Influence of the L-PBF Process Atmosphere on the Microstructure and Tensile Properties of AISI 318LN Duplex Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
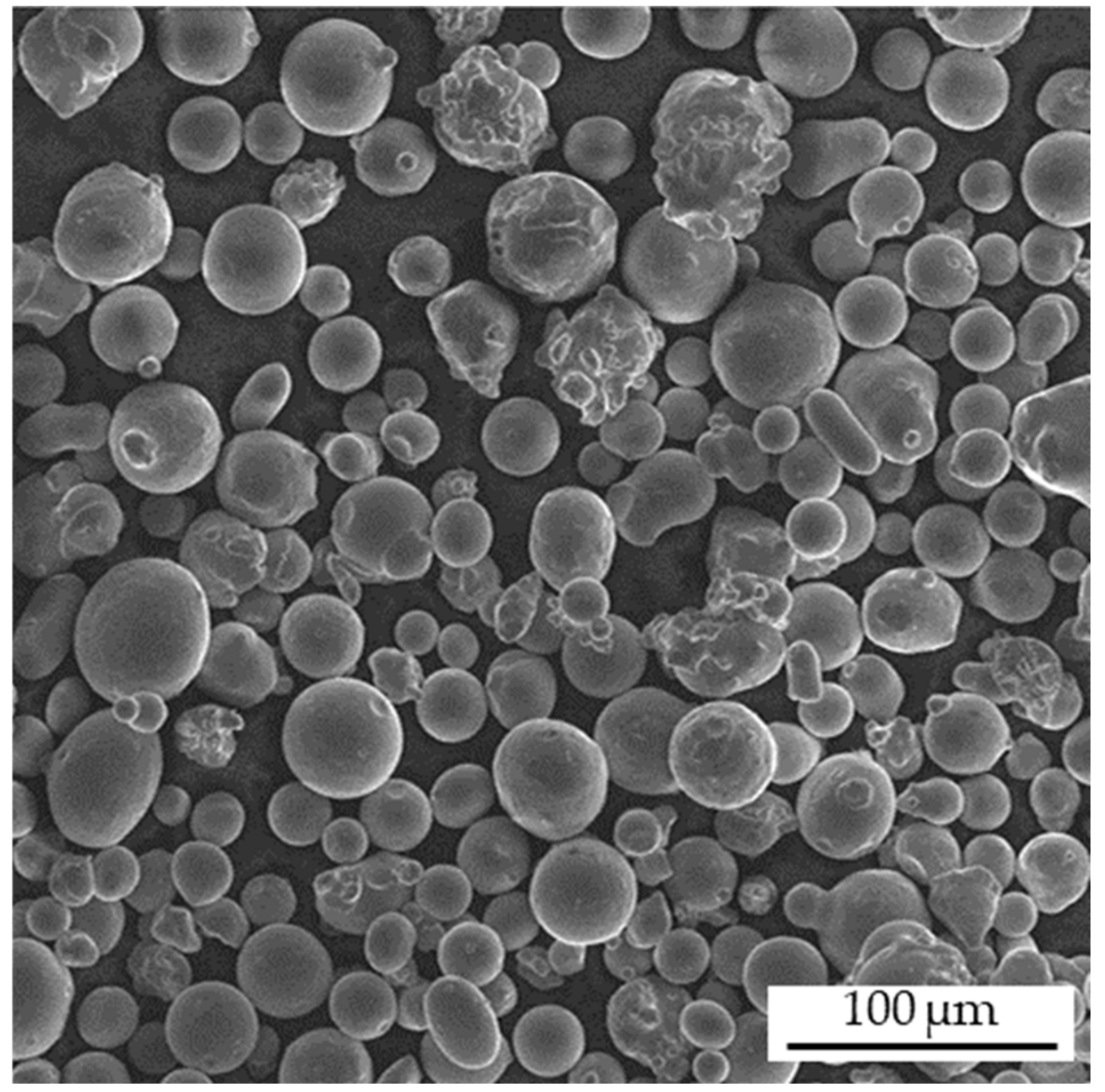
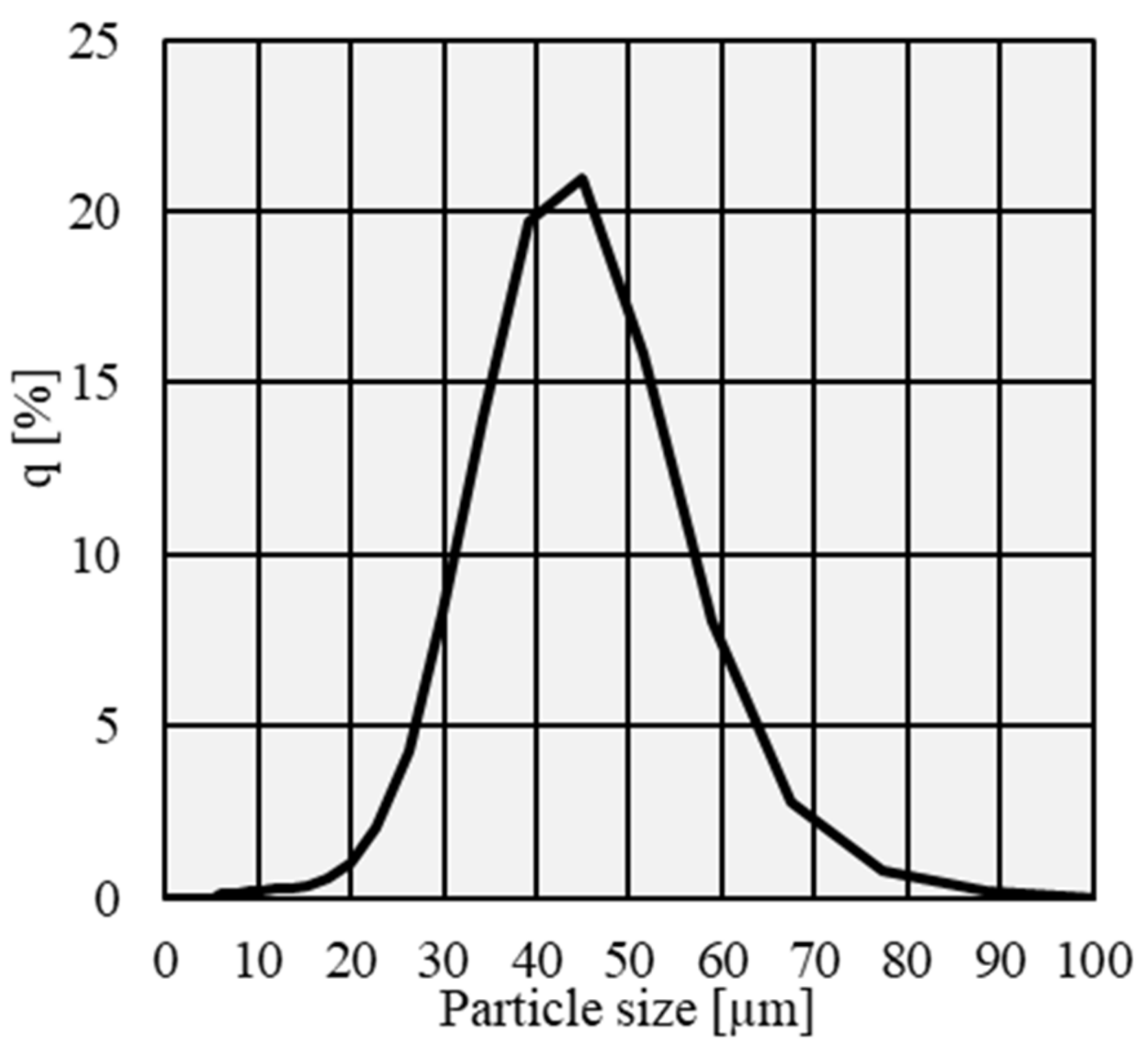
2.1. Material
2.2. L-PBF Processing and Specimen Production
2.3. Microstructural Analysis
2.4. Post-Processing by Solution-Annealing and Hot Isostatic Pressing
2.5. Tensile and Hardness Testing
2.6. Specimen Nomenclature
3. Results and Discussion
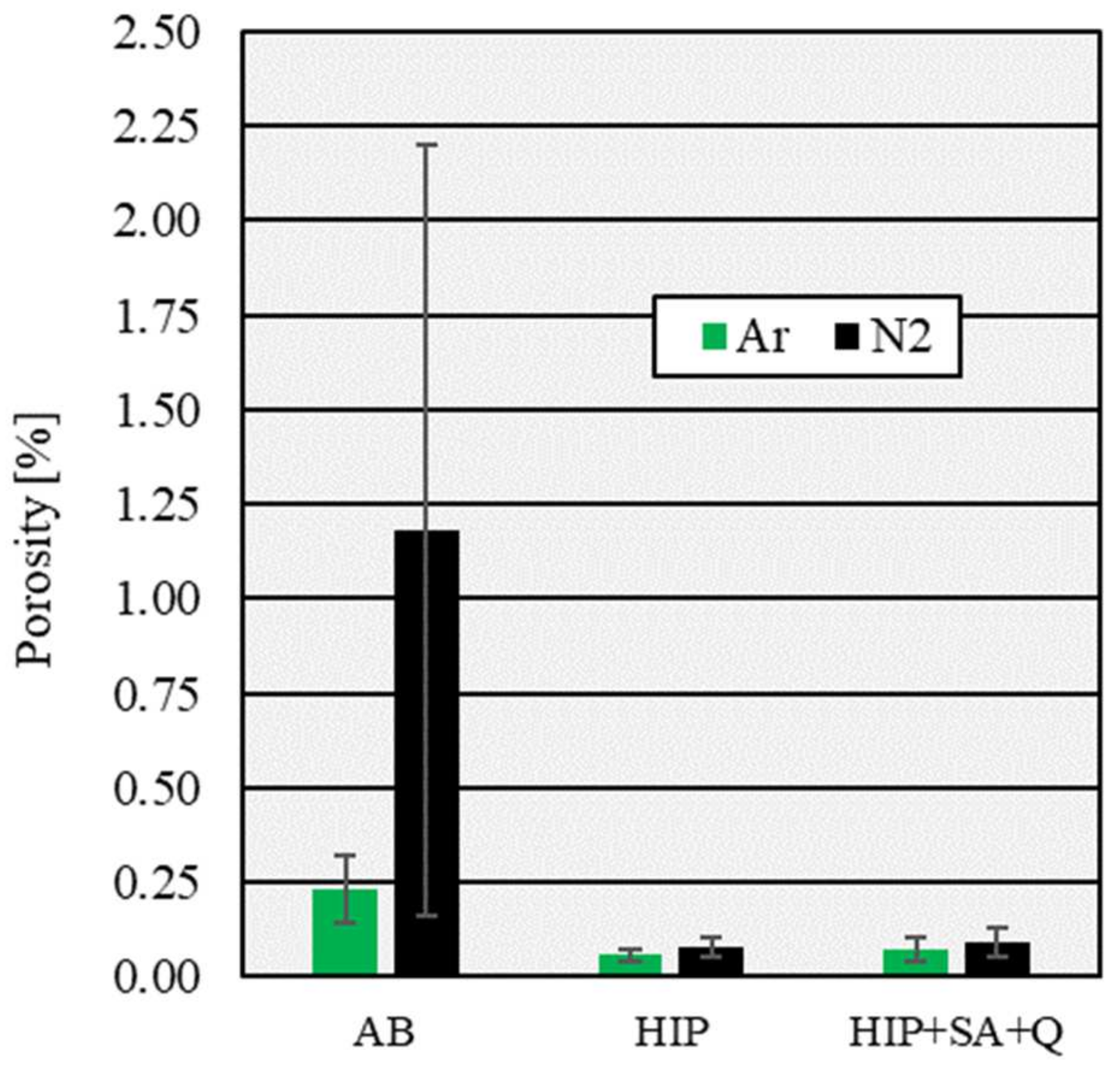
3.1. Influence of Process Atmosphere on Porosity
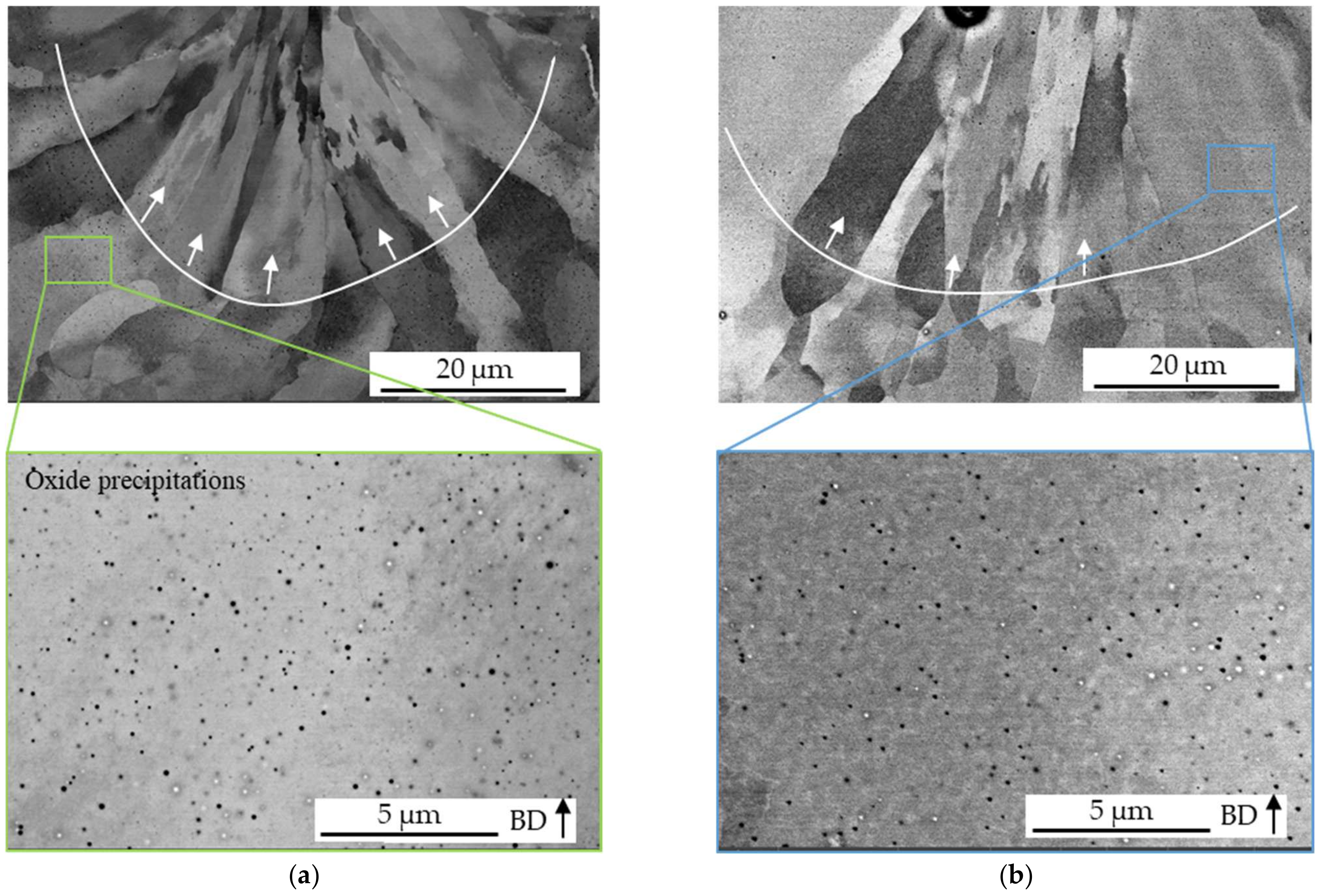
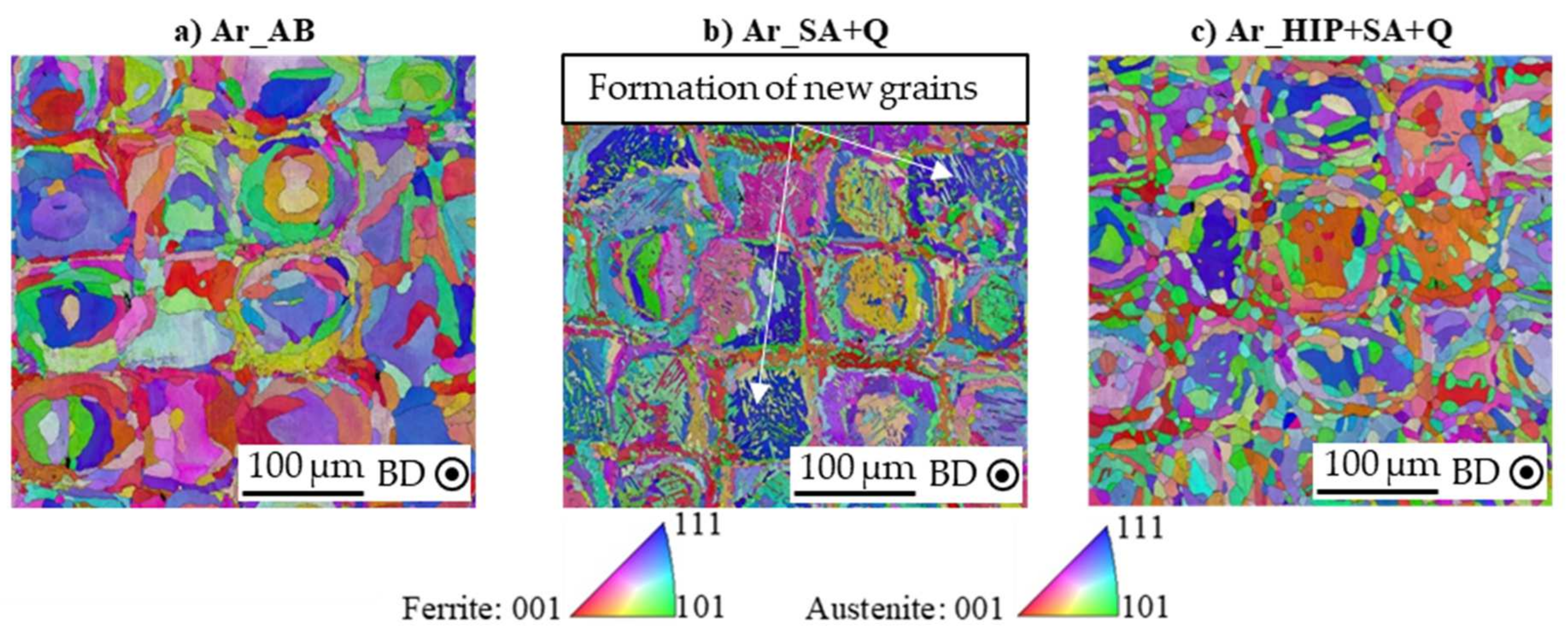
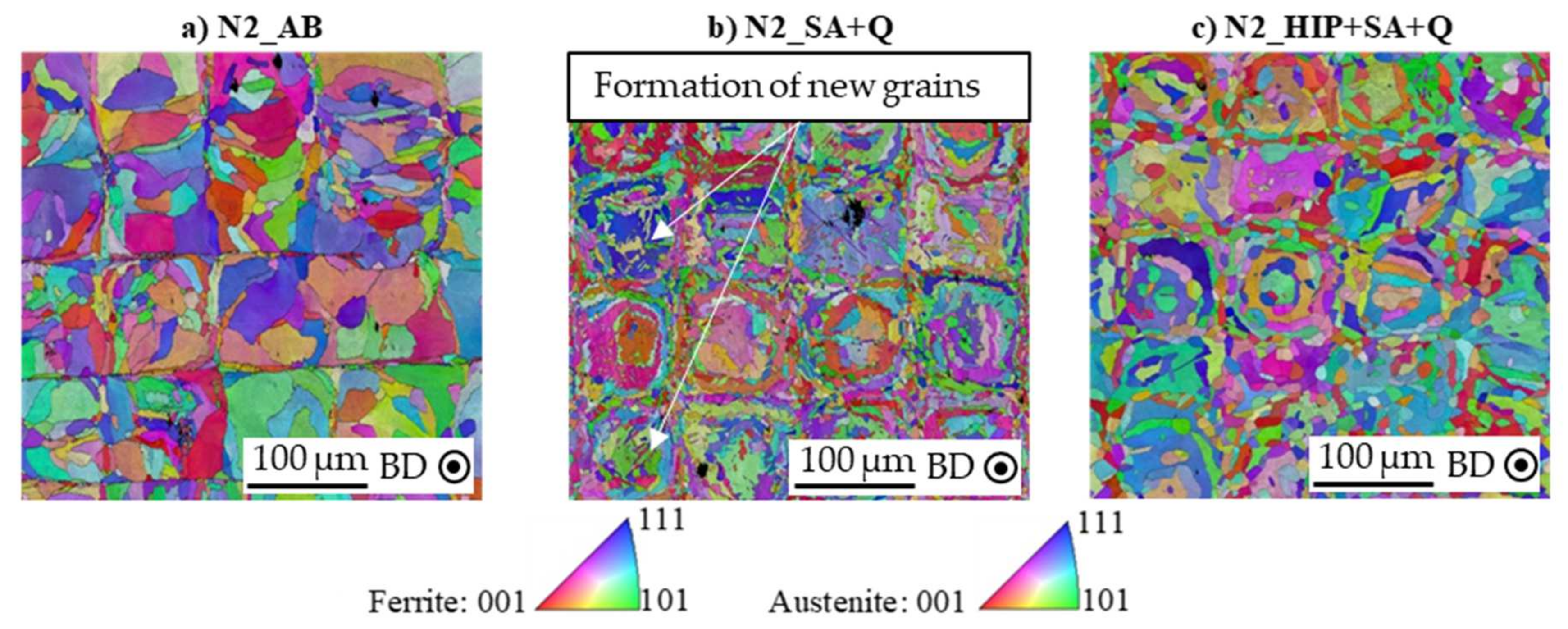
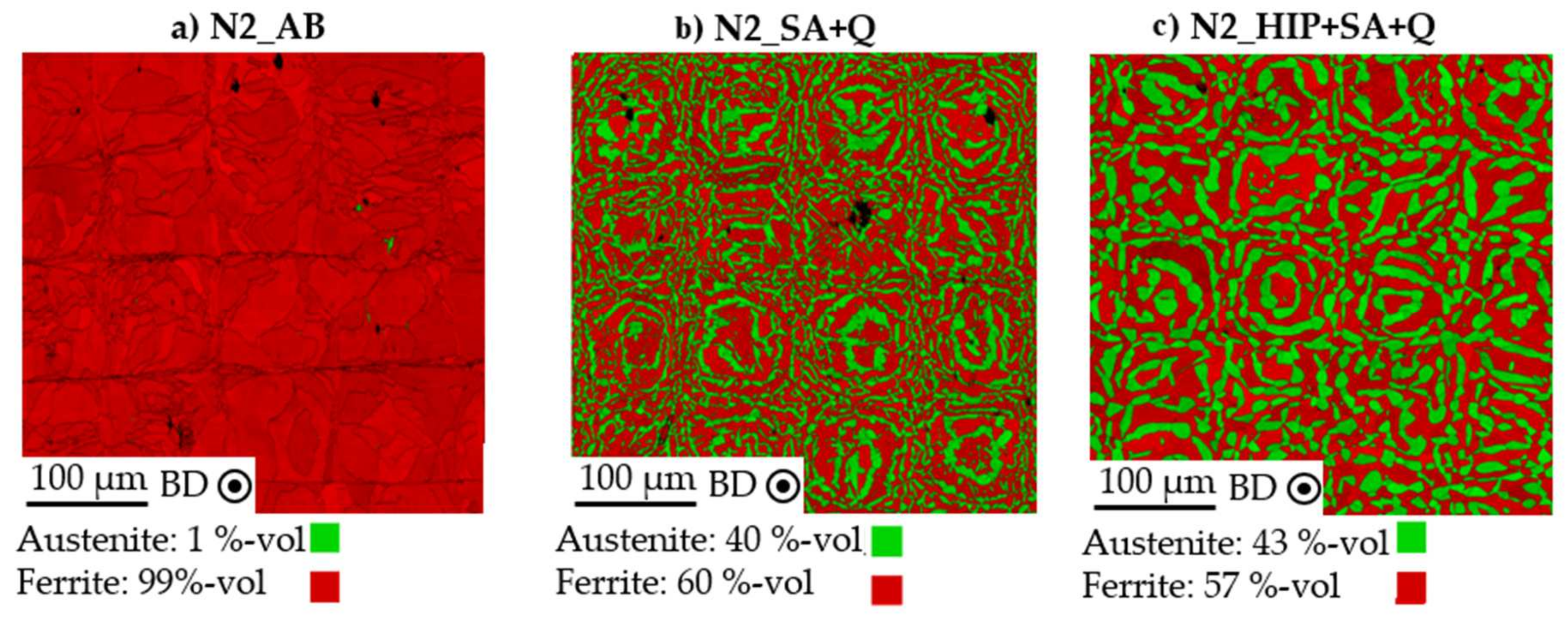
3.2. Influence of Process Atmosphere on Grain Structure and Orientation
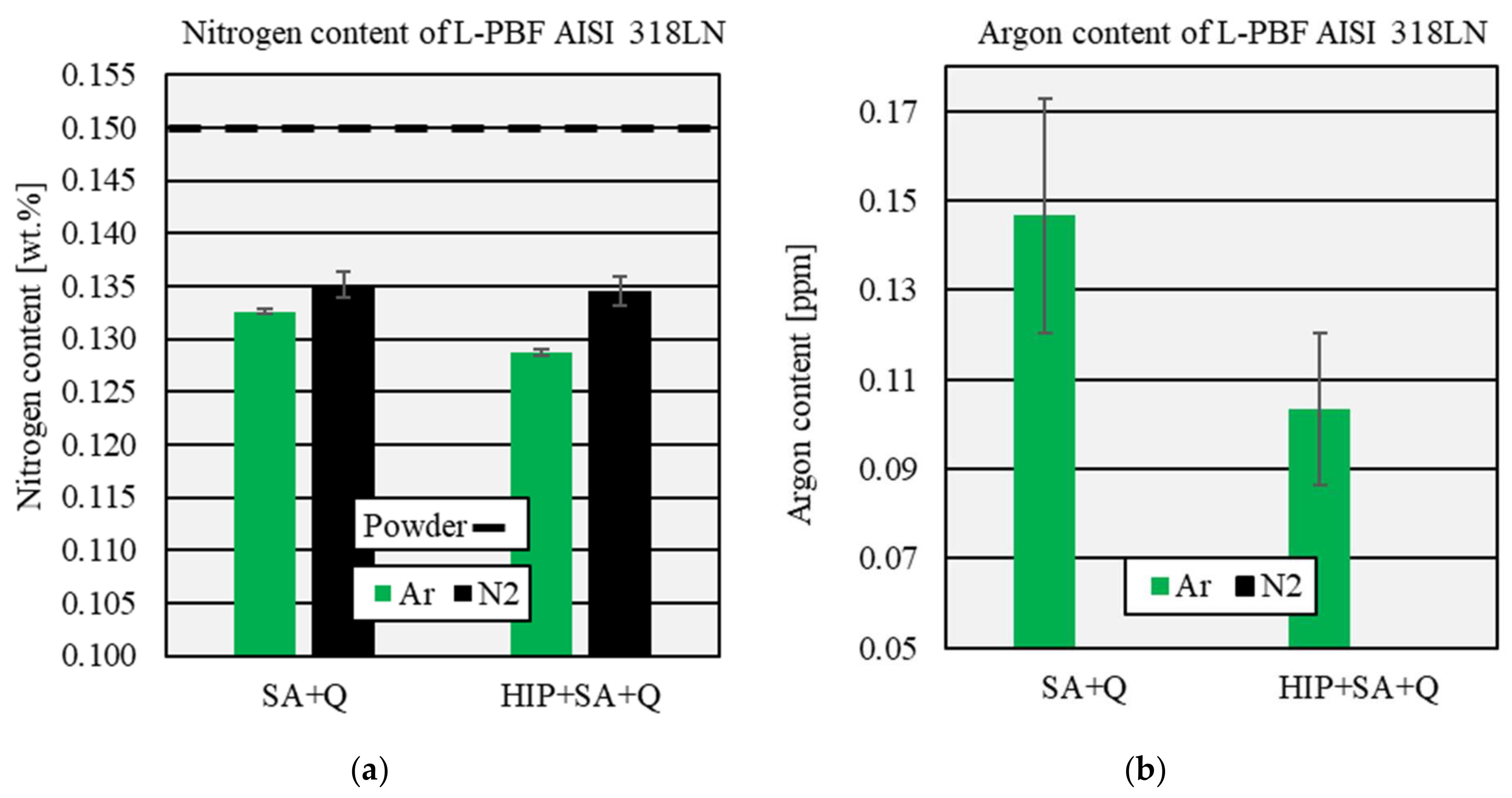
3.3. Influence of the Process Atmosphere on Nitrogen and Argon Content
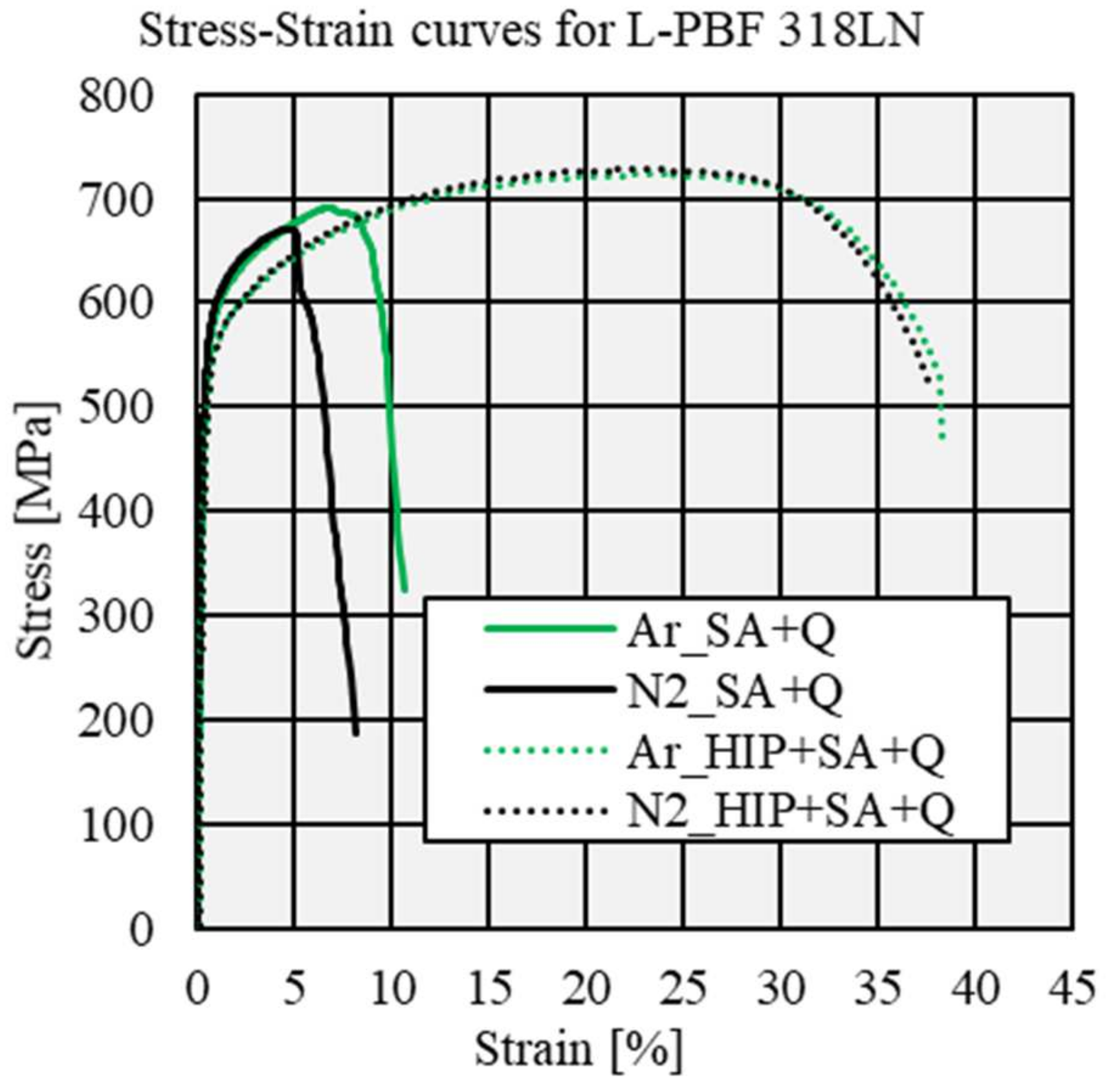
3.4. Influence of Process Atmosphere on Static–Mechanical Properties
4. Conclusions
- Specimens processed under a nitrogen protective atmosphere have a lower density in the as-built condition. The predominant form of porosity in these specimens is large and irregularly formed LOF pores. The use of nitrogen leads to higher retained nitrogen concentrations. Specimens processed under an argon atmosphere form more spherical gas pores