Experimental and Numerical Assessment of the Hot Sheet Formability of Martensitic Stainless Steels
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial State of Test Material
2.2. Experimental Methods
2.2.1. Procedure and Parameter Setup for the Thermomechanical Treatment of MSS
2.2.2. Definition of the Forming Temperature
2.2.3. Experimental Hot Deep Drawing Test
2.2.4. Determination of the Maximum Drawing Force and Maximum Drawing Depth
2.2.5. Determination of the Maximum Hardness of Specimen
2.3. Thermophysical Calculations
2.4. Structure of the FE-Model
2.5. Statistical Study–Analysis of Variance
3. Results
3.1. Martensitic Stainless Steel Alloys
3.2. Cooling Behaviour of Austenized MSS
3.3. Phase Transformation by Thermophysical Calculations
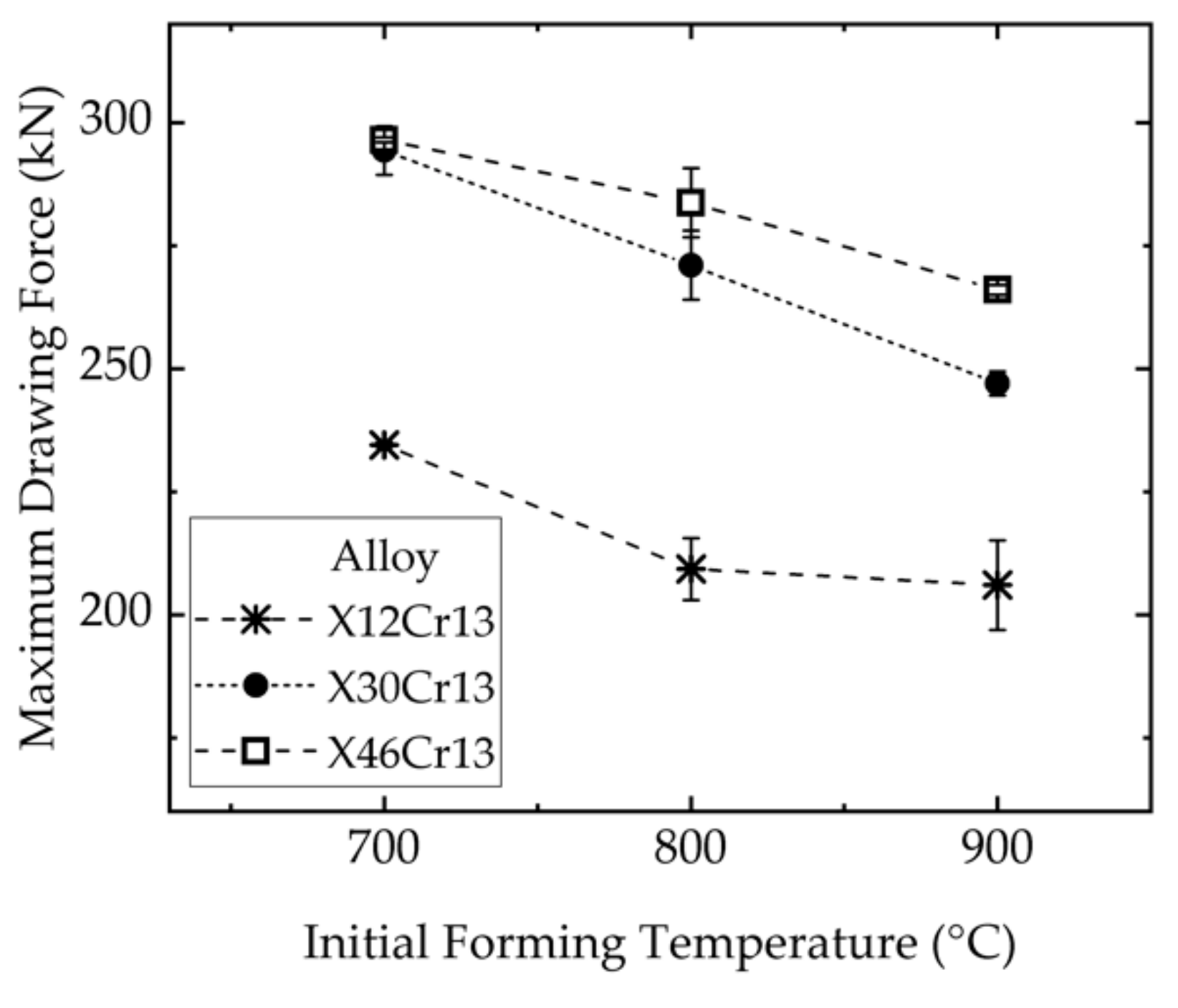
3.4. Influence of the Alloy Composition and the Forming Temperature on the Forming Forces
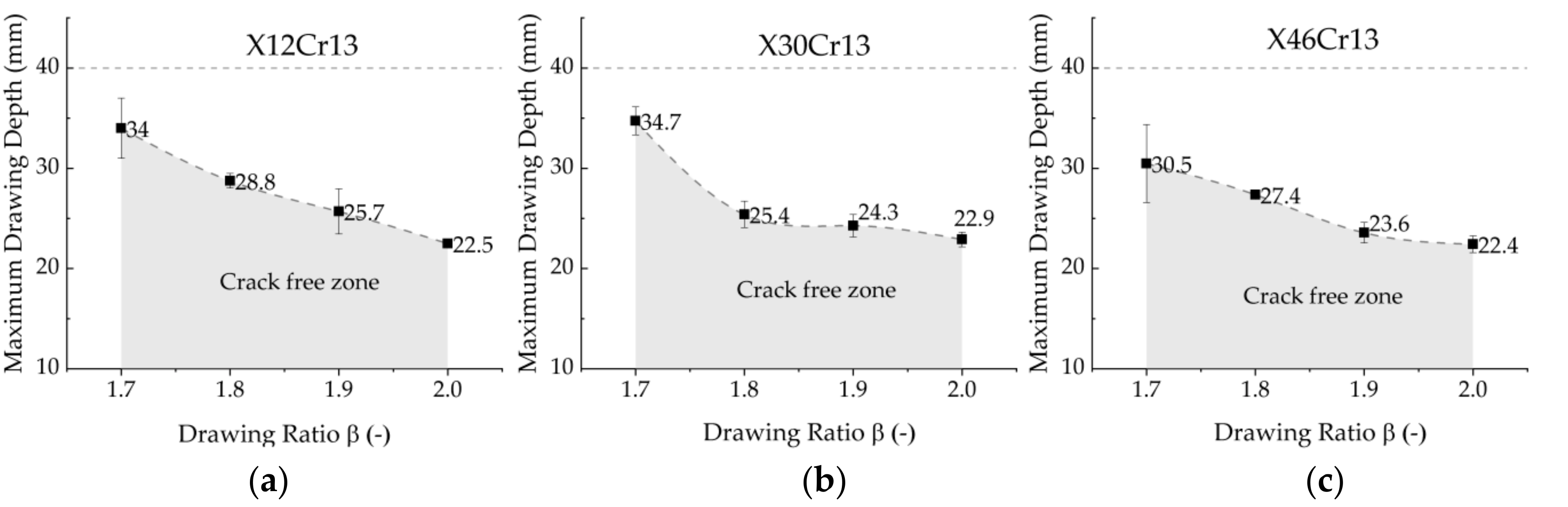
3.5. Influence of the Blank Diameter on the Maximum Drawing Depth
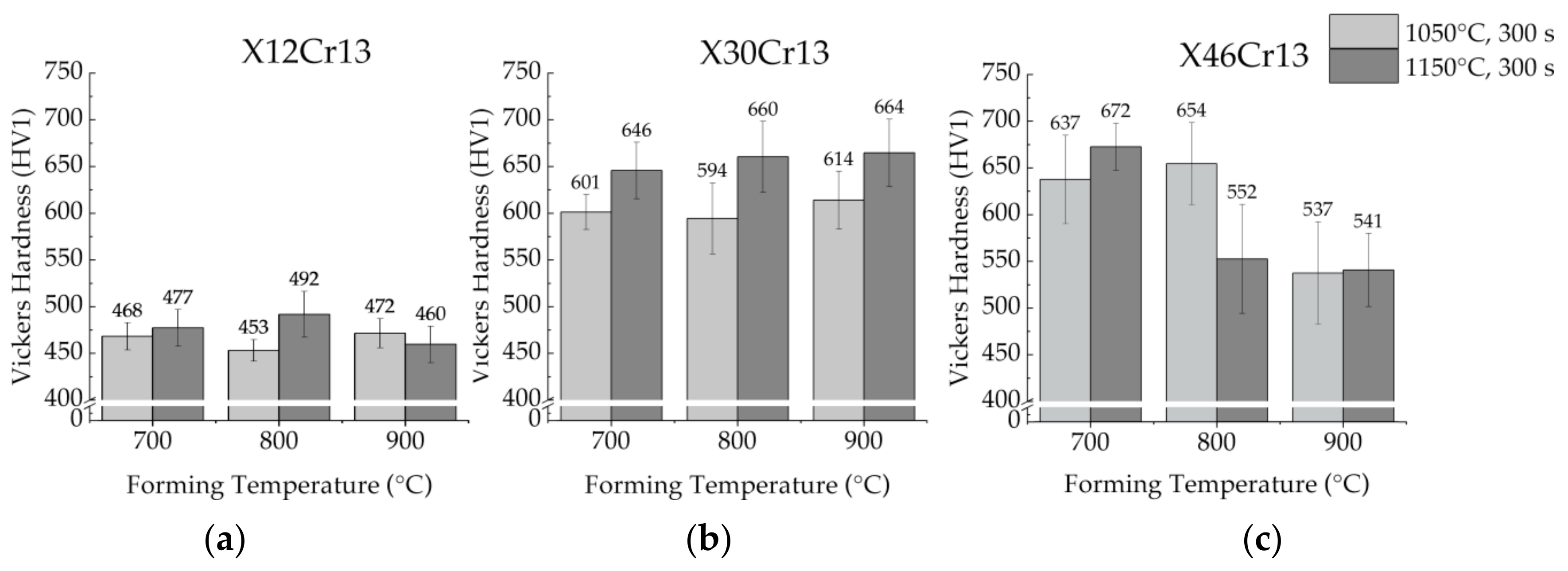
3.6. Influence of the Austenitzation Temperature and the Forming Temperature on the Hardness
3.7. Influence of the Austenitzation Time and the Forming Temperature on the Hardness
4. FE-Simulation of the Thermomechanical Treatment for MSS
