On Coating Techniques for Surface Protection: A Review
Abstract
1. Introduction
2. Reliable Coating Methods
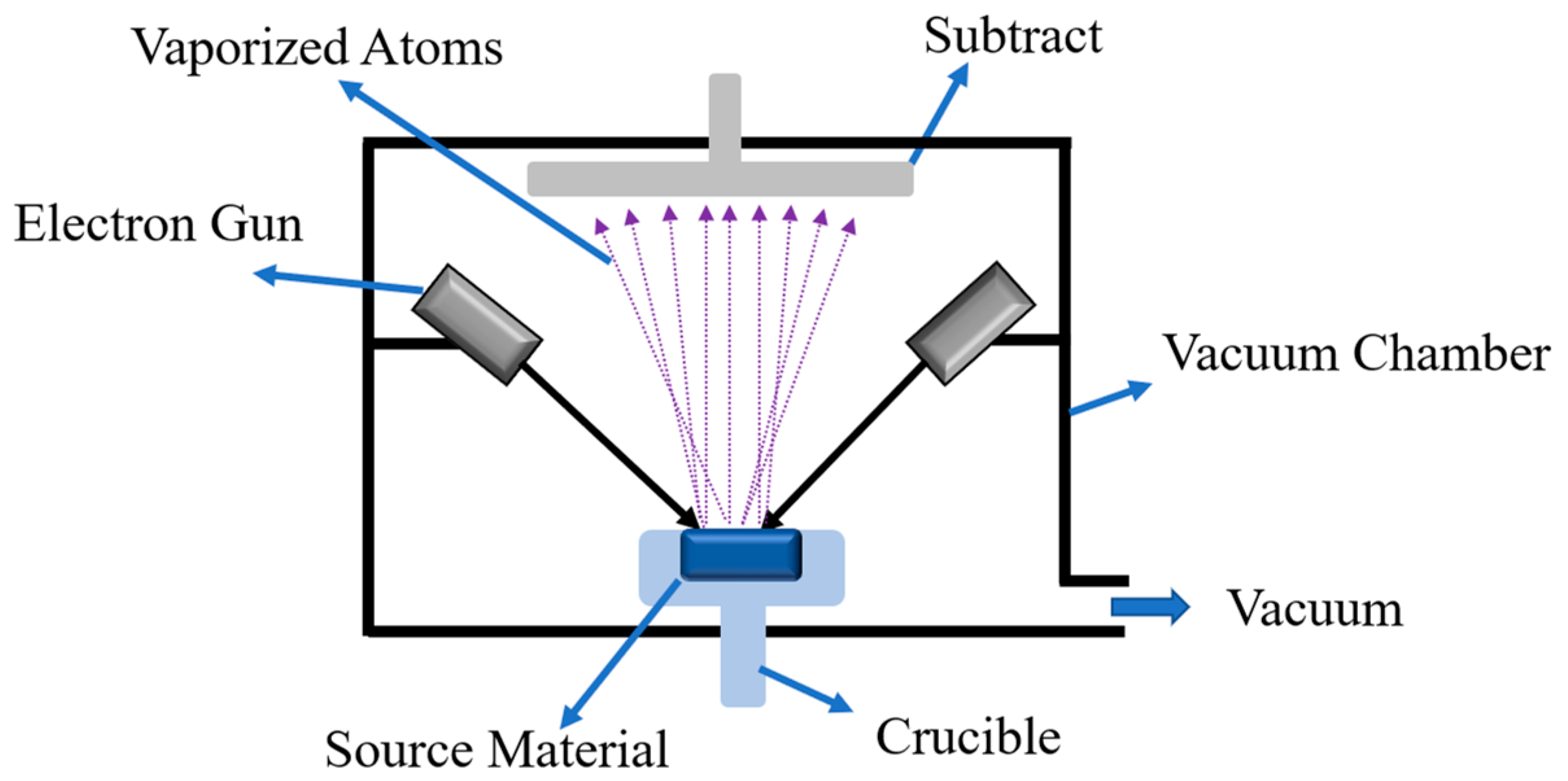
2.1. Physical Vapor Deposition (PVD) Coating