due to argon pick-up during the L-PBF process. The difference in density is aligned to higher values by a densification process via HIP. The density remains constant upon additional solution-annealing and quenching;
- A fully ferritic microstructure is achieved in the as-built condition when argon is used as a process gas. A small phase fraction of austenite in the range of 1% is achieved when nitrogen is used. The differences in grain size and orientation between specimens processed under argon and nitrogen are negligible;
- The main influencing factor on tensile strength in the solution-annealed and quenched condition is porosity. Specimens processed under nitrogen show a lower density compared to specimens processed under argon, thus resulting in lower ultimate tensile and yield strength, as well as elongation. HIP neglects the influence of porosity on static–mechanical properties. The remaining argon content of 1 ppm in specimens processed under an argon protective atmosphere does not alter the mechanical properties or elongation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Moyle, M.; Primig, S. Additive manufacturing of steels: A review of achievements and challenges. J. Mater. Sc. 2021, 56, 64–107. [Google Scholar] [CrossRef]
- Gunn, R.N. Duplex Stainless Steels: Microstructure, Properties and Applications; Abington: Cambridge, UK, 1997. [Google Scholar]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Tillmann, W.; Tröster, T.; Hoyer, K.-P.; Schaper, M. Duplex stainless steel fabricated by selective laser melting—Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Davidson, K.; Singamneni, S. Selective Laser Melting of Duplex Stainless Steel Powders: An Investigation. Mater. Manuf. Processes 2016, 31, 1543–1555. [Google Scholar] [CrossRef]
- Kunz, J.; Boontanom, A.; Herzog, S.; Suwanpinij, P.; Kaletsch, A.; Broeckmann, C. Influence of hot isostatic pressing post-treatment on the microstructure and mechanical behavior of standard and super duplex stainless steel produced by laser powder bed fusion. Mater. Sci. Eng. A 2020, 794, 139806. [Google Scholar] [CrossRef]
- Papula, S.; Song, M.; Pateras, A.; Chen, X.-B.; Brandt, M.; Easton, M.; Yagodzinskyy, Y.; Virkkunen, I.; Hänninen, H. Selective Laser Melting of Duplex Stainless Steel 2205: Effect of Post-Processing Heat Treatment on Microstructure, Mechanical Properties, and Corrosion Resistance. Materials 2019, 12, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Technology and Challenges in Additive Manufacturing of Duplex Stainless Steels. Biointerface Res. Appl. Chem. 2021, 12, 1110–1119. [CrossRef]
- Köhler, M.L.; Kunz, J.; Herzog, S.; Kaletsch, A.; Broeckmann, C. Microstructure analysis of novel LPBF-processed duplex stainless steels correlated to their mechanical and corrosion properties. Mater. Sci. Eng. A 2021, 801, 140432. [Google Scholar] [CrossRef]