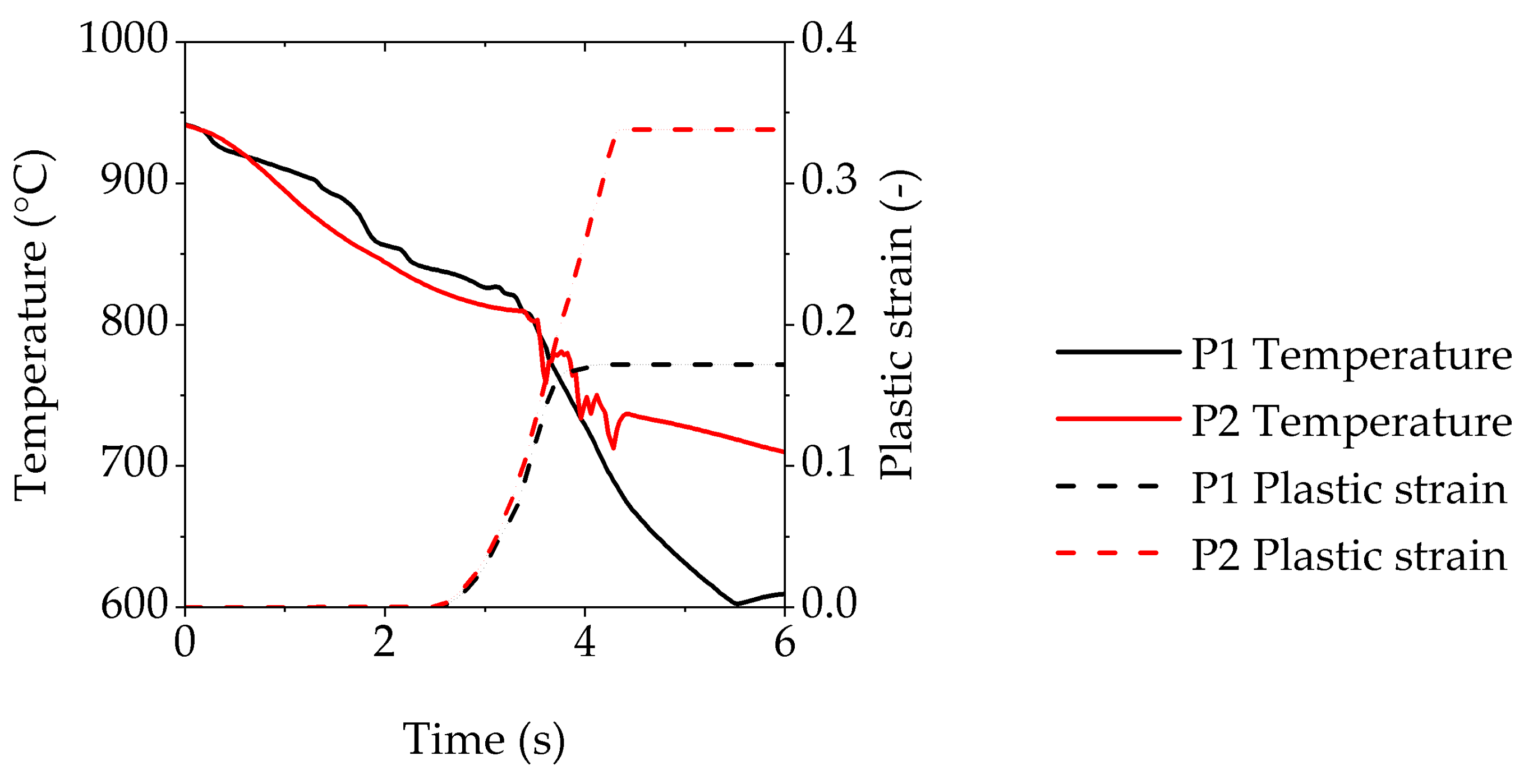
4.1. Distribution of the Plastic Strain in the Cup
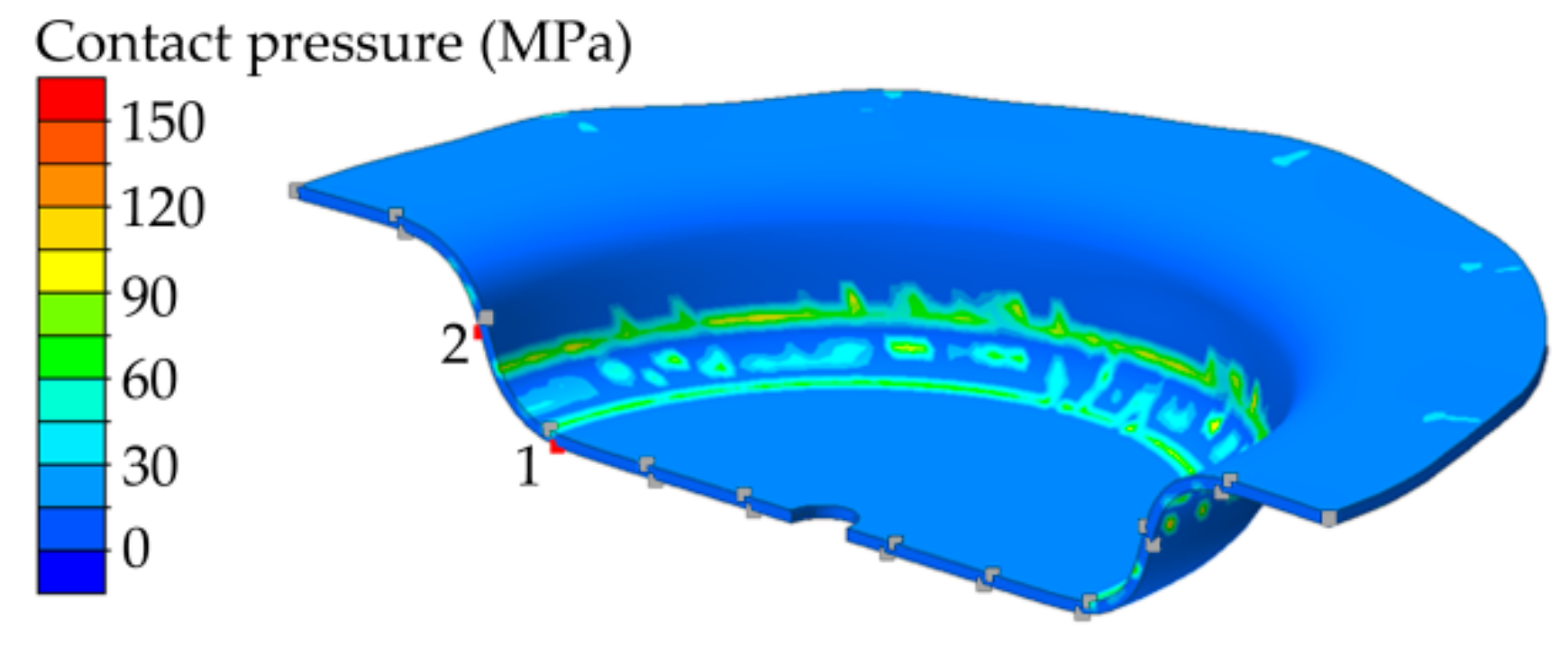
4.2. Distribution of the Contact Pressure between Workpiece and Tool during Forming
4.3. Simultaneous Cooling and Forming Behaviour during the Thermomechanical Treatment
5. Statistical Study—Analysis of Variance
5.1. Resulting Vickers Hardness
5.2. Resulting Maximum Drawing Depth
6. Discussion
6.1. Influence of the Alloy Composition and the Process Parameters on the Forming Forces
6.2. Influence of the Process Parameters on the Maximum Drawing Depth
6.3. Influence of the Process Parameters on the Mean Hardness
7. Conclusions
- Deep drawability:
- Forming forces:
- Hardenability:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gümpel, P. Rostfreie Stähle—Grundwissen, Konstruktion und Verarbeitungshinweise, Bd. 493; Expert Verlag: Renningen-Malmsheim, Germany, 1996. [Google Scholar]
- McGuire, M. Chapter 9 Martensitic stainless steels. In Stainless Steels for Design Engineers, 1st ed.; ASM International: Materials Park, OH, USA, 2008; pp. 133–135. [Google Scholar]
