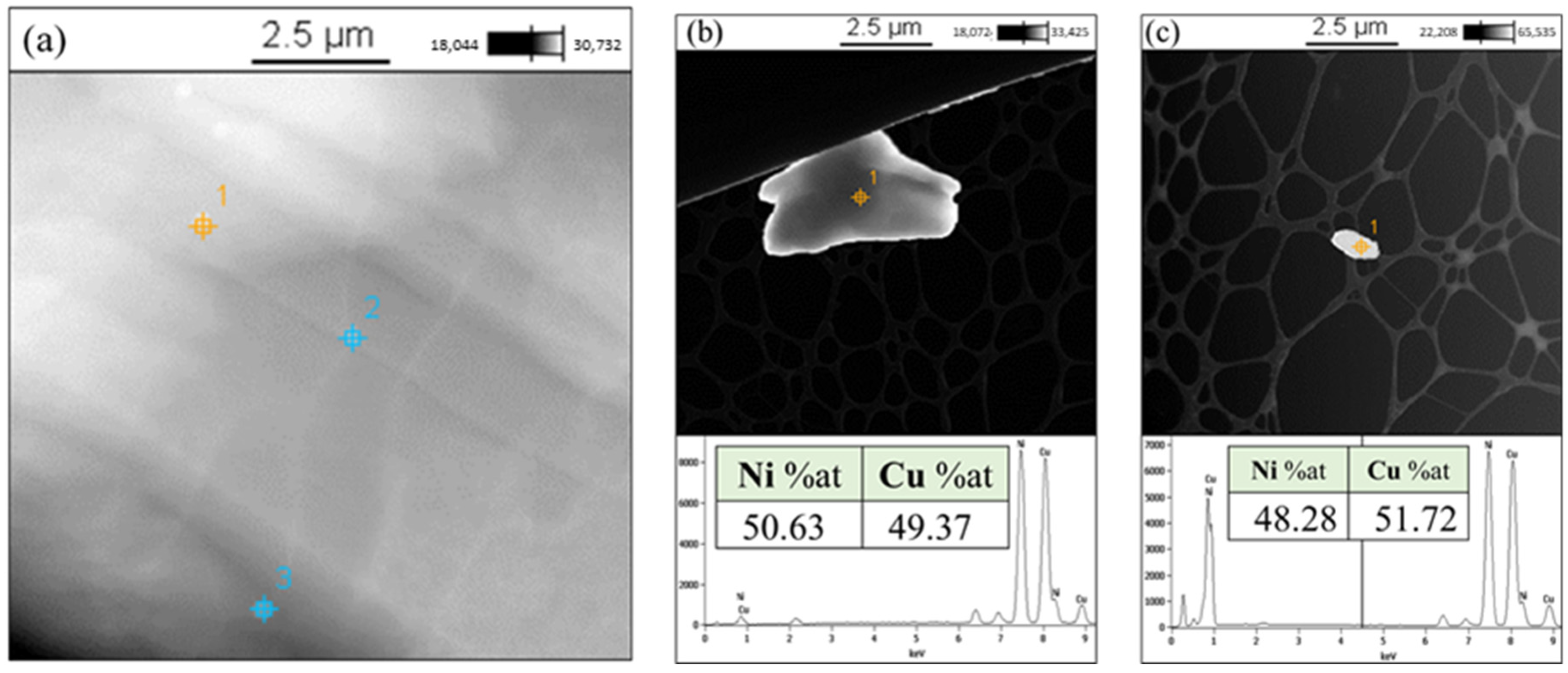
3.1. Investigation of Monel Powder
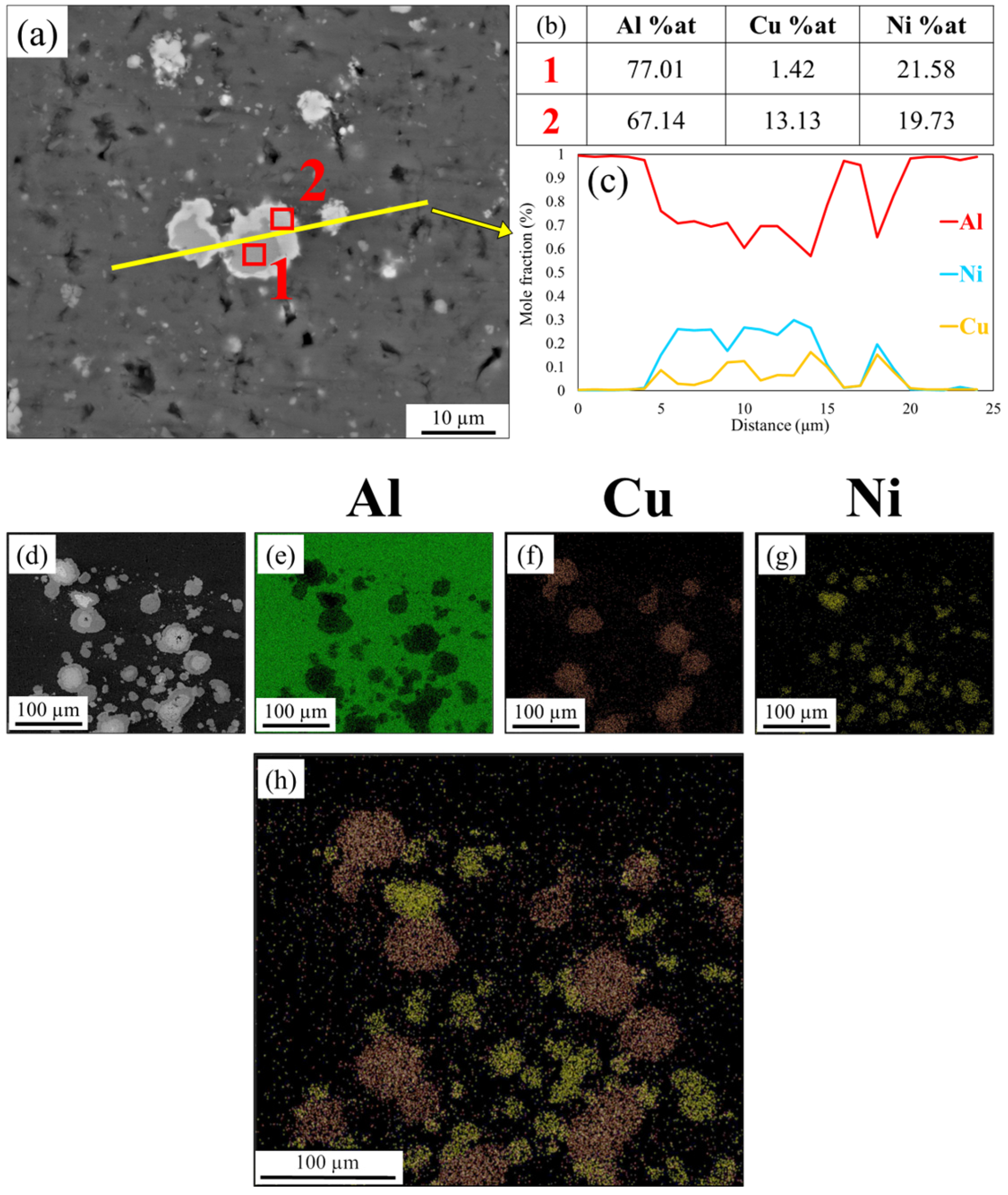
3.2. SEM Images
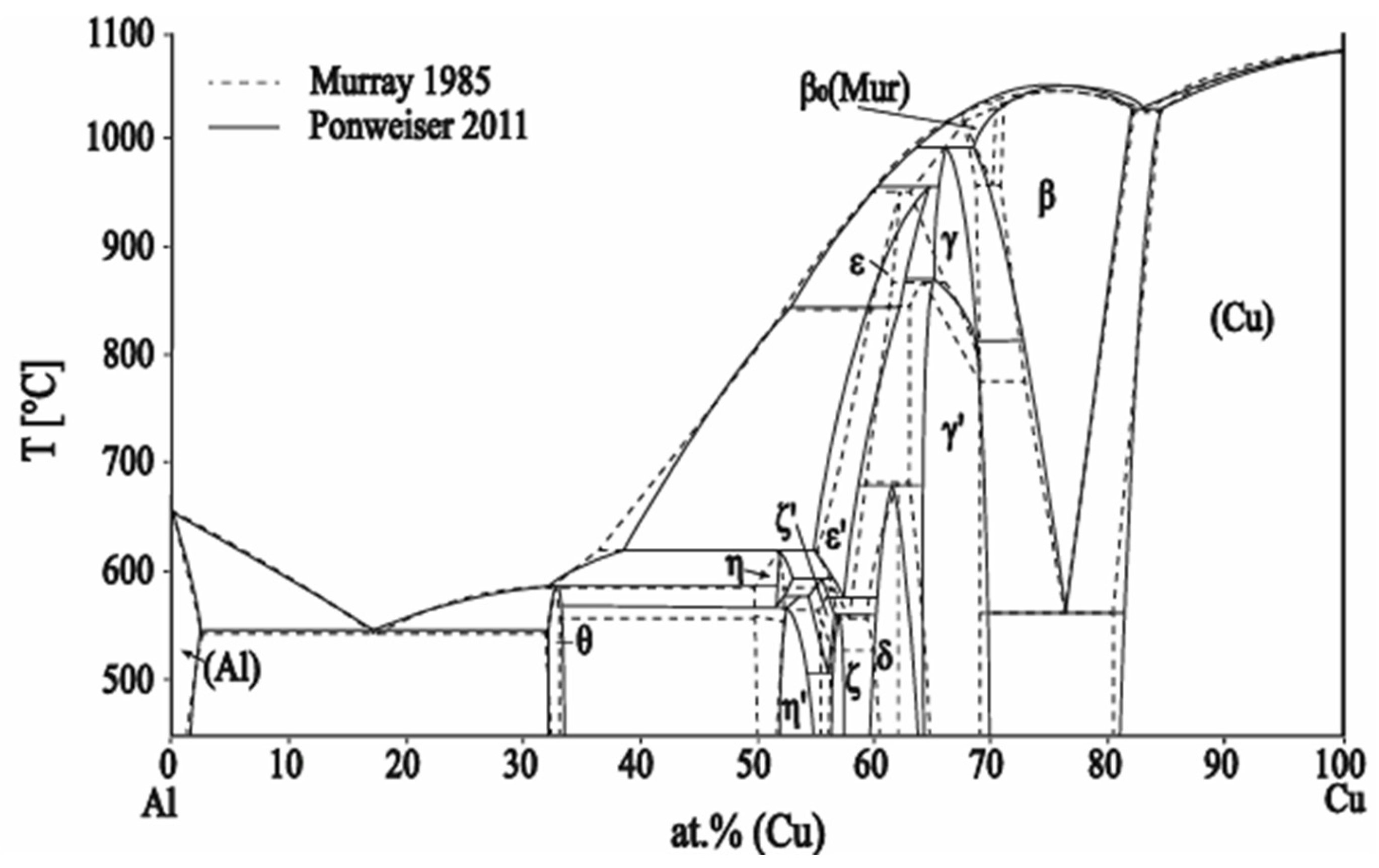
3.3. Diffusion Calculations
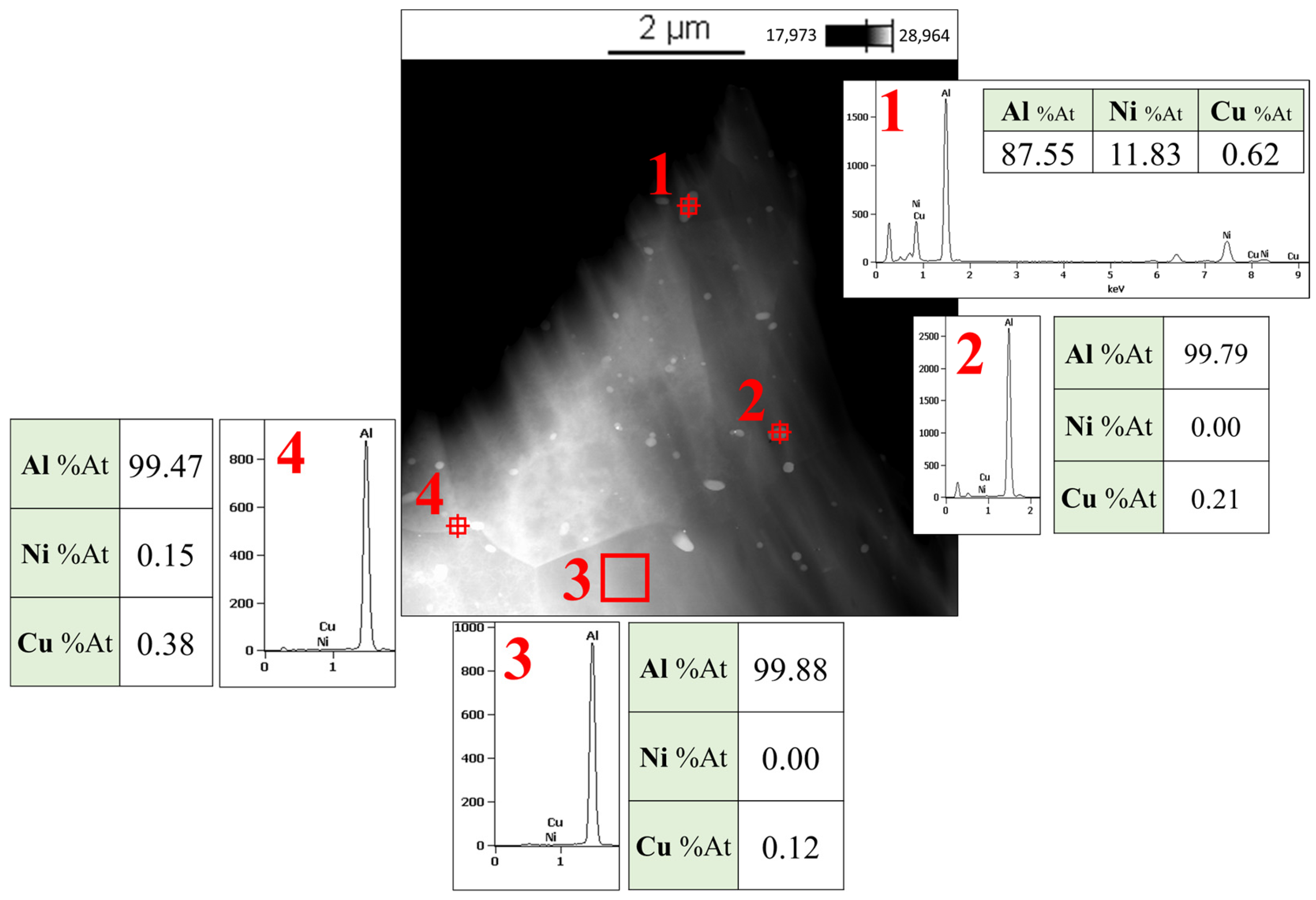