- Hertzman, S.; Charles, J. On the effect of nitrogen on duplex stainless steels. Rev. Metall. 2011, 108, 413–425. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Metallogr. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.; Agrawal, A.; Mandal, A.; Podder, A.S. Characteristics and Manufacturability of Duplex Stainless Steel: A Review. Trans. Indian Inst. Met. 2021, 74, 1089–1098. [Google Scholar] [CrossRef]
- Brandi, S.D.; Schön, C.G. A Thermodynamic Study of a Constitutional Diagram for Duplex Stainless Steels. J. Phase Equilib. Diffus. 2017, 38, 268–275. [Google Scholar] [CrossRef]
- Park, Y.-H.; Lee, Z.-H. The effect of nitrogen and heat treatment on the microstructure and tensile properties of 25Cr–7Ni–1.5Mo–3W–xN duplex stainless steel castings. Mater. Sci. Eng. A 2001, 297, 78–84. [Google Scholar] [CrossRef]
- Frehser, J.; Kubisch, C. Metallurgie und Eigenschaften unter hohem Druck erschmolzener stickstoffhaltiger Stähle. Berg-Hüttenmännische Mon. 1963, 11, 369–380. [Google Scholar]
- Becker, L.; Röttger, A.; Boes, J.; Weber, S.; Theisen, W. Processing of a newly developed nitrogen-alloyed ferritic-austenitic stainless steel by laser powder bed fusion—Microstructure and properties. Addit. Manuf. 2021, 46, 102185. [Google Scholar] [CrossRef]