- Meza-García, E.; Rautenstrauch, A.; Kräusel, V.; Landgrebe, D. Tailoring of mechanical properties of a side sill part made of martensitic stainless steel by press hardening. In AIP Conference Proceedings, Proceedings of the 19th International ESAFORM Conference on Material Forming, Nantes, France, 27 April 2016; Chinesta, F., Cueto, E., Abisset-Chavanne, E., Eds.; AIP Publishing: Melville, NY, USA, 2016; Volume 1769, pp. 1300015-1–1300015-5. [Google Scholar]
- Meza-García, E.; Rautenstrauch, A.; Kräusel, V.; Landgrebe, D. Press hardening of a martensitic stainless steel sheet alloy for manufacturing a side sill demonstrator with tailored properties. In Proceedings of the 5th International Conference on Hot Sheet Metal Forming of High Performance Steel CHS2, Toronto, ON, Canada, 31 May–3 June 2015; Steinhoff, K., Oldenburg, M., Prakash, B., Eds.; Publisher: Verlag Wissenschaftliche Scripten, Auerbach, Germany, 2015; pp. 765–773. [Google Scholar]
- Karbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Garcia, C.; Alvarez, L.F. Optimization of the properties obtained by quenching in martensitic stainless steels X30-40Cr13 and X40-60CrMoV14. J. Mater. Sci. 1993, 28, 1264–1268. [Google Scholar] [CrossRef]