3.4. TEM Images
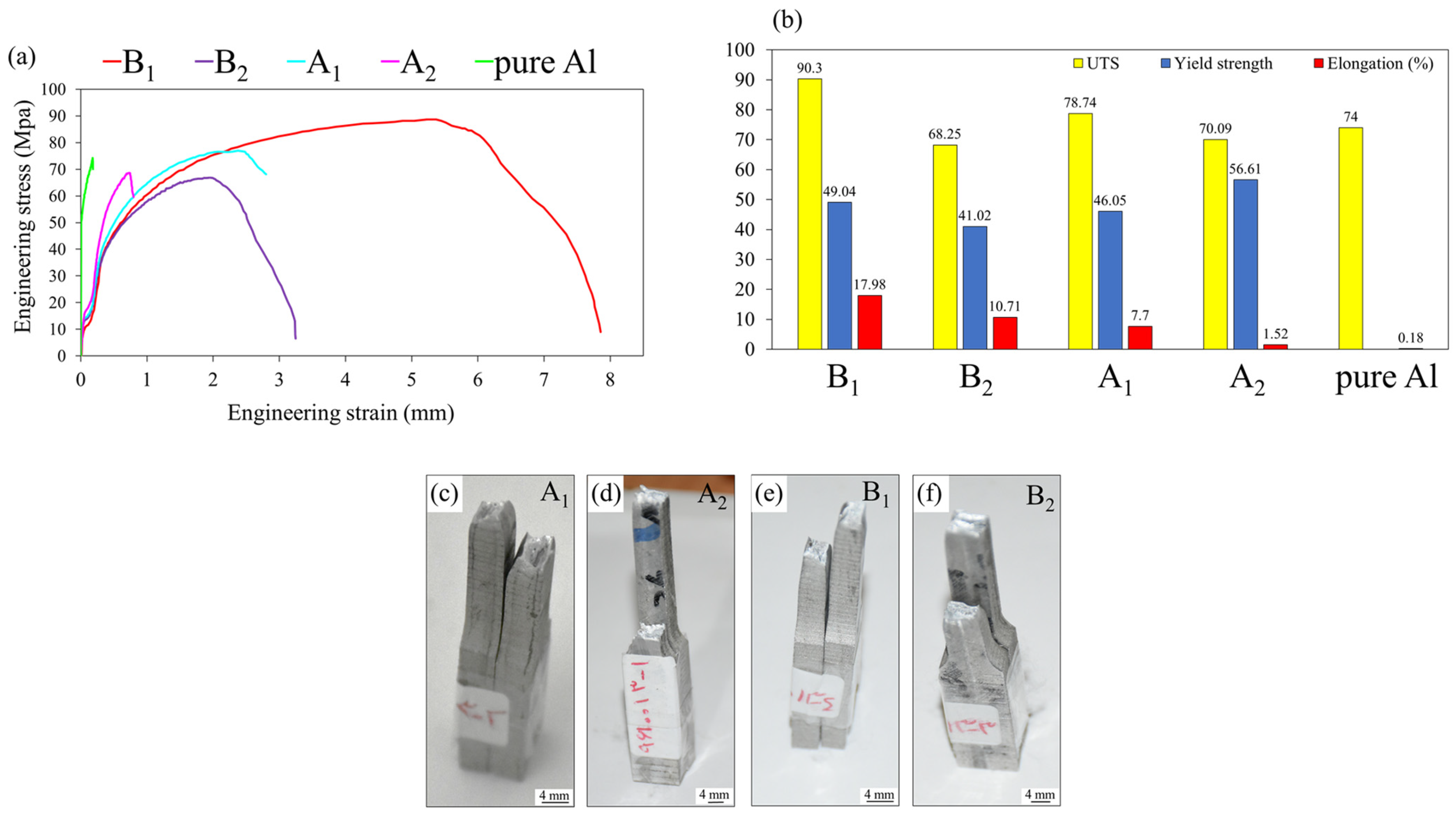
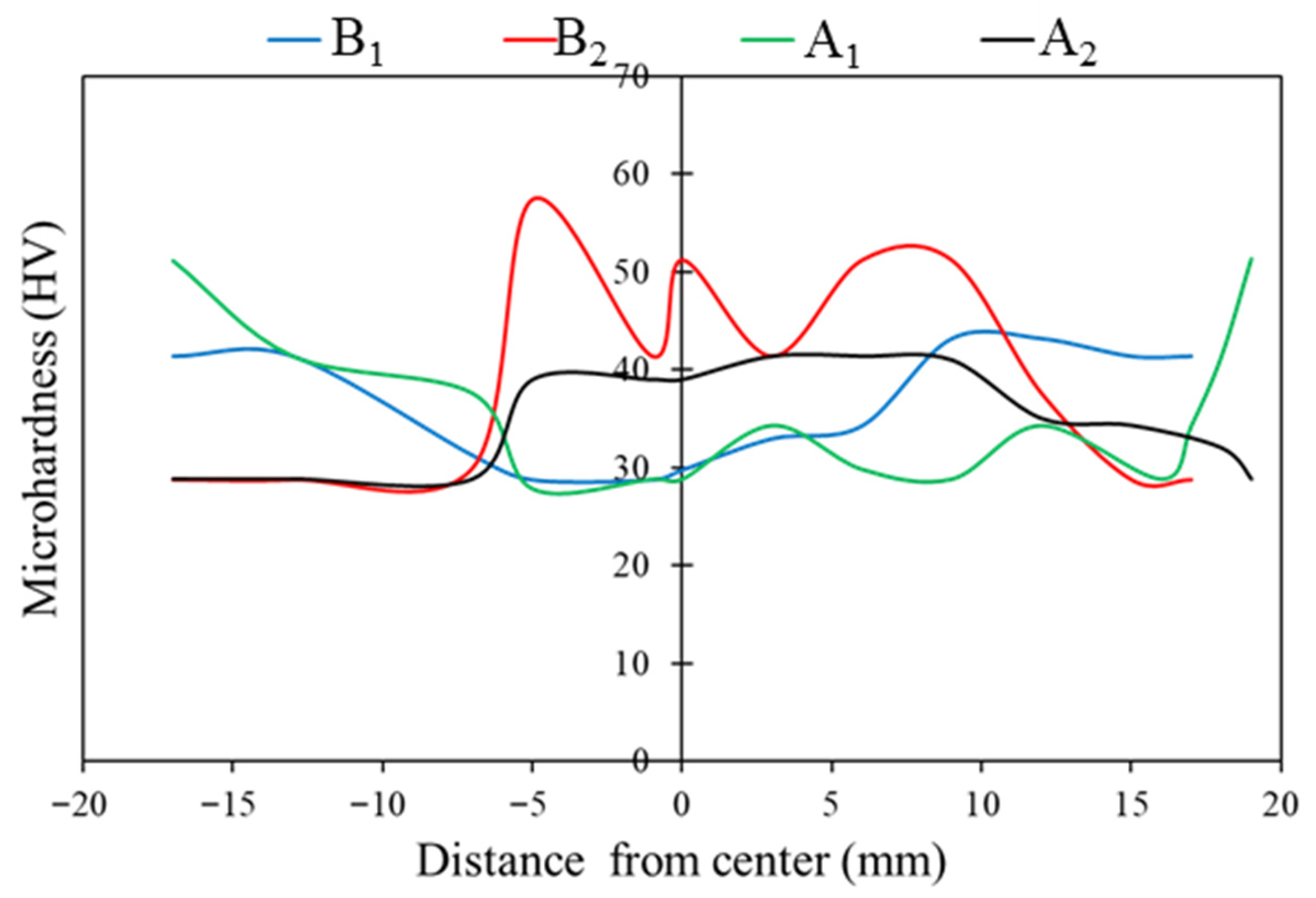
3.5. Tensile Strength and Microhardness
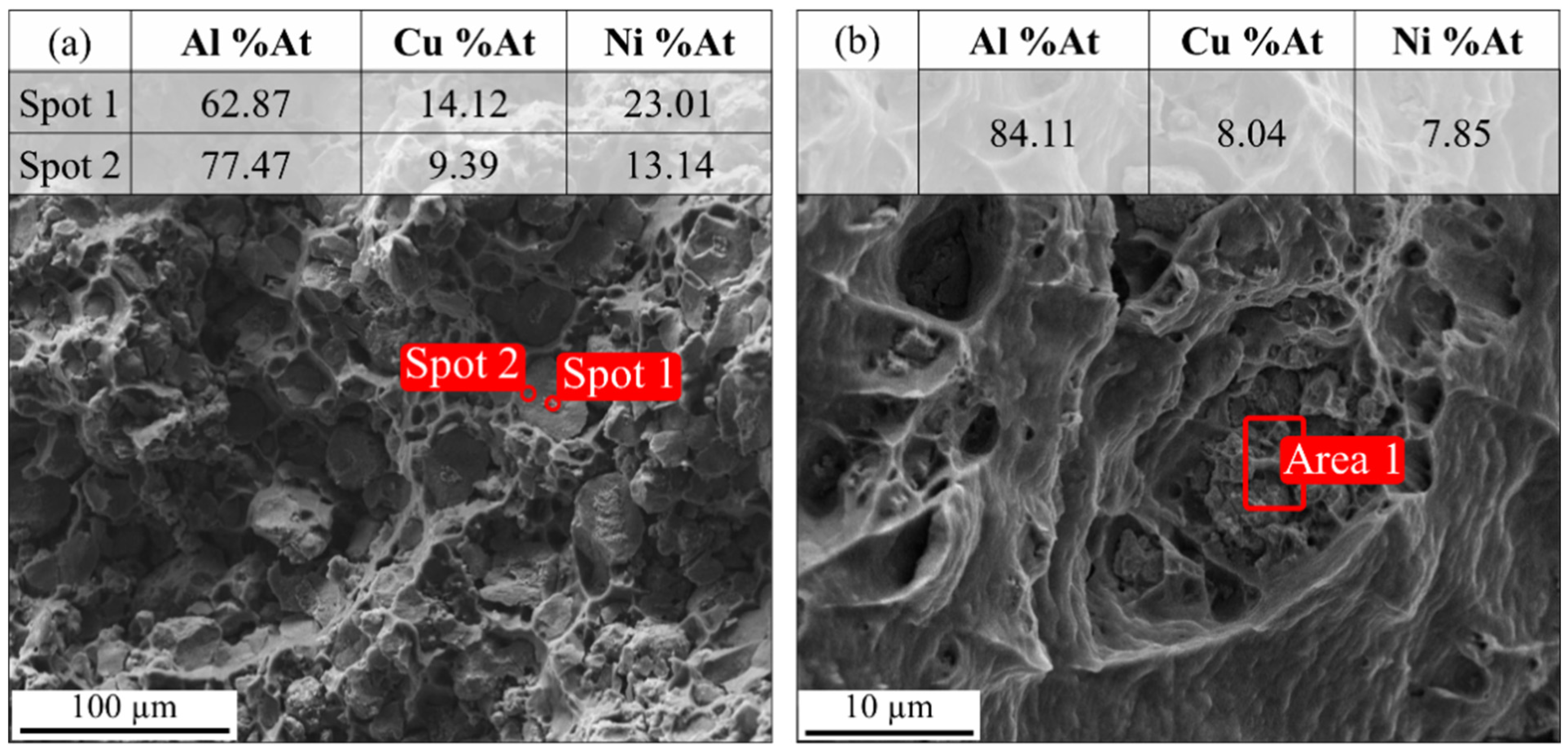
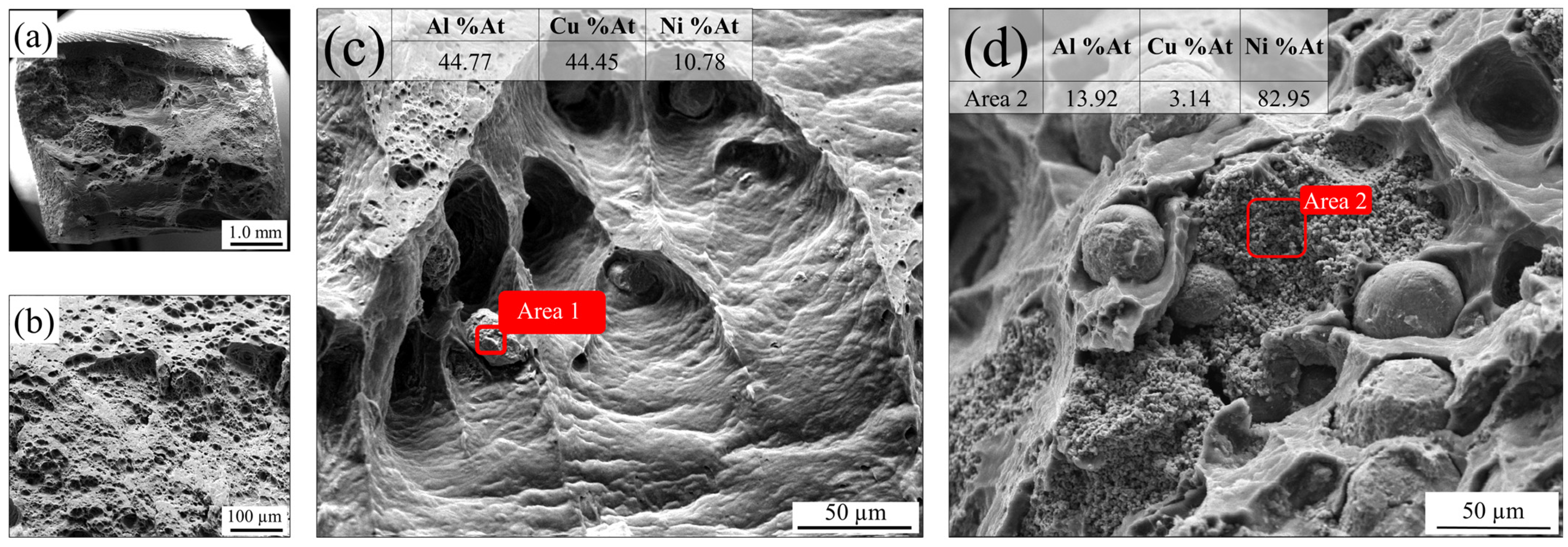