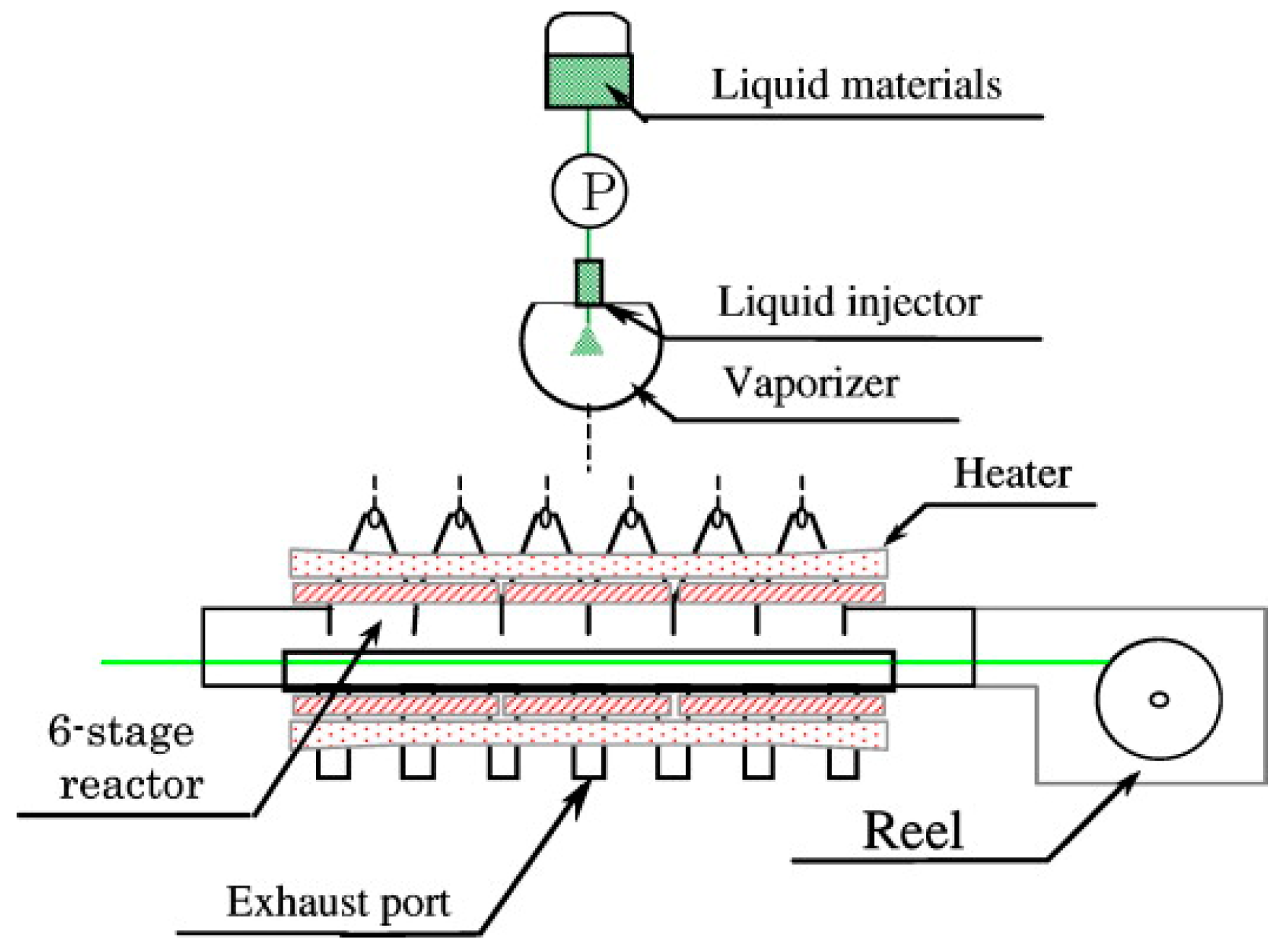
2.2. Chemical Vapor Deposition (CVD) Coating
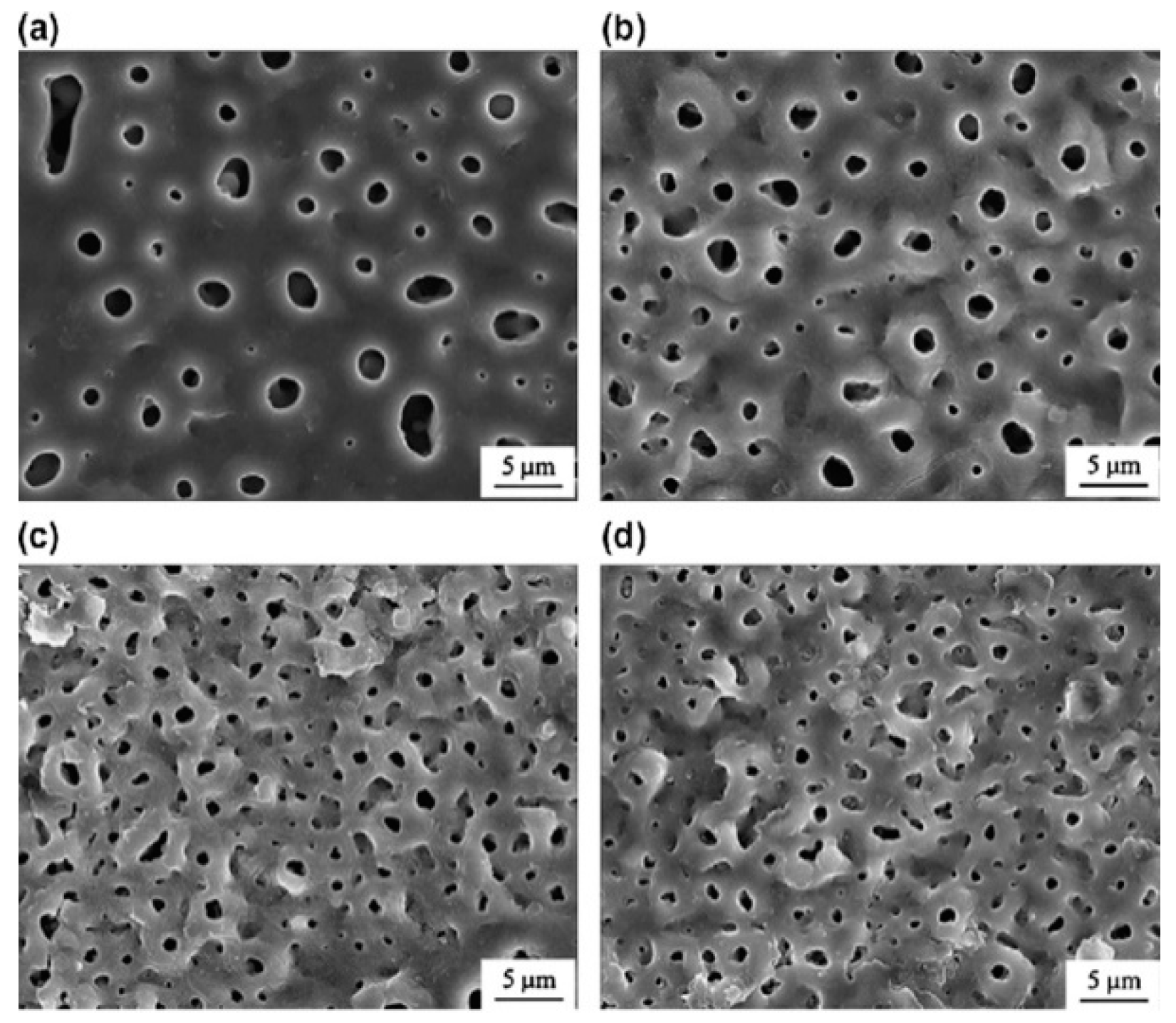