- Alvarez, L.F.; Garcia, C.; Lopez, V. Continuous cooling transformation in martensitic stainless steel. ISIJ Int. 1994, 34, 516–521. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, Z.; Li, X.; Miodownik, A.P.; Schillé, J.-P. Using JMatPro to model materials properties and behavior. JOM 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Landgrebe, D.; Rautenstrauch, A.; Kunke, A.; Polster, S.; Kriechenbauer, S.; Mauermann, R. The effect of cushion-ram pulsation on hot stamping. AIP Conf. Proc. 2016, 1769, 070014. [Google Scholar] [CrossRef]
- Kusumi, K.; Nomura, N.; Yamamoto, S.; Nakata, M.; Abe, M.; Suehiro, M. Improvement of cylindrical deep drawability in hot stamping. Procedia Eng. 2014, 81, 1719–1724. [Google Scholar] [CrossRef][Green Version]
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals; Academic Press Inc.: Cambridge, MA, USA, 1970; Volume 4, p. 344. [Google Scholar]
- Saunders, N.; Miodownik, A.P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide; Pergamon Materials Series; Elsevier: London, UK, 1998; Volume 1, p. 478. [Google Scholar]
- Diekmann, U. Calculation of steel data using JMatPro. In Proceedings of the 2nd International Conference on Recent Trends in Structural Materials (COMAT 2012), Plzeň, Czech Republic, 21–22 November 2012; Dzugan, J., Ed.; Thomas Reuters Database: Toronto, ON, Canada, 2012. [Google Scholar]