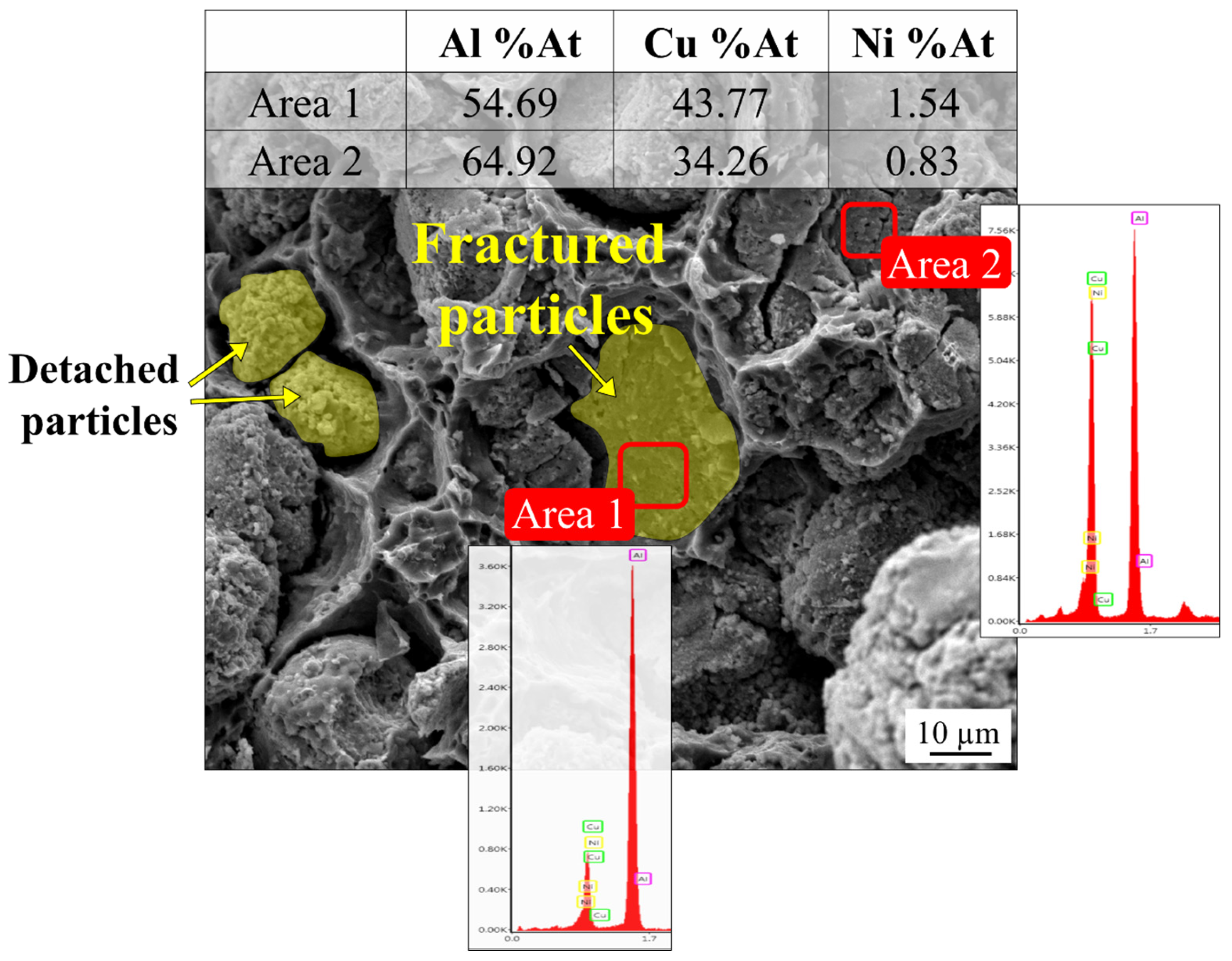
3.6. Fractography
4. Conclusions
- Ni-Cu solid solution (Monel particles) with an average crystallite size of 23 nm was obtained after mechanical alloying of Ni and Cu particles.