2.3. Micro-Arc Oxidation (MAO) Coating
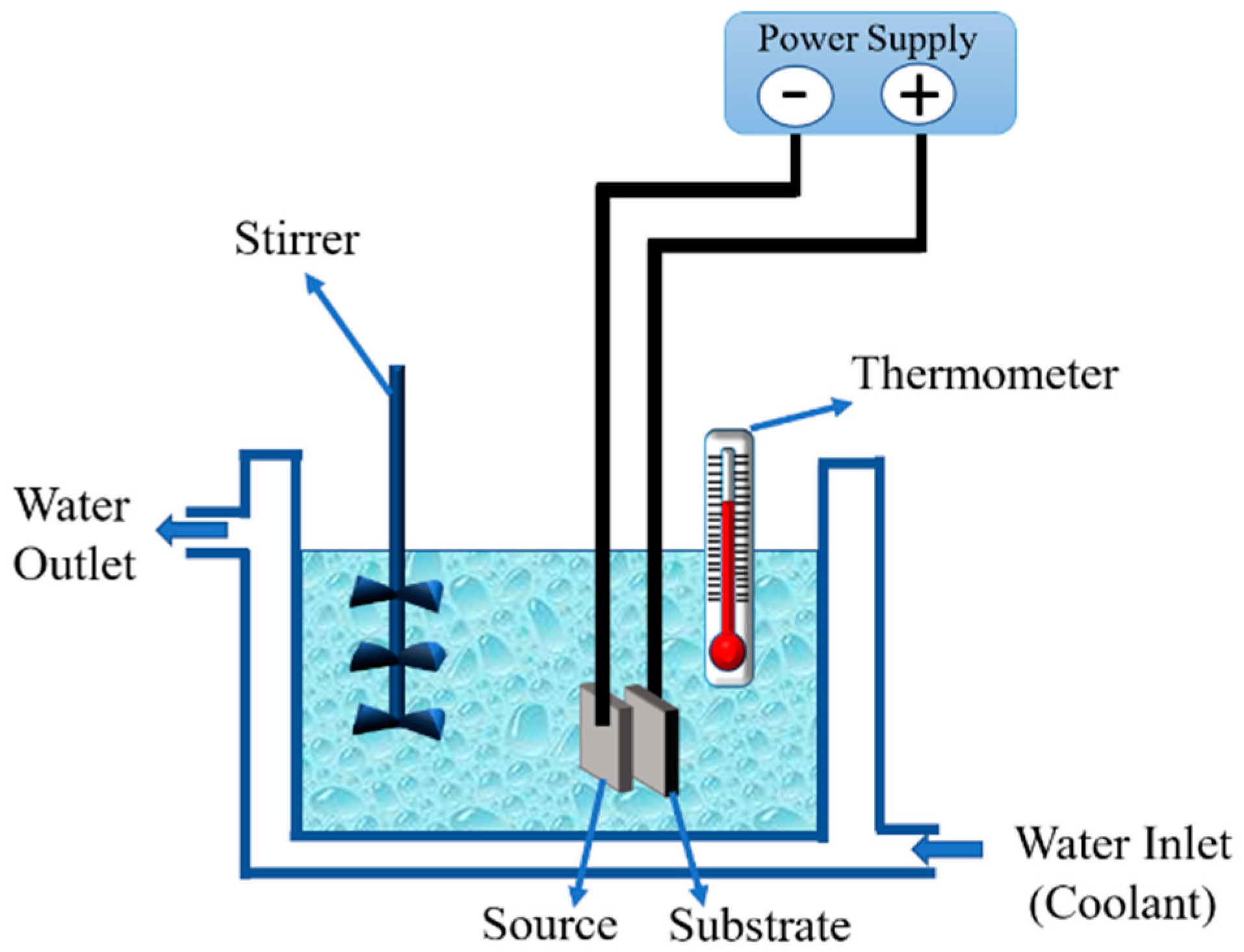