- Guo, Z.; Saunders, N.; Miodownik, P.; Schillé, J.P. Modelling phase transformations and material properties critical to the prediction of distortion during the heat treatment of steels. Int. J. Microstruct. Mater. Prop. 2009, 4, 187–195. [Google Scholar] [CrossRef]
- Meza-García, E.; Birnbaum, P.; Landgraf, P.; Grund, T.; Lampke, T.; Kräusel, V. Thermomechanical Treatment of Martensitic Stainless Steels Sheets and Its Effects on Their Deep Drawability and Resulting Hardness in Press Hardening. Metals 2020, 10, 1536. [Google Scholar] [CrossRef]
- Spittel, M.; Spittel, M. Steel symbol/number: Datasheet X12Cr13/1.4006;X46Cr13/1.4034. In Landolt-Börnstein—Group VIII Advanced Materials and Technologies; Warlimont, H., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2009; Volume 2C1. [Google Scholar]

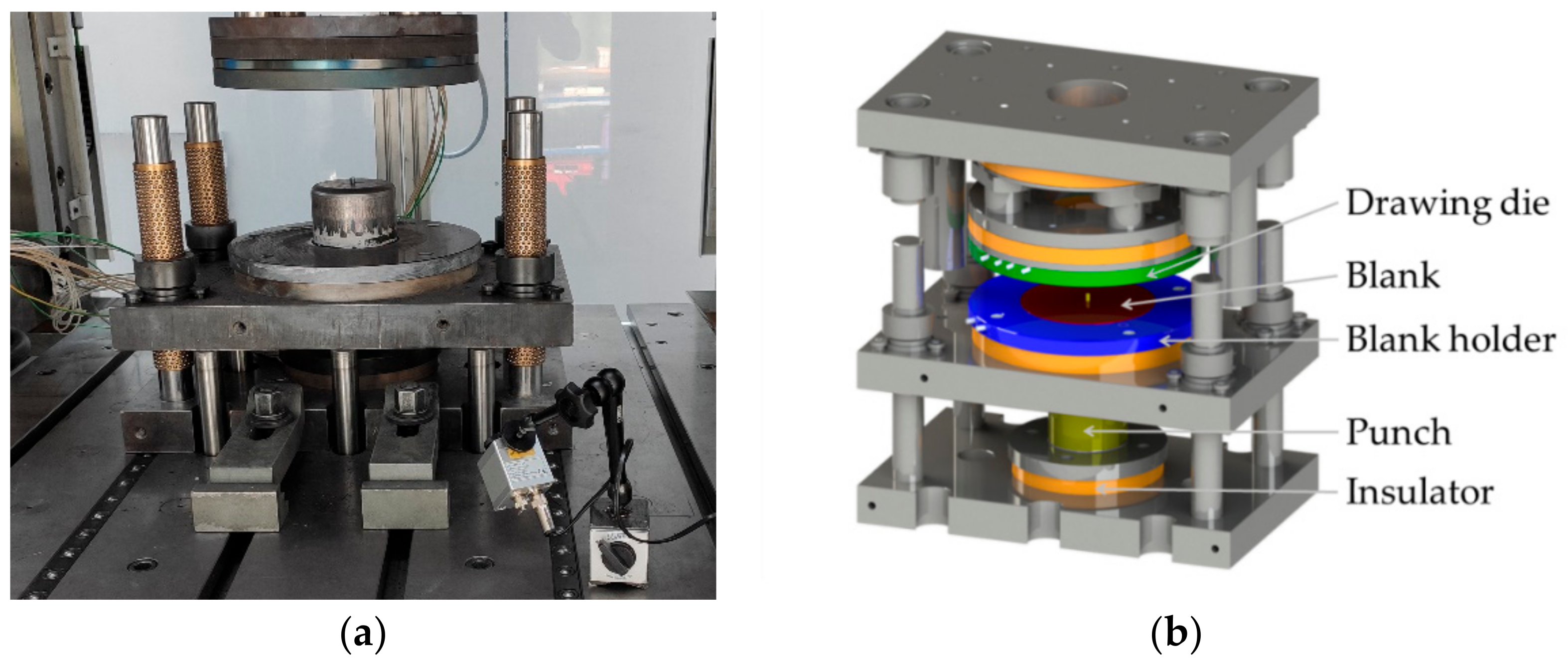
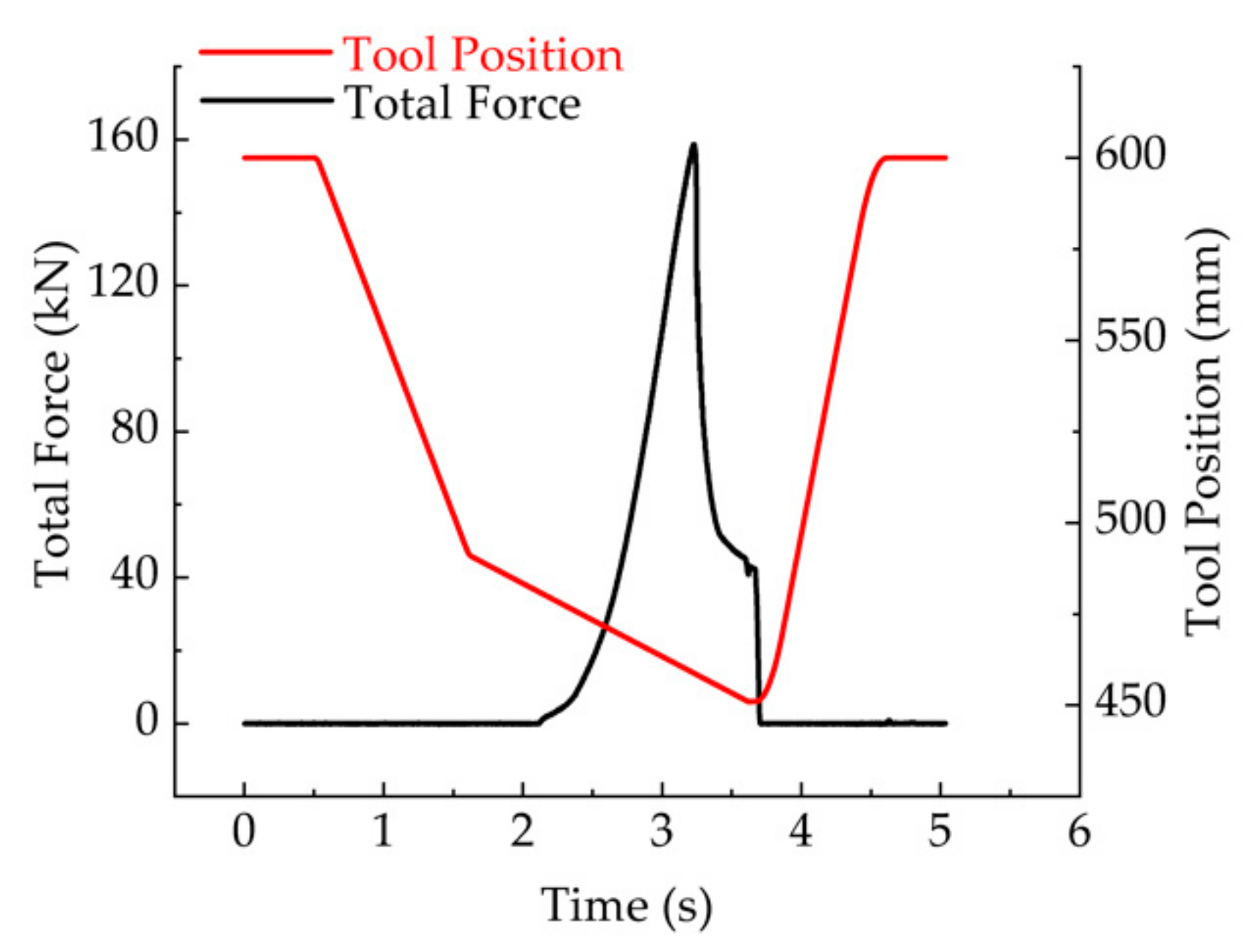


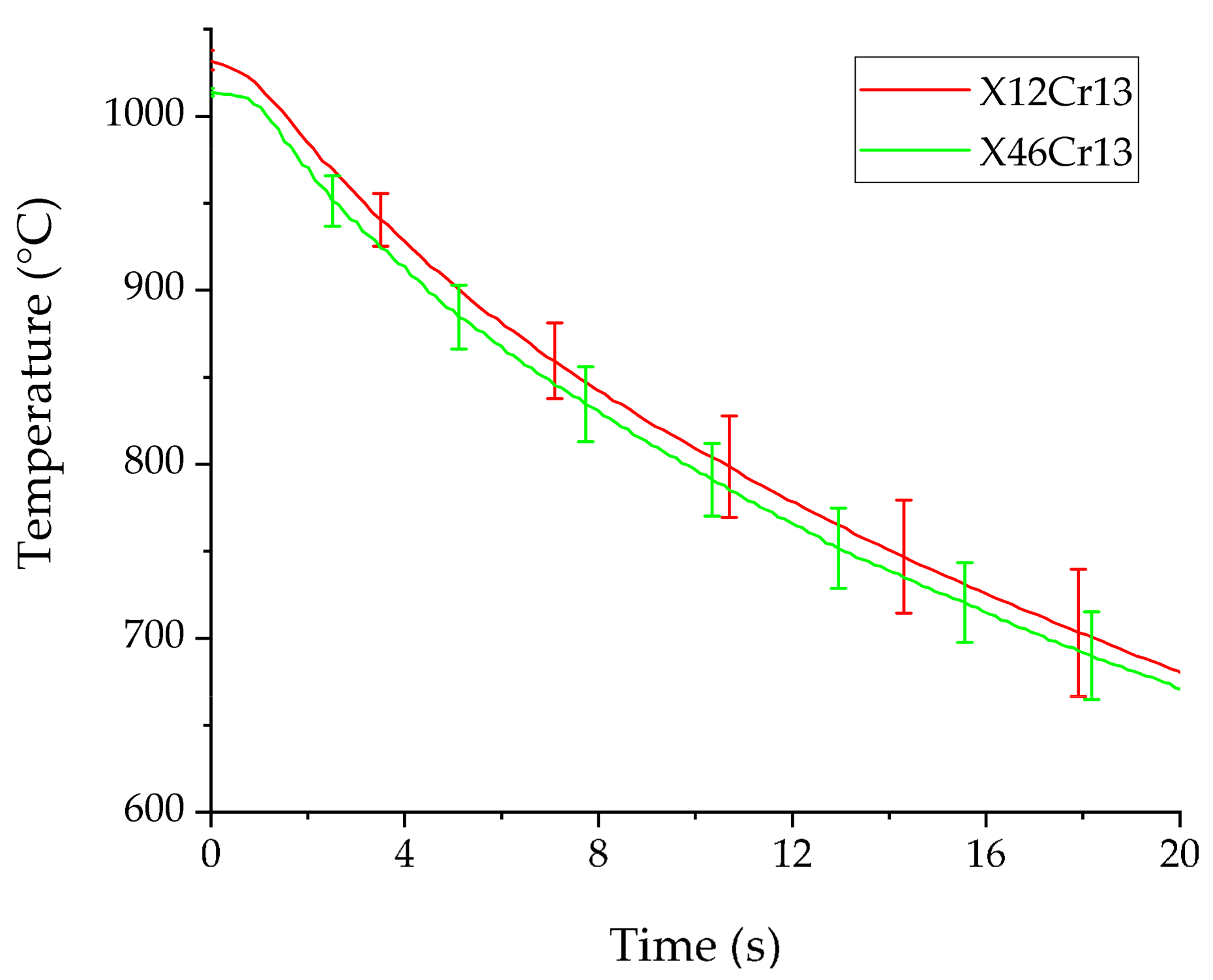
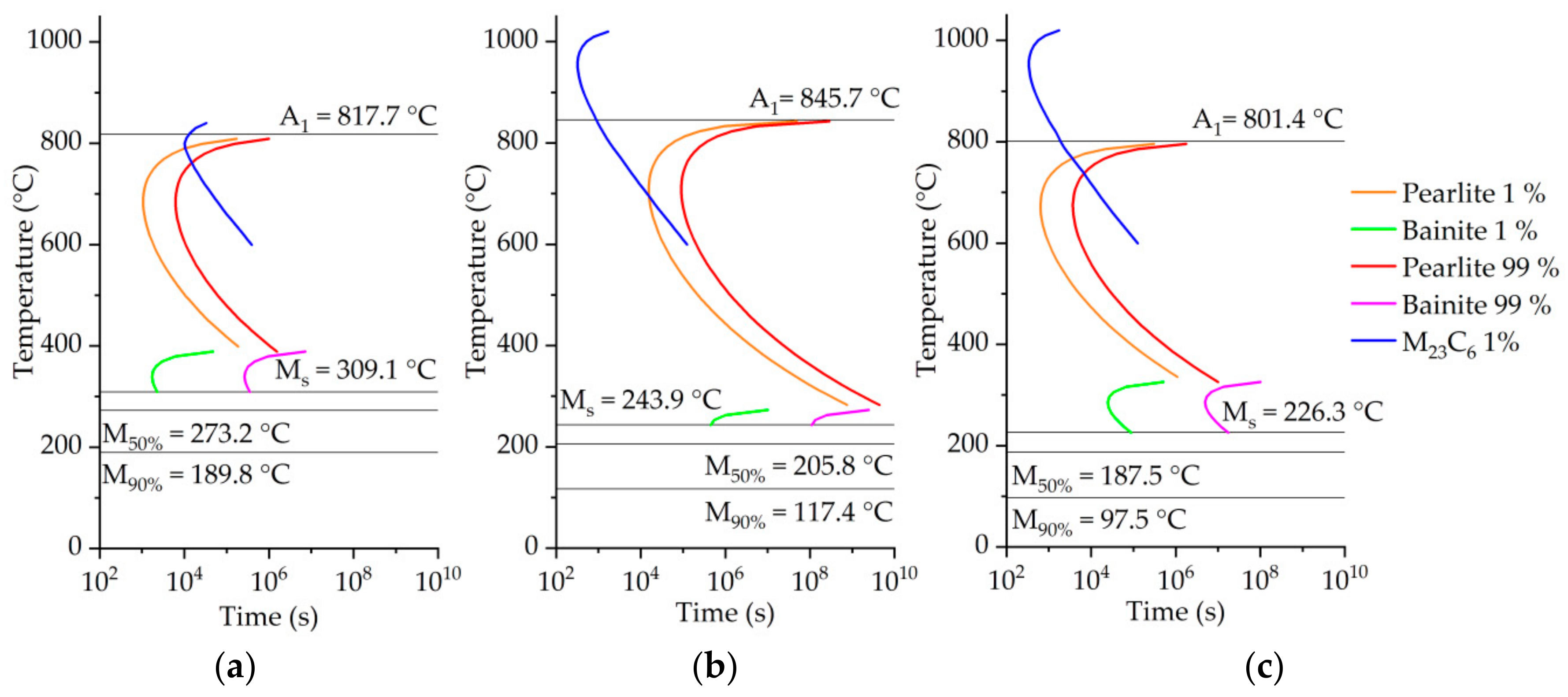


| Austenitization Temperature Tγ (°C) | Austenitization Time tdwell (s) | Initial Forming Temperature (°C) | Blank Diameter (mm) | Flange Gap (mm) | Ram Speed (mm/s) |
|---|---|---|---|---|---|
| 1050, 1150 | 120, 300 | 700, 800, 900 | 170, 180, 190, 200 | 2.5 | 20 |