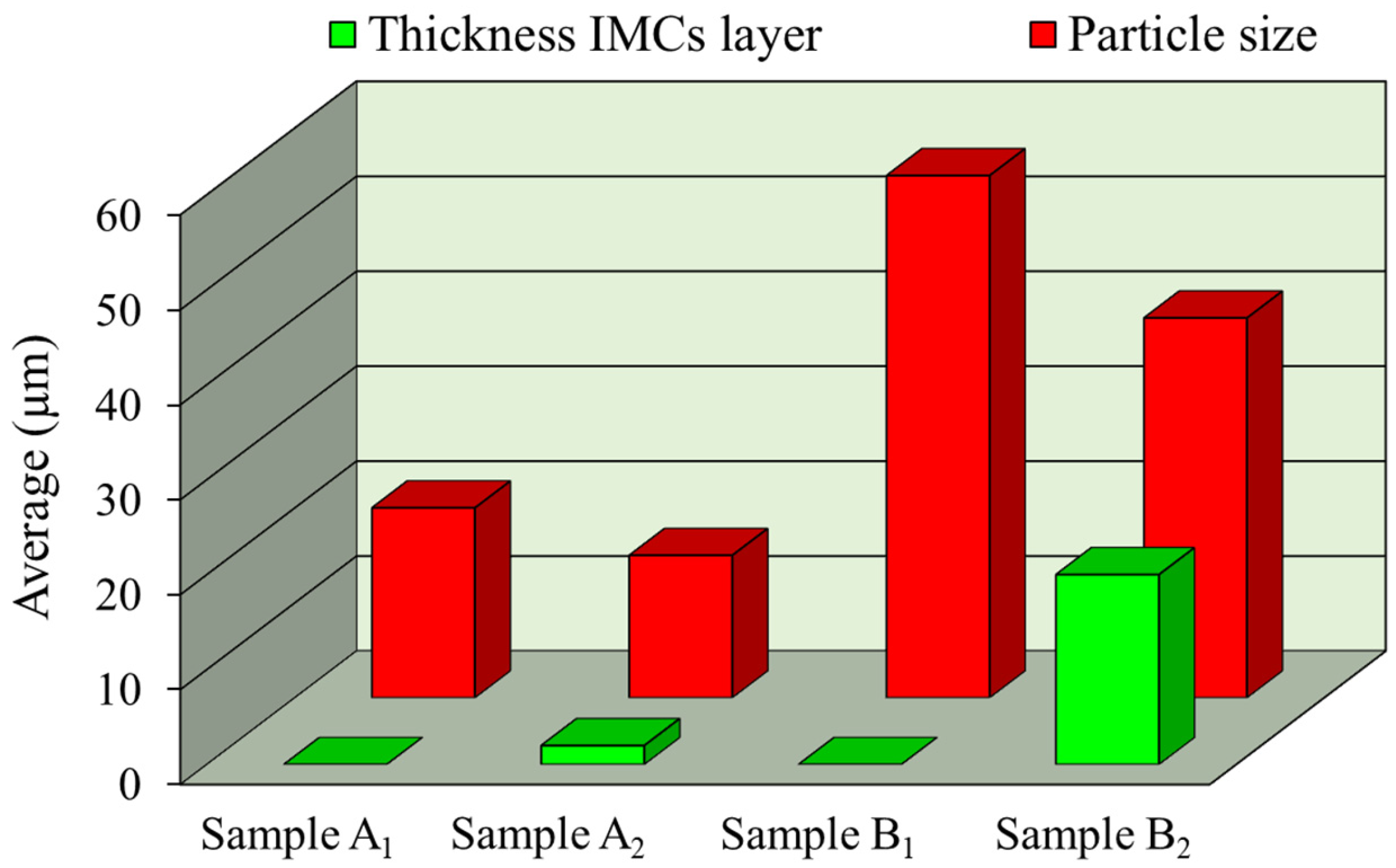
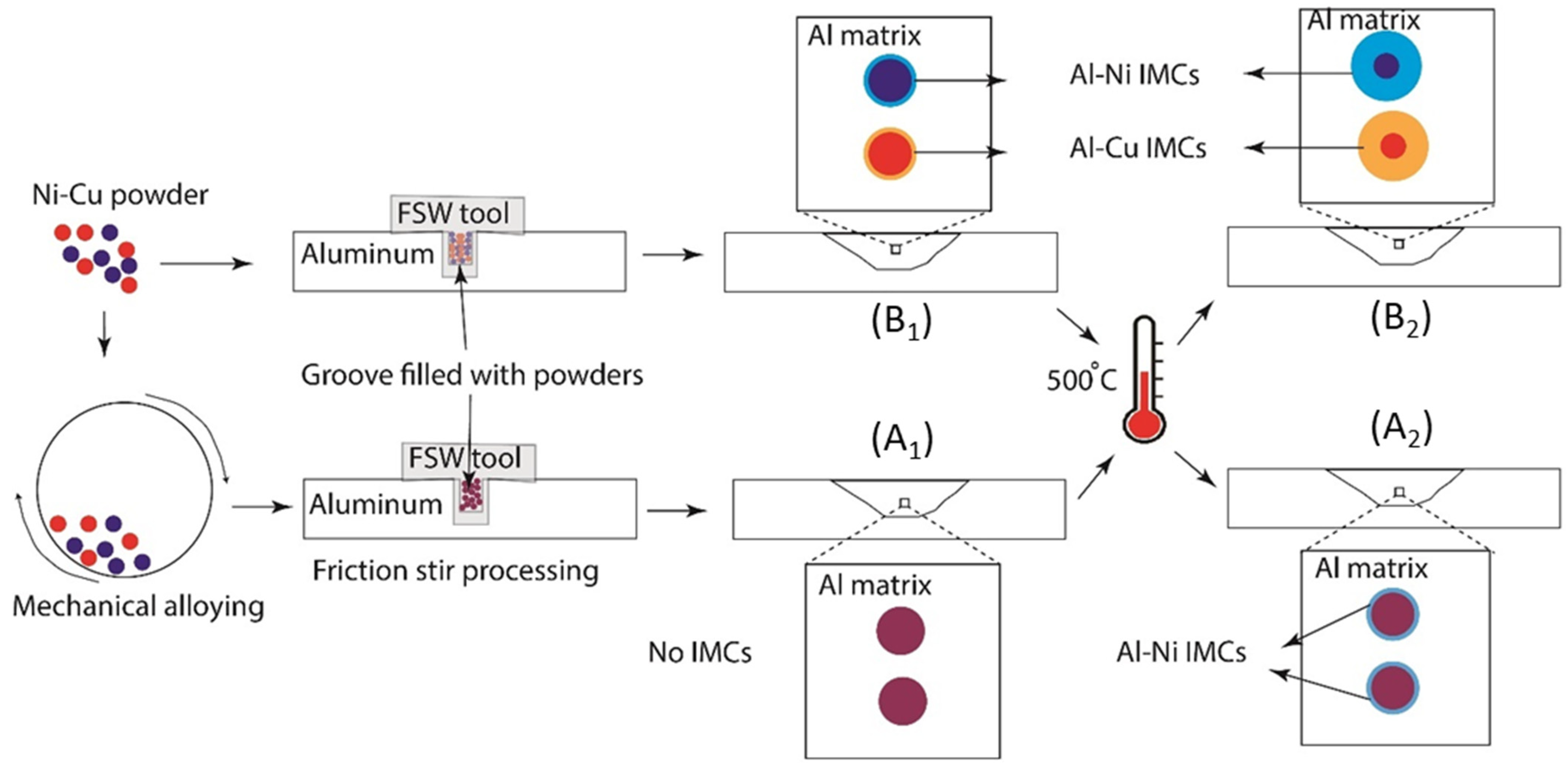
- In the Al-matrix composite obtained by FSP using an initial mixture of Ni and Cu particles, IMCs of Al-Cu and Al-Ni were formed around Cu and Ni particles, respectively. The thickness of the IMCs was approximately between 2 and 25 microns. No mixing of Cu and Ni particles occurred during FSP.
- In the Al-matrix composite obtained by FSP using mechanically alloyed Ni-Cu particles present as Monel, no IMCs were formed around the particles. This was attributed to the hindering effect of the solid solution on the diffusion of Ni and Cu elements.