- Boes, J.; Röttger, A.; Becker, L.; Theisen, W. Processing of gas-nitrided AISI 316L steel powder by laser powder bed fusion—Microstructure and properties. Addit. Manuf. 2019, 30, 100836. [Google Scholar] [CrossRef]
- Boes, J.; Röttger, A.; Theisen, W. Microstructure and properties of high-strength C + N austenitic stainless steel processed by laser powder bed fusion. Addit. Manuf. 2020, 32, 101081. [Google Scholar] [CrossRef]
- Valiente Bermejo, M.A.; Thalavai Pandian, K.; Axelsson, B.; Harati, E.; Kisielewicz, A.; Karlsson, L. Microstructure of laser metal deposited duplex stainless steel: Influence of shielding gas and heat treatment. Weld. World 2021, 65, 525–541. [Google Scholar] [CrossRef]
- Pauzon, C.; Leicht, A.; Klement, U.; Forêt, P.; Hryha, E. Effect of the Process Gas and Scan Speed on the Properties and Productivity of Thin 316L Structures Produced by Laser-Powder Bed Fusion. Metall. Mater. Trans. A 2020, 51, 5339–5350. [Google Scholar] [CrossRef]
- Pauzon, C.; Hryha, E.; Forêt, P.; Nyborg, L. Effect of argon and nitrogen atmospheres on the properties of stainless steel 316 L parts produced by laser-powder bed fusion. Mater. Des. 2019, 179, 107873. [Google Scholar] [CrossRef]
- Ch, S.R.; Raja, A.; Nadig, P.; Jayaganthan, R.; Vasa, N.J. Influence of working environment and built orientation on the tensile properties of selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2019, 750, 141–151. [Google Scholar] [CrossRef]