| Austenitization Temperature (Tγ) (°C) | Austenitization Time (tdwell) (s) | Initial Forming Temperature (°C) | Blank Diameter (mm) | Flange Gap (mm) | Ram Speed (mm/s) |
|---|---|---|---|---|---|
| 1050 | 300 | 700, 800, 900 | 170 | 2.5 | 20 |
| Alloy | C | Cr | Si | Mo | Mn | P | S | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|
| X12Cr13 | 0.11 | 11.54 | 0.50 | 0.06 | 0.38 | 0.021 | <0.001 | 0.33 | 0.18 |
| X30Cr13 | 0.36 | 13.47 | 0.47 | 0.94 | 0.42 | 0.021 | 0.002 | 0.38 | 0.06 |
| X46Cr13 | 0.48 | 13.62 | 0.47 | 0.02 | 0.63 | 0.026 | <0.001 | 0.17 | 0.23 |
| Alloy | Condition | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Fracture Elongation A50mm (%) |
|---|---|---|---|---|
| X12Cr13 | Supply condition | 518 ± 19 | 651 ± 19 | 17 ± 0.1 |
| X30Cr13 | Supply condition | 361 ± 6 | 611 ± 5 | 26 ± 0.2 |
| X46Cr13 | Supply condition | 371 ± 3 | 633 ± 3 | 26 ± 0.1 |
| Input Parameter | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Alloy | 2 | 159,651.02 | 79,825.51 | 78.74 | 5.46 × 10−6 |
| Austenitization | 2 | 7344.17 | 3672.08 | 3.62 | 0.08 |
| Forming temperature | 2 | 3406.55 | 1703.28 | 1.68 | 0.25 |
| Alloy-Austenitization | 4 | 10,915.19 | 2728.80 | 2.69 | 0.11 |
| Alloy-Forming temperature | 4 | 7230.37 | 1807.59 | 1.78 | 0.22 |