- The fracture of the composites occurred in ductile mode, exhibiting dimples on the fracture surface. The particles inside the dimples debond from the matrix when the interface was weak (In samples without heat treatment). The particles failed in a brittle manner in the samples that were heat-treated due to the formation of brittle IMCs at their interface with the matrix.
- Solid solutioning is an effective way to control IMC growth in metal-matrix composites with metallic reinforcements. This results from the lowering of the activity of the metallic elements through solid solution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khodabakhshi, F.; Simchi, A.; Kokabi, A. Surface modifications of an aluminum-magnesium alloy through reactive stir friction processing with titanium oxide nanoparticles for enhanced sliding wear resistance. Surf. Coat. Technol. 2017, 309, 114–123. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Rohatgi, P.K.; Asthana, R.; Das, S. Solidification, structures, and properties of cast metal-ceramic particle composites. Int. Met. Rev. 1986, 31, 115–139. [Google Scholar] [CrossRef]
- Geng, R.; Qiu, F.; Jiang, Q. Reinforcement in Al matrix composites: A review of strengthening behavior of nano-sized particles. Adv. Eng. Mater. 2018, 20, 1701089. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Ostovan, F.; Matori, K.A.; Toozandehjani, M.; Oskoueian, A.; Yusoff, H.M.; Yunus, R.; Ariff, A.H.M. Microstructural evaluation of ball-milled nano Al2O3 particulate-reinforced aluminum matrix composite powders. Int. J. Mater. Res. 2015, 106, 636–640. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Aziz, S.A.; Mamat, S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, F.; Matori, K.A.; Toozandehjani, M.; Oskoueian, A.; Yusoff, H.M.; Yunus, R.; Ariff, A.H.M.; Quah, H.J.; Lim, W.F. Effects of CNTs content and milling time on mechanical behavior of MWCNT-reinforced aluminum nanocomposites. Mater. Chem. Phys. 2015, 166, 160–166. [Google Scholar] [CrossRef]
- Adiga, K.; Herbert, M.A.; Rao, S.S.; Shettigar, A. Applications of reinforcement particles in the fabrication of aluminium metal matrix composites by friction stir processing—A review. Manuf. Rev. 2022, 9, 26. [Google Scholar] [CrossRef]
- Tong, X.C. Advanced Materials for Thermal Management of Electronic Packaging; Springer Science & Business Media: New York, NY, USA, 2011; Volume 30. [Google Scholar]
- Tong, X.C. High thermal conductivity metal matrix composites. In Advanced Materials for Thermal Management of Electronic Packaging; Springer: New York, NY, USA, 2011; pp. 233–276. [Google Scholar]
- Efzan, M.N.E.; Syazwani, N.S.; Abdullah, M.M.A.B. Fabrication method of aluminum matrix composite (AMCs): A review. Key Eng. Mater. 2016, 700, 102–110. [Google Scholar] [CrossRef]
- Josyula, S.K.; Narala, S.K.R. Machinability enhancement of stir cast Al-TiCp composites under cryogenic condition. Mater. Manuf. Process. 2017, 32, 1764–1774. [Google Scholar] [CrossRef]
- Myalski, J.; Posmyk, A. Processing of sliding hybrid composites with aluminum alloy matrix containing solid lubricants. Mater. Manuf. Process. 2016, 31, 1324–1332. [Google Scholar] [CrossRef]
- Gurusamy, P.; Prabu, S.B.; Paskaramoorthy, R. Influence of processing temperatures on mechanical properties and microstructure of squeeze cast aluminum alloy composites. Mater. Manuf. Process. 2015, 30, 367–373. [Google Scholar] [CrossRef]
- Soltani, N.; Sadrnezhaad, S.K.; Bahrami, A. Manufacturing wear-resistant 10Ce-TZP/Al2O3 nanoparticle aluminum composite by powder metallurgy processing. Mater. Manuf. Process. 2014, 29, 1237–1244. [Google Scholar] [CrossRef]
- Zeren, A. Effect of the graphite content on the tribological properties of hybrid Al/SiC/Gr composites processed by powder metallurgy. Ind. Lubr. Tribol. 2015, 67, 262–268. [Google Scholar] [CrossRef]