- Rakesh, C.S.; Priyanka, N.; Jayaganthan, R.; Vasa, N.J. Effect of build atmosphere on the mechanical properties of AlSi10Mg produced by selective laser melting. Mater. Today Proc. 2018, 5, 17231–17238. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, L.C.; Fang, M.H.; Sercombe, T.B. The effect of atmosphere on the structure and properties of a selective laser melted Al–12Si alloy. Mater. Sci. Eng. A 2014, 597, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.; Gu, D. Effect of metal vaporization behavior on keyhole-mode surface morphology of selective laser melted composites using different protective atmospheres. Appl. Surf. Sci. 2015, 355, 310–319. [Google Scholar] [CrossRef]
- Dong, J.; Liu, S.; Chen, H.; Li, D.; Zhang, T.; Chen, C.; Zhou, K. Effect of atmosphere on the microstructure and properties of additively manufactured tungsten. Mater. Sci. Technol. 2020, 36, 1988–1996. [Google Scholar] [CrossRef]
- Pauzon, C.; Markström, A.; Dubiez-Le Goff, S.; Hryha, E. Effect of the Process Atmosphere Composition on Alloy 718 Produced by Laser Powder Bed Fusion. Metals 2021, 11, 1254. [Google Scholar] [CrossRef]
- Traore, S.; Schneider, M.; Koutiri, I.; Coste, F.; Fabbro, R.; Charpentier, C.; Lefebvre, P.; Peyre, P. Influence of gas atmosphere (Ar or He) on the laser powder bed fusion of a Ni-based alloy. J. Mater. Processing Technol. 2021, 288, 116851. [Google Scholar] [CrossRef]