| Austenitization-Forming temperature | 4 | 1323.33 | 330.83 | 0.33 | 0.85 |
| Error | 8 | 8110.44 | 1013.80 | ||
| Total | 26 | 197,981.07 |
| Input Parameter | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Alloy | 2 | 159,651.02 | 79,825.51 | 78.74 | 5.46 × 10−6 |
| Austenitization | 1 | 4485.71 | 4485.71 | 4.42 | 0.07 |
| Dwell time | 1 | 2858.46 | 2858.46 | 2.82 | 0.13 |
| Forming temp. | 2 | 3406.55 | 1703.28 | 1.68 | 0.25 |
| Alloy-Austenitization | 2 | 2191.47 | 1095.73 | 1.08 | 0.38 |
| Alloy-Dwell time | 2 | 8723.72 | 4361.86 | 4.30 | 0.05 |
| Alloy-Forming temperature | 4 | 7230.37 | 1807.59 | 1.78 | 0.22 |
| Austenitization-Forming temperature | 2 | 227.48 | 113.74 | 0.11 | 0.89 |
| Dwell time-Forming temp. | 2 | 1095.85 | 547.92 | 0.54 | 0.60 |
| Error | 8 | 8110.44 | 1013.80 | ||
| Total | 26 | 197,981.07 |
| Input Parameter | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Alloy | 2 | 17.36 | 8.68 | 0.78 | 0.49 |
| Austenitization | 2 | 147.48 | 73.74 | 6.59 | 0.02 |
| Forming temperature | 2 | 83.52 | 41.76 | 3.73 | 0.07 |
| Alloy-Austenitization | 4 | 27.39 | 6.85 | 0.61 | 0.67 |
| Alloy-Forming temperature | 4 | 89.86 | 22.46 | 2.01 | 0.19 |
| Austenitization-Forming temperature | 4 | 69.90 | 17.47 | 1.56 | 0.27 |