- Singh, L.K.; Maiti, A.; Maurya, R.S.; Laha, T. Al alloy nanocomposite reinforced with physically functionalized carbon nanotubes synthesized via spark plasma sintering. Mater. Manuf. Process. 2016, 31, 733–738. [Google Scholar] [CrossRef]
- Goo, B.-C. Al/SiCp brake discs produced by dissimilar cast-bonding. Mater. Manuf. Process. 2016, 31, 1318–1323. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, B.; Mei, H.; Wu, H. Microstructure and wear resistance of semisolid TiB2/7050 composites produced by serpentine tube pouring technique. Mater. Manuf. Process. 2016, 31, 1029–1036. [Google Scholar] [CrossRef]
- Kumar, C.S.; Yadav, D.; Bauri, R.; Ram, G.J. Effects of ball milling and particle size on microstructure and properties 5083 Al–Ni composites fabricated by friction stir processing. Mater. Sci. Eng. A 2015, 645, 205–212. [Google Scholar] [CrossRef]
- Shorowordi, K.; Laoui, T.; Haseeb, A.; Celis, J.; Froyen, L. Microstructure and interface characteristics of B4C, SiC and Al2O3 reinforced Al matrix composites: A comparative study. J. Mater. Process. Technol. 2003, 142, 738–743. [Google Scholar] [CrossRef]
- Yadav, D.; Bauri, R. Nickel particle embedded aluminium matrix composite with high ductility. Mater. Lett. 2010, 64, 664–667. [Google Scholar] [CrossRef]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R Rep. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Charandabi, F.K.; Jafarian, H.R.; Mahdavi, S.; Javaheri, V.; Heidarzadeh, A. Modification of microstructure, hardness, and wear characteristics of an automotive-grade Al-Si alloy after friction stir processing. J. Adhes. Sci. Technol. 2021, 35, 2696–2709. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Cao, X.; Larose, S.; Wanjara, P. Review of tools for friction stir welding and processing. Can. Met. Q. 2012, 51, 250–261. [Google Scholar] [CrossRef]
- Wang, G.-Q.; Zhao, Y.-H.; Tang, Y.-Y. Research progress of bobbin tool friction stir welding of aluminum alloys: A review. Acta Met. Sin. Engl. Lett. 2020, 33, 13–29. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Xiong, J.; Huang, F.; Zhang, F.; Raza, S.H. Joining aluminum to titanium alloy by friction stir lap welding with cutting pin. Mater. Charact. 2012, 71, 1–5. [Google Scholar] [CrossRef]
- Krishnan, M.M.; Maniraj, J.; Deepak, R.; Anganan, K. Prediction of optimum welding parameters for FSW of aluminium alloys AA6063 and A319 using RSM and ANN. Mater. Today Proc. 2018, 5, 716–723. [Google Scholar] [CrossRef]
- Jain, V.K.S.; Varghese, J.; Muthukumaran, S. Effect of first and second passes on microstructure and wear properties of titanium dioxide-reinforced aluminum surface composite via friction stir processing. Arab. J. Sci. Eng. 2019, 44, 949–957. [Google Scholar] [CrossRef]
- Eftekhari, M.; Movahedi, M.; Kokabi, A.H. Microstructure, strength, and wear behavior relationship in Al-Fe3O4 nanocomposite produced by multi-pass friction stir processing. J. Mater. Eng. Perform. 2017, 26, 3516–3530. [Google Scholar] [CrossRef]
- Panaskar, N.J.; Sharma, A. Surface modification and nanocomposite layering of fastener-hole through friction-stir processing. Mater. Manuf. Process. 2014, 29, 726–732. [Google Scholar] [CrossRef]
- Madhu, H.C.; Kumar, P.A.; Perugu, C.S.; Kailas, S.V. Microstructure and mechanical properties of friction stir process derived Al-TiO2 nanocomposite. J. Mater. Eng. Perform. 2018, 27, 1318–1326. [Google Scholar] [CrossRef]