- DIN EN 10088-3:2014-12; Nichtrostende Stähle_- Teil_3: Technische Lieferbedingungen für Halbzeug, Stäbe, Walzdraht, Gezogenen Draht, Profile und Blankstahlerzeugnisse aus Korrosionsbeständigen Stählen für Allgemeine Verwendung. Deutsche Fassung EN_10088-3:2014; Beuth Verlag GmbH: Berlin, Germany, 2014.
- SS 118000:2018; Powder Metallurgy—Hot Isostatic Pressing—Argon Detection Using Gas Chromatography and mass Spectrometry Techniques. Swedish Institute for Standards: Stockholm, Sweden, 2018.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- DIN EN ISO 6892-1:2020-06; Metallische Werkstoffe_-Zugversuch_-Teil_1: Prüfverfahren bei Raumtemperatur (ISO_6892-1:2019); Deutsche Fassung EN_ISO_6892-1:2019. Beuth Verlag GmbH: Berlin, Germany, 2019.
- DIN 50125:2021-08; Prüfung Metallischer Werkstoffe_- Zugproben. Beuth Verlag GmbH: Berlin, Germany, 2021.
- Carassus, H.; Morvan, H.; Haugou, G.; Guerin, J.-D.; Sadat, T.; Guerard, S.; Markiewicz, E. On the effects of build orientation, strain rate sensitivity and sample thickness on the mechanical behavior of 316l Stainless Steel manufactured by Selective Laser Melting. EPJ Web Conf. 2021, 250, 5009. [Google Scholar] [CrossRef]
- DIN EN ISO 6507-1:2018-07; Metallische Werkstoffe_-Härteprüfung nach Vickers_-Teil_1: Prüfverfahren (ISO_6507-1:2018); Deutsche Fassung EN_ISO_6507-1:2018. Beuth Verlag GmbH: Berlin, Germany, 2018.