| Error | 8 | 89.54 | 11.19 | ||
| Total | 26 | 525.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birnbaum, P.; Meza-García, E.; Landgraf, P.; Grund, T.; Lampke, T.; Kräusel, V. Experimental and Numerical Assessment of the Hot Sheet Formability of Martensitic Stainless Steels. J. Manuf. Mater. Process. 2020, 4, 122. https://doi.org/10.3390/jmmp4040122
Birnbaum P, Meza-García E, Landgraf P, Grund T, Lampke T, Kräusel V. Experimental and Numerical Assessment of the Hot Sheet Formability of Martensitic Stainless Steels. Journal of Manufacturing and Materials Processing. 2020; 4(4):122. https://doi.org/10.3390/jmmp4040122
Chicago/Turabian StyleBirnbaum, Peter, Enrique Meza-García, Pierre Landgraf, Thomas Grund, Thomas Lampke, and Verena Kräusel. 2020. "Experimental and Numerical Assessment of the Hot Sheet Formability of Martensitic Stainless Steels" Journal of Manufacturing and Materials Processing 4, no. 4: 122. https://doi.org/10.3390/jmmp4040122
APA StyleBirnbaum, P., Meza-García, E., Landgraf, P., Grund, T., Lampke, T., & Kräusel, V. (2020). Experimental and Numerical Assessment of the Hot Sheet Formability of Martensitic Stainless Steels. Journal of Manufacturing and Materials Processing, 4(4), 122. https://doi.org/10.3390/jmmp4040122