- Khorrami, M.S.; Kazeminezhad, M.; Miyashita, Y.; Kokabi, A.H. Improvement in the mechanical properties of Al/SiC nanocomposites fabricated by severe plastic deformation and friction stir processing. Int. J. Miner. Met. Mater. 2017, 24, 297–308. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Ikeuchi, K.; Takahashi, M. Fabrication of SiC particle reinforced composite on aluminium surface by friction stir processing. Sci. Technol. Weld. Join. 2008, 13, 607–618. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Takahashi, M.; Shibayanagi, T.; Ikeuchi, K. Effect of friction stir processing tool probe on fabrication of SiC particle reinforced composite on aluminium surface. Sci. Technol. Weld. Join. 2009, 14, 413–425. [Google Scholar] [CrossRef]
- Liu, Q.; Ke, L.; Liu, F.; Huang, C.; Xing, L. Microstructure and mechanical property of multi-walled carbon nanotubes reinforced aluminum matrix composites fabricated by friction stir processing. Mater. Des. 2013, 45, 343–348. [Google Scholar] [CrossRef]
- Thangarasu, A.; Murugan, N.; Dinaharan, I.; Vijay, S.J. Microstructure and microhardness of AA1050/TiC surface composite fabricated using friction stir processing. Sadhana 2012, 37, 579–586. [Google Scholar] [CrossRef]
- Dixit, M.; Newkirk, J.W.; Mishra, R.S. Properties of friction stir-processed Al 1100–NiTi composite. Scr. Mater. 2007, 56, 541–544. [Google Scholar] [CrossRef]
- Ahmadifard, S.; Kazemi, S.; Momeni, A. A356/TiO2 nanocomposite fabricated by friction stir processing: Microstructure, mechanical properties and tribologic behavior. JOM 2018, 70, 2626–2635. [Google Scholar] [CrossRef]
- Shojaeefard, M.H.; Akbari, M.; Asadi, P.; Khalkhali, A. The effect of reinforcement type on the microstructure, mechanical properties, and wear resistance of A356 matrix composites produced by FSP. Int. J. Adv. Manuf. Technol. 2017, 91, 1391–1407. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Atapour, M.; Ashrafi, A. Fabrication and wear characterization of an A413/Ni surface metal matrix composite fabricated via friction stir processing. Mater. Des. 2015, 83, 471–482. [Google Scholar] [CrossRef]
- Eskandari, H.; Taheri, R.; Khodabakhshi, F. Friction-stir processing of an AA8026-TiB2-Al2O3 hybrid nanocomposite: Microstructural developments and mechanical properties. Mater. Sci. Eng. A 2016, 660, 84–96. [Google Scholar] [CrossRef]
- Dinaharan, I. Influence of ceramic particulate type on microstructure and tensile strength of aluminum matrix composites produced using friction stir processing. J. Asian Ceram. Soc. 2016, 4, 209–218. [Google Scholar] [CrossRef]
- Ostovan, F.; Hasanzadeh, E.; Toozandehjani, M.; Shafiei, E.; Jamaludin, K.R.; Amrin, A.B. A combined friction stir processing and ball milling route for fabrication Al5083-Al2O3 nanocomposite. Mater. Res. Express 2019, 6, 065012. [Google Scholar] [CrossRef]
- Narimani, M.; Lotfi, B.; Sadeghian, Z. Investigating the microstructure and mechanical properties of Al-TiB2 composite fabricated by Friction Stir Processing (FSP). Mater. Sci. Eng. A 2016, 673, 436–442. [Google Scholar] [CrossRef]
- Ungár, T. The Meaning of Size Obtained from Broadened X-ray Diffraction Peaks. Adv. Eng. Mater. 2003, 5, 323–329. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, C.; Chen, J.; Chen, A.; Jia, L.; Xie, H.; Lu, Z. Characterizations on Precipitations in the Cu-Rich Corner of Cu-Ni-Al Ternary Phase Diagram. Crystals 2023, 13, 274. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Brown, A.; Ashby, M. Correlations for diffusion constants. Acta Metall. 1980, 28, 1085–1101. [Google Scholar] [CrossRef]
- Wazzan, A.R. Lattice and grain boundary self-diffusion in nickel. J. Appl. Phys. 1965, 36, 3596–3599. [Google Scholar] [CrossRef]
- Jönsson, B. Ferromagnetic ordering and diffusion of carbon and nitrogen in bcc Cr-Fe-Ni alloys. Int. J. Mater. Res. 1994, 85, 498–501. [Google Scholar] [CrossRef]
- Belova, I.; Sohn, Y.; Murch, G. Measurement of tracer diffusion coefficients in an interdiffusion context for multicomponent alloys. Philos. Mag. Lett. 2015, 95, 416–424. [Google Scholar] [CrossRef]