- Martin, A.A.; Calta, N.P.; Khairallah, S.A.; Wang, J.; Depond, P.J.; Fong, A.Y.; Thampy, V.; Guss, G.M.; Kiss, A.M.; Stone, K.H.; et al. Dynamics of pore formation during laser powder bed fusion additive manufacturing. Nat. Commun. 2019, 10, 1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iams, A.D.; Keist, J.S.; Giannuzzi, L.A.; Palmer, T.A. The Evolution of Oxygen-Based Inclusions in an Additively Manufactured Super-Duplex Stainless Steel. Metall. Mater. Trans. A 2021, 52, 3401–3412. [Google Scholar] [CrossRef]
- Broeckmann, C. Hot Isostatic Pressing: HIP’17; Hot Isostatic Pressing; Materials Research Forum LLC.: Millersville, PA, USA, 2019; pp. 169–181. [Google Scholar]
- Kaletsch, A.; Qin, S.; Herzog, S.; Broeckmann, C. Influence of high initial porosity introduced by laser powder bed fusion on the fatigue strength of Inconel 718 after post-processing with hot isostatic pressing. Addit. Manuf. 2021, 47, 102331. [Google Scholar] [CrossRef]
- Kunz, J.; Herzog, S.; Kaletsch, A.; Broeckmann, C. Influence of initial defect density on mechanical properties of AISI H13 hot-work tool steel produced by laser powder bed fusion and hot isostatic pressing. Powder Metall. 2021, 65, 1–12. [Google Scholar] [CrossRef]
- Shiyas, K.A.; Ramanujam, R. A review on post processing techniques of additively manufactured metal parts for improving the material properties. Mater. Today Proc. 2021, 46, 1429–1436. [Google Scholar] [CrossRef]
- Peng, X.; Kong, L.; Fuh, J.Y.H.; Wang, H. A Review of Post-Processing Technologies in Additive Manufacturing. J. Manuf. Mater. Processing 2021, 5, 38. [Google Scholar] [CrossRef]
- Lavery, N.P.; Cherry, J.; Mehmood, S.; Davies, H.; Girling, B.; Sackett, E.; Brown, S.; Sienz, J. Effects of hot isostatic pressing on the elastic modulus and tensile properties of 316L parts made by powder bed laser fusion. Mater. Sci. Eng. A 2017, 693, 186–213. [Google Scholar] [CrossRef] [Green Version]
- Liverani, E.; Lutey, A.H.A.; Ascari, A.; Fortunato, A. The effects of hot isostatic pressing (HIP) and solubilization heat treatment on the density, mechanical properties, and microstructure of austenitic stainless steel parts produced by selective laser melting (SLM). Int. J. Adv. Manuf. Technol. 2020, 107, 109–122. [Google Scholar] [CrossRef]
- Kluczyński, J.; Śnieżek, L.; Grzelak, K.; Oziębło, A.; Perkowski, K.; Torzewski, J.; Szachogłuchowicz, I.; Gocman, K.; Wachowski, M.; Kania, B. Hot isostatic pressing influence on the mechanical properties of selectively laser-melted 316L. Bull. Pol. Acad. Sci. Tech. Sci. 2020, 68, 1413–1424. [Google Scholar]






| Combustion Analysis | Optical Emission Spectroscopy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | C | N | Si | Mn | P | S | Cr | Mo | Ni | Fe |
| Powder in this study | 0.02 | 0.15 | 0.70 | 1.10 | 0.01 | 0.01 | 21.30 | 2.50 | 5.00 | Bal. |
| EN 10088-3 | <0.03 | 0.10–0.22 | <1.00 | <2.00 | <0.035 | <0.015 | 21.00–23.00 | 2.50–3.50 | 4.50–6.50 | Bal. |
| Gas | Condition | |||
|---|---|---|---|---|
| AB | SA + Q | HIP | HIP + SA + Q | |
| Argon (HV10) | 355.1 ± 11.4 | 249.9 ± 6.1 | 237.3 ± 2.8 | 234.4 ± 3.1 |
| Nitrogen (HV10) | 358.1 ± 9.6 | 247.6 ± 3.2 | 237.4 ± 6.4 | 235.9 ± 2.8 |
| Gas | Condition | Rm [MPa] | Rp0.2 [MPa] | Ag [%] | A [%] |
|---|---|---|---|---|---|
| Argon | SA + Q | 692.2 ± 20.6 | 531.9 ± 7.6 | 6.6 ± 1.3 | 9.8 ± 1.3 |
| HIP + SA + Q | 724.9 ± 2.1 | 500.1 ± 3.5 | 22.8 ± 0.4 | 38.0 ±0.5 | |
| Nitrogen | SA + Q | 603.7 ± 48.8 | 488.0 ± 20.9 | 3.0 ± 1.2 | 5.6 ± 1.8 |
| HIP + SA + Q | 728.5 ± 2.2 | 495.2 ±6.3 | 23.2 ± 1.0 | 37.2 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirz, M.; Herzog, S.; Broeckmann, C.; Kaletsch, A. Influence of the L-PBF Process Atmosphere on the Microstructure and Tensile Properties of AISI 318LN Duplex Stainless Steel. J. Manuf. Mater. Process. 2022, 6, 32. https://doi.org/10.3390/jmmp6020032
Mirz M, Herzog S, Broeckmann C, Kaletsch A. Influence of the L-PBF Process Atmosphere on the Microstructure and Tensile Properties of AISI 318LN Duplex Stainless Steel. Journal of Manufacturing and Materials Processing. 2022; 6(2):32. https://doi.org/10.3390/jmmp6020032
Chicago/Turabian StyleMirz, Markus, Simone Herzog, Christoph Broeckmann, and Anke Kaletsch. 2022. "Influence of the L-PBF Process Atmosphere on the Microstructure and Tensile Properties of AISI 318LN Duplex Stainless Steel" Journal of Manufacturing and Materials Processing 6, no. 2: 32. https://doi.org/10.3390/jmmp6020032
APA StyleMirz, M., Herzog, S., Broeckmann, C., & Kaletsch, A. (2022). Influence of the L-PBF Process Atmosphere on the Microstructure and Tensile Properties of AISI 318LN Duplex Stainless Steel. Journal of Manufacturing and Materials Processing, 6(2), 32. https://doi.org/10.3390/jmmp6020032