- Schulz, E.A.; Mehta, A.; Belova, I.V.; Murch, G.E.; Sohn, Y. Simultaneous measurement of isotope-free tracer diffusion coefficients and interdiffusion coefficients in the Cu-Ni system. J. Phase Equilibria Diffus. 2018, 39, 862–869. [Google Scholar] [CrossRef]
- BWierzba, B.; Skibiński, W. The interdiffusion in copper-nickel alloys. J. Alloys Compd. 2016, 687, 104–108. [Google Scholar] [CrossRef]
- Karakulak, E.; Koç, F.G.; Yamanoglu, R.; Zeren, M. Mechanical properties of hypoeutectic Al-Ni alloys with Al3Ni intermetallics. Mater. Test. 2016, 58, 117–121. [Google Scholar] [CrossRef]
- Zobac, O.; Kroupa, A.; Zemanova, A.; Richter, K.W. Experimental Description of the Al-Cu Binary Phase Diagram. Met. Mater. Trans. A 2019, 50, 3805–3815. [Google Scholar] [CrossRef]
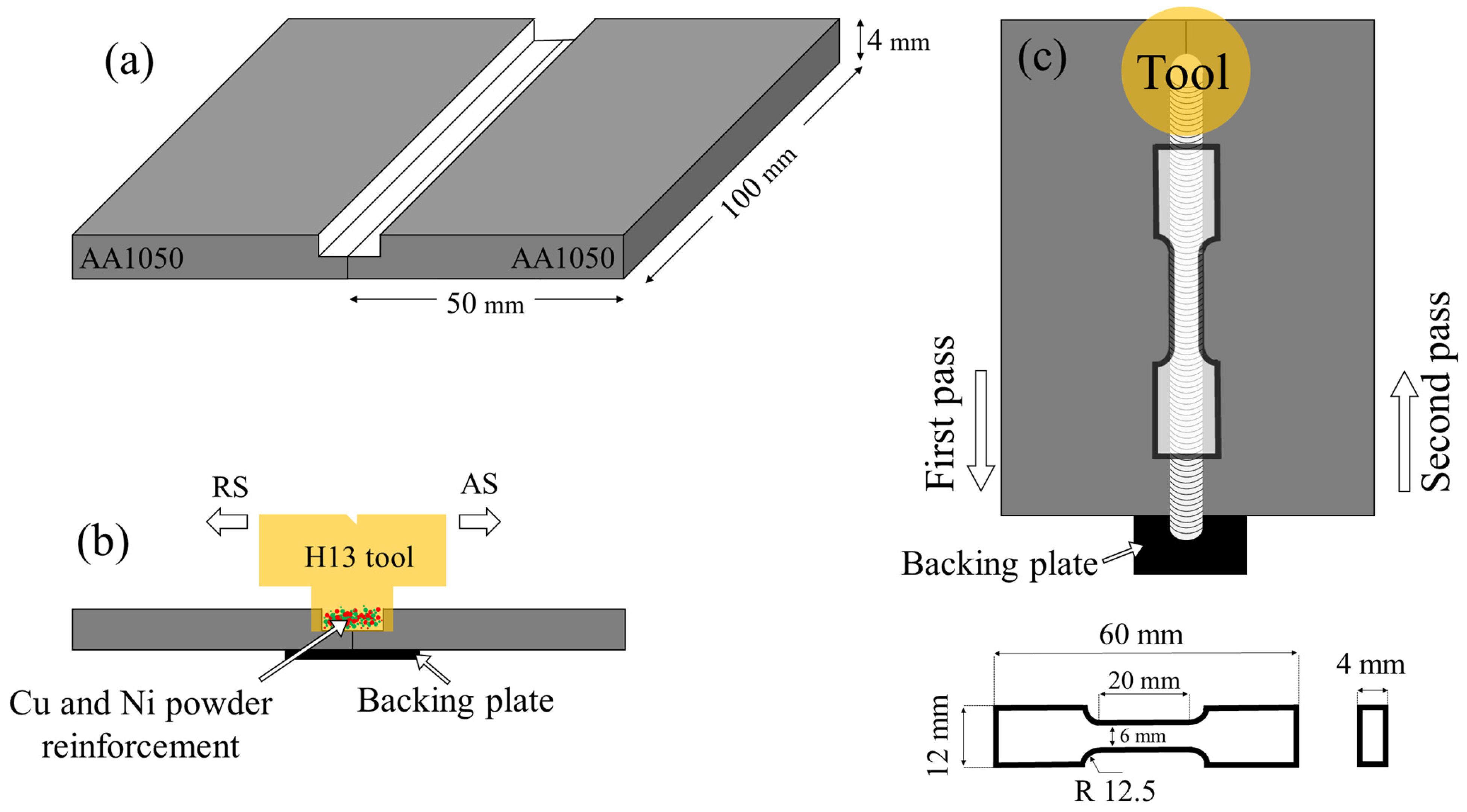

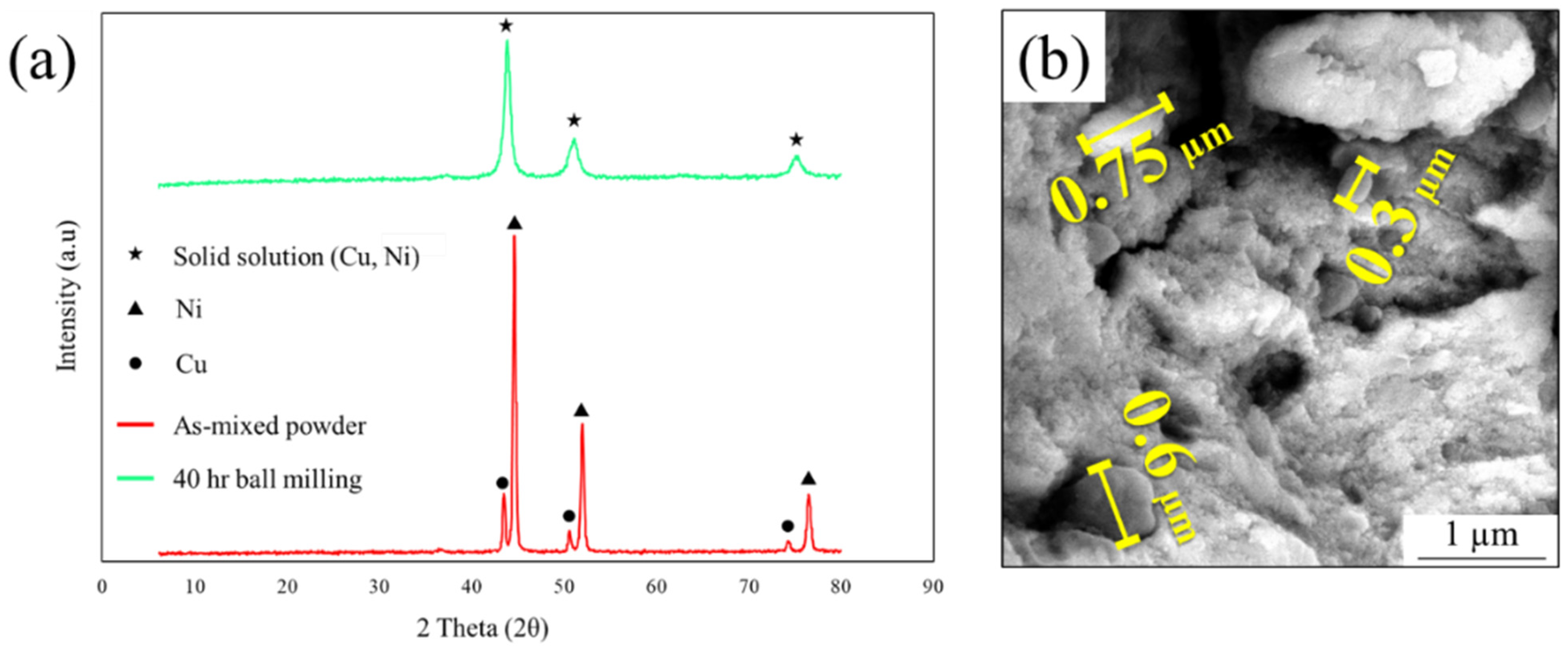
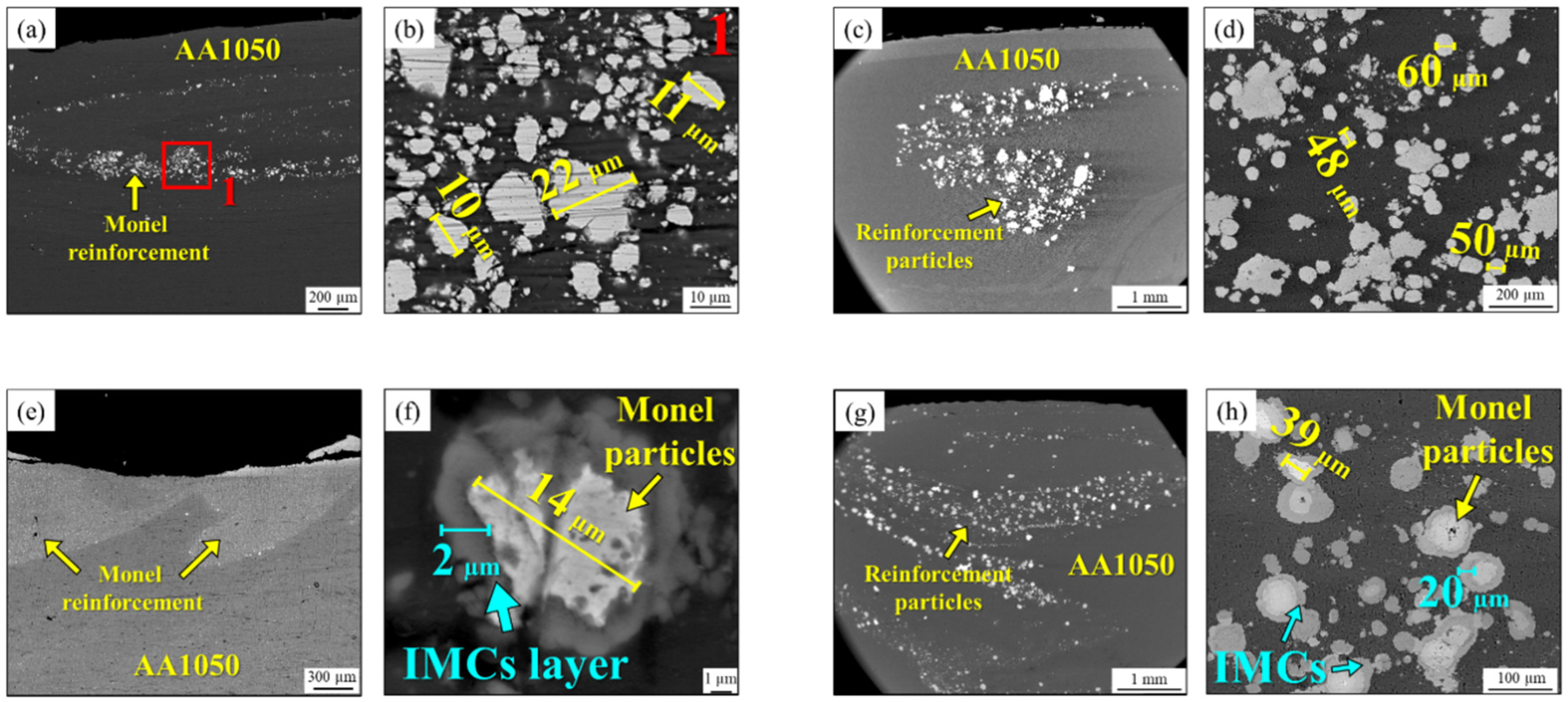
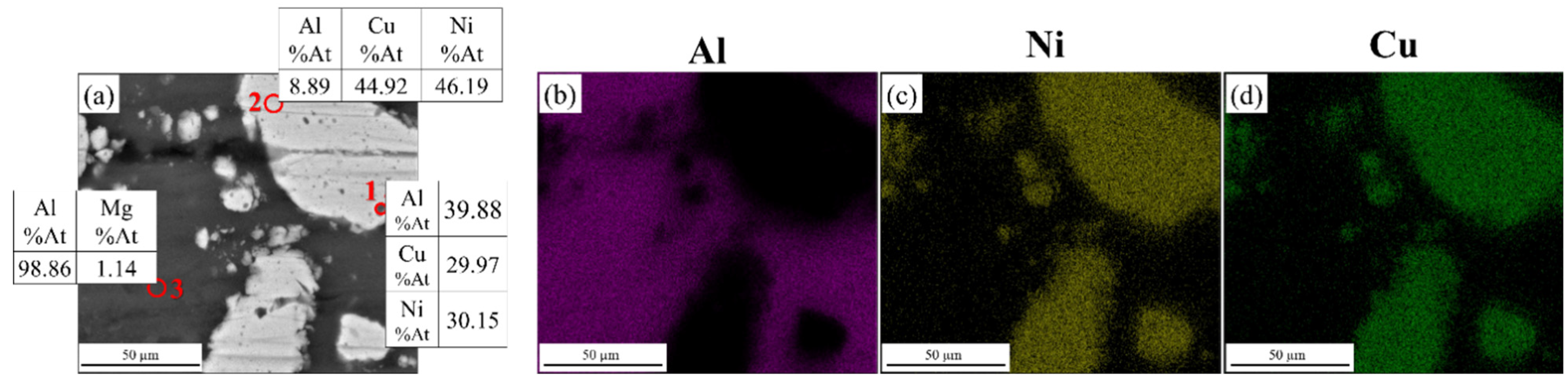
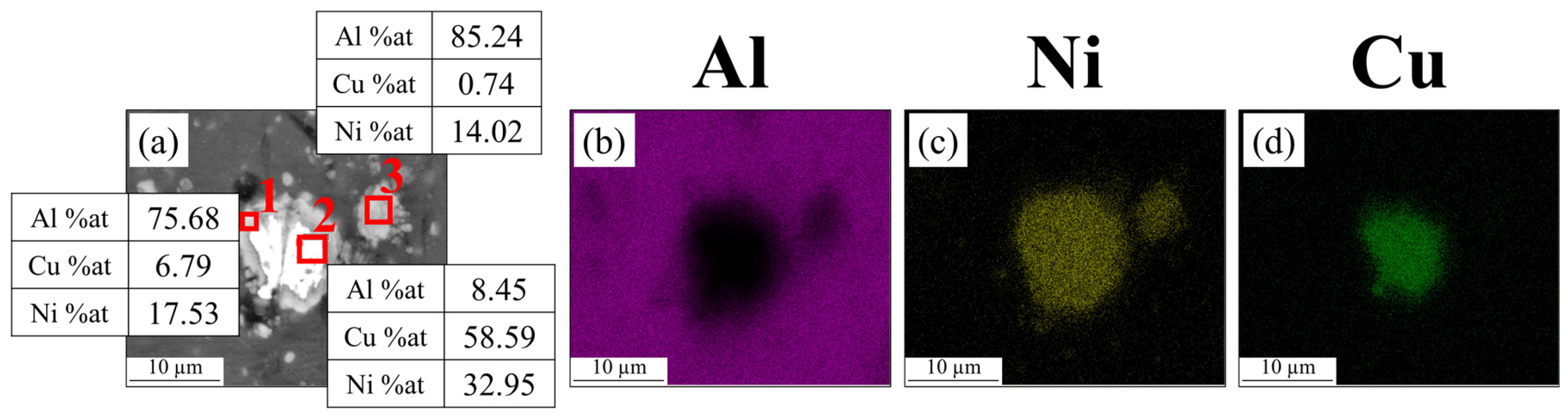
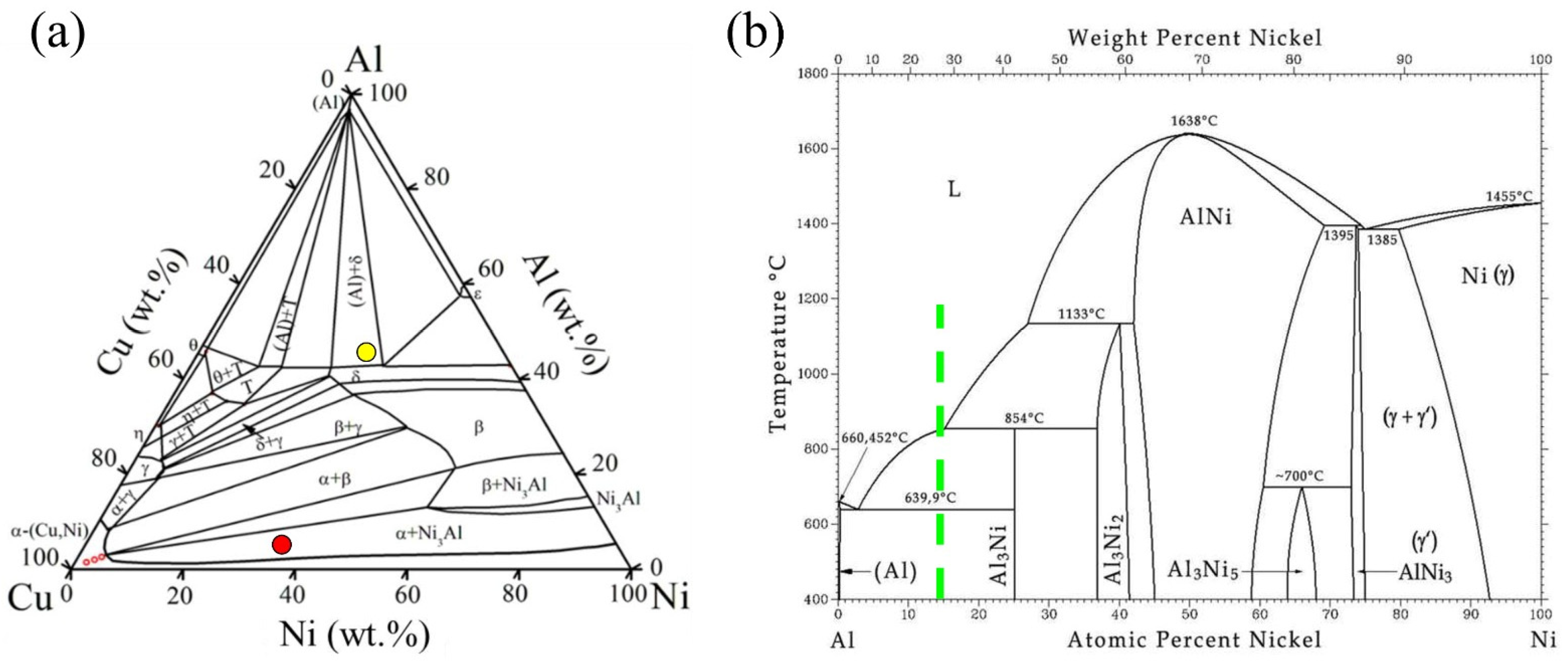
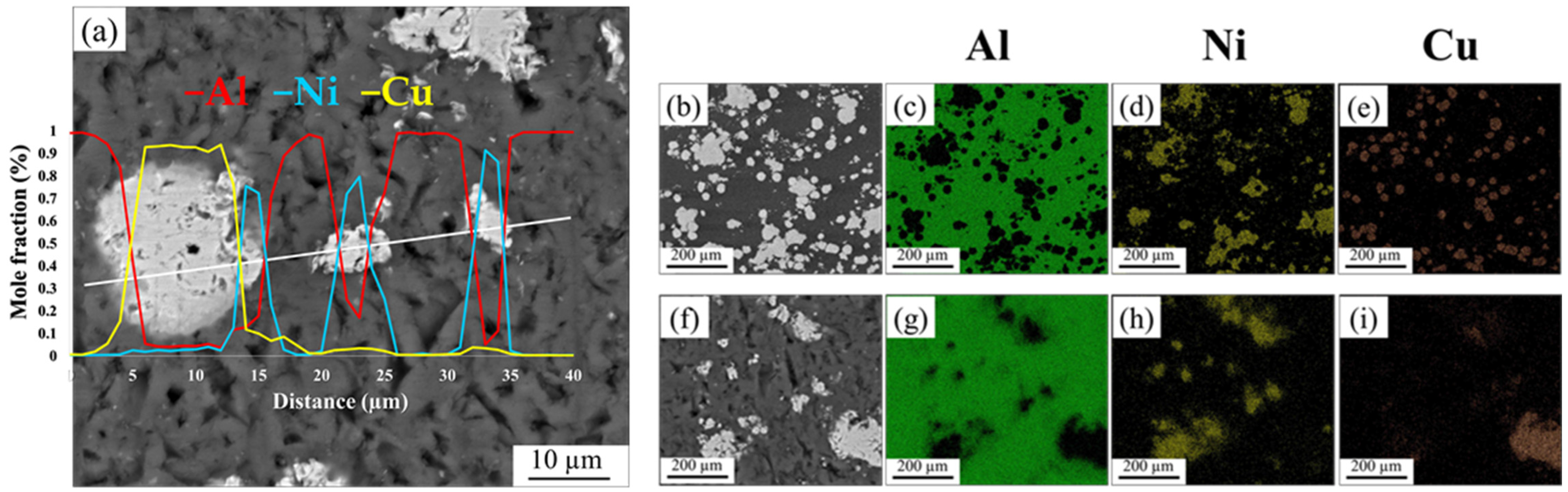
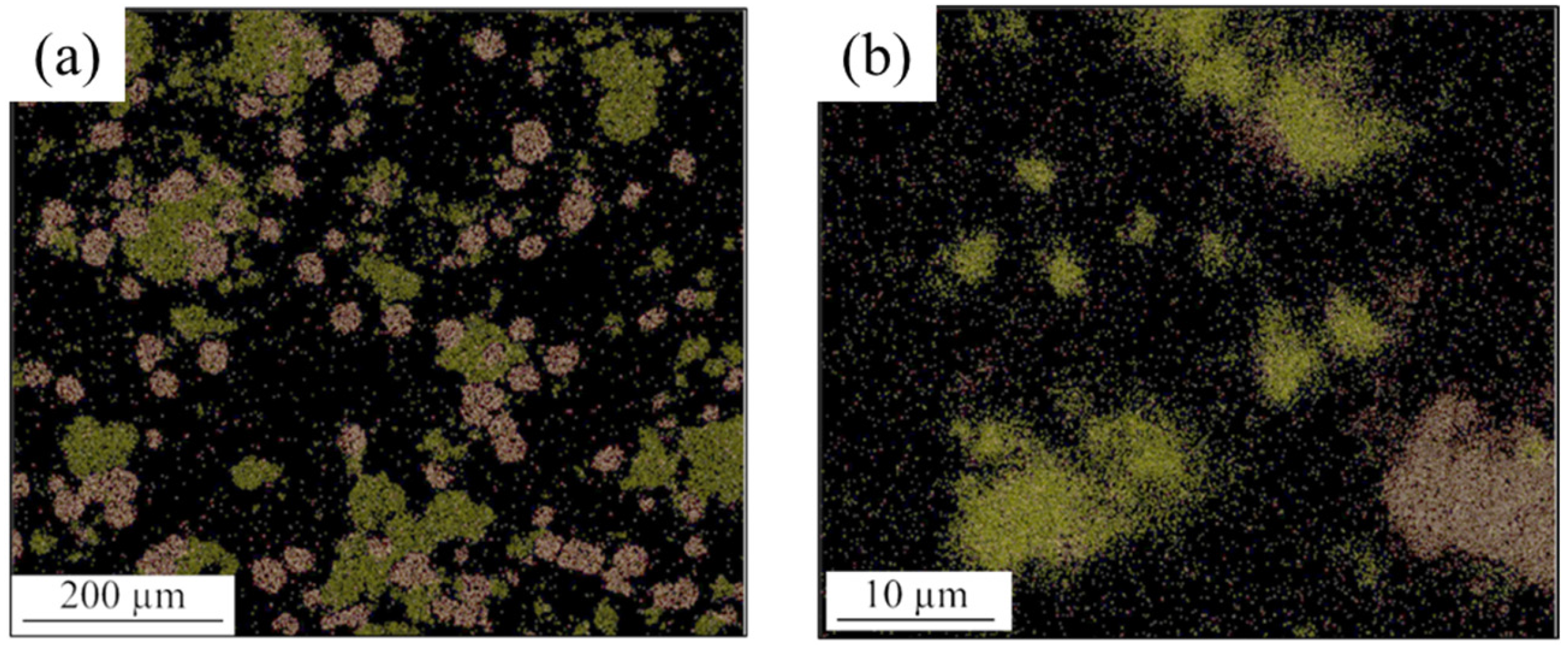


| Elements (%at) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ti | Zn | Mg | Cu | Si | Mn | Al | Fe | |
| AA1050 | 0.028 | 0.029 | 0.056 | 0.021 | 0.241 | 0.025 | 99.406 | 0.194 |
| Hardness (Brinell) | Yield strength (Mpa) | Tensile strength (Mpa) | ||||||
| AA1050-O | 21 | 28 | 76 | |||||
| AA1050-H14 | 34 | 103 | 11 | |||||
| Pieces Name | Remark/Heat Treatment Condition | Images |
|---|---|---|
| A1 | Mechanical Alloyed reinforcement/As-weld |  |
| A2 | Mechanical Alloyed reinforcement/500 °C for 2 h |  |
| B1 | Without Mechanical Alloying/As-weld |  |
| B2 | Without Mechanical Alloying/500 °C for 2 h |  |
| Peak | 2θ (°) | θ (°) | First peak | (h k l) | |
|---|---|---|---|---|---|
| 1 | 43 | 21.5 | 0.134 | 1 | (1 1 1) |
| 2 | 50 | 25 | 0.179 | 1.34 | (2 0 0) |
| 3 | 74 | 37 | 0.361 | 2.69 | (2 2 0) |
| Peak | (h k l) | d (A°) | ɑ (A°) | |
|---|---|---|---|---|
| 1 | (1 1 1) | 3 | 2.10 | 3.64 |
| 2 | (2 0 0) | 4 | 1.82 | 3.64 |
| 3 | (2 2 0) | 8 | 1.28 | 3.62 |
| Peak | 2θ (°) | θ (°) | FWHM (°) | β (rad) | cosθ | D (nm) |
|---|---|---|---|---|---|---|
| 1 | 43 | 21.5 | 0.3 | 0.00524 | 0.933 | 26.3 |
| 2 | 50 | 25 | 0.35 | 0.00611 | 0.906 | 22.8 |
| 3 | 74 | 37 | 0.4 | 0.00698 | 0.798 | 19.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesari, M.B.; Beygi, R.; Bayrami, A.; Kasaei, M.M.; Mehrizi, M.Z.; Marques, E.A.S.; da Silva, L.F.M. Hindering Effect of Solid-Solutioning on Intermetallic Growth in Aluminum–Matrix Composite Reinforced with Mechanically Alloyed Ni-Cu Particles. J. Manuf. Mater. Process. 2025, 9, 364. https://doi.org/10.3390/jmmp9110364
Hesari MB, Beygi R, Bayrami A, Kasaei MM, Mehrizi MZ, Marques EAS, da Silva LFM. Hindering Effect of Solid-Solutioning on Intermetallic Growth in Aluminum–Matrix Composite Reinforced with Mechanically Alloyed Ni-Cu Particles. Journal of Manufacturing and Materials Processing. 2025; 9(11):364. https://doi.org/10.3390/jmmp9110364
Chicago/Turabian StyleHesari, Masih Bolhasani, Reza Beygi, Ali Bayrami, Mohammad Mehdi Kasaei, Majid Zarezade Mehrizi, Eduardo A. S. Marques, and Lucas F. M. da Silva. 2025. "Hindering Effect of Solid-Solutioning on Intermetallic Growth in Aluminum–Matrix Composite Reinforced with Mechanically Alloyed Ni-Cu Particles" Journal of Manufacturing and Materials Processing 9, no. 11: 364. https://doi.org/10.3390/jmmp9110364
APA StyleHesari, M. B., Beygi, R., Bayrami, A., Kasaei, M. M., Mehrizi, M. Z., Marques, E. A. S., & da Silva, L. F. M. (2025). Hindering Effect of Solid-Solutioning on Intermetallic Growth in Aluminum–Matrix Composite Reinforced with Mechanically Alloyed Ni-Cu Particles. Journal of Manufacturing and Materials Processing, 9(11), 364. https://doi.org/10.3390/jmmp9110364