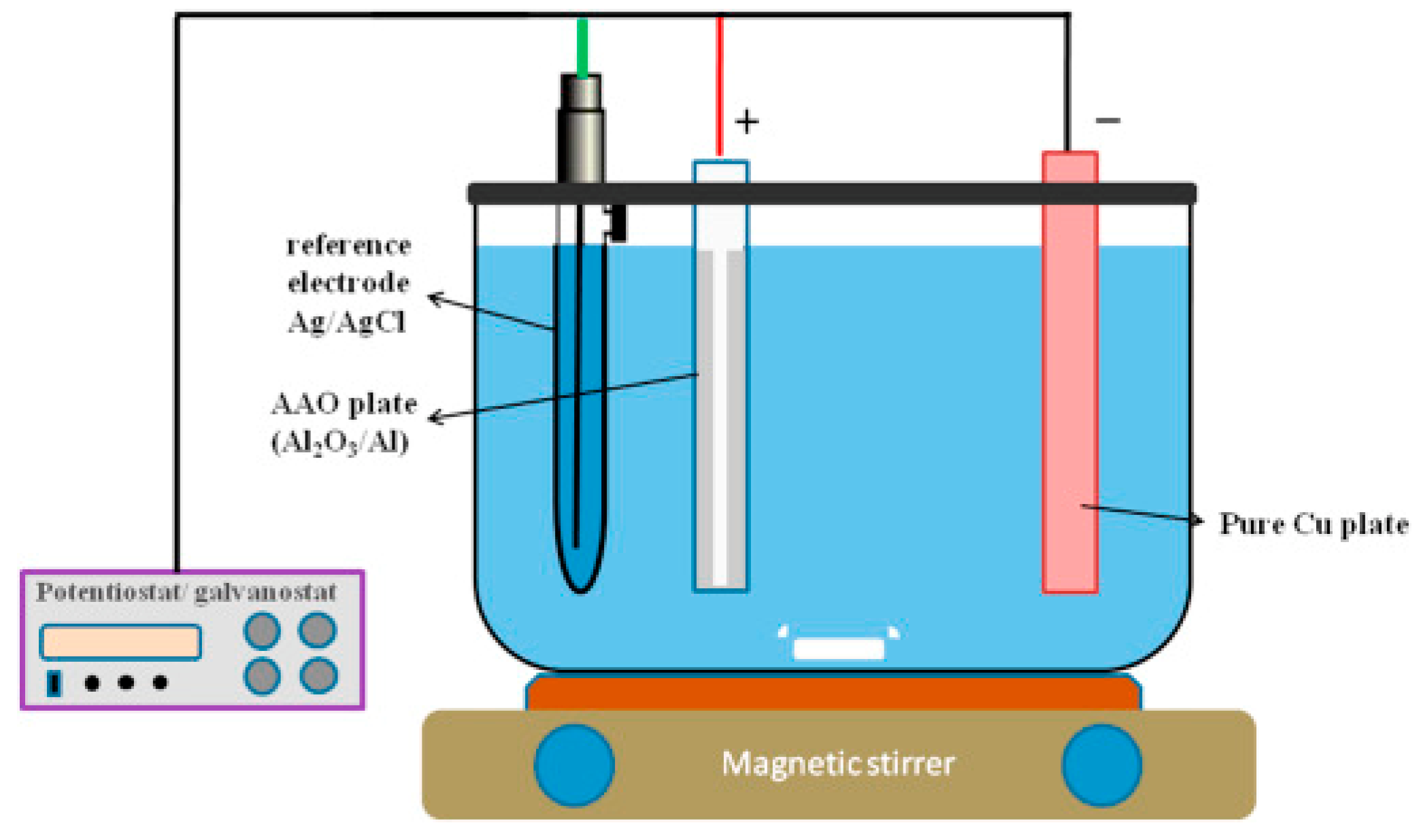
2.4. Electrodeposition Coating
2.4.1. Electrolytic Deposition (ELD) Coating
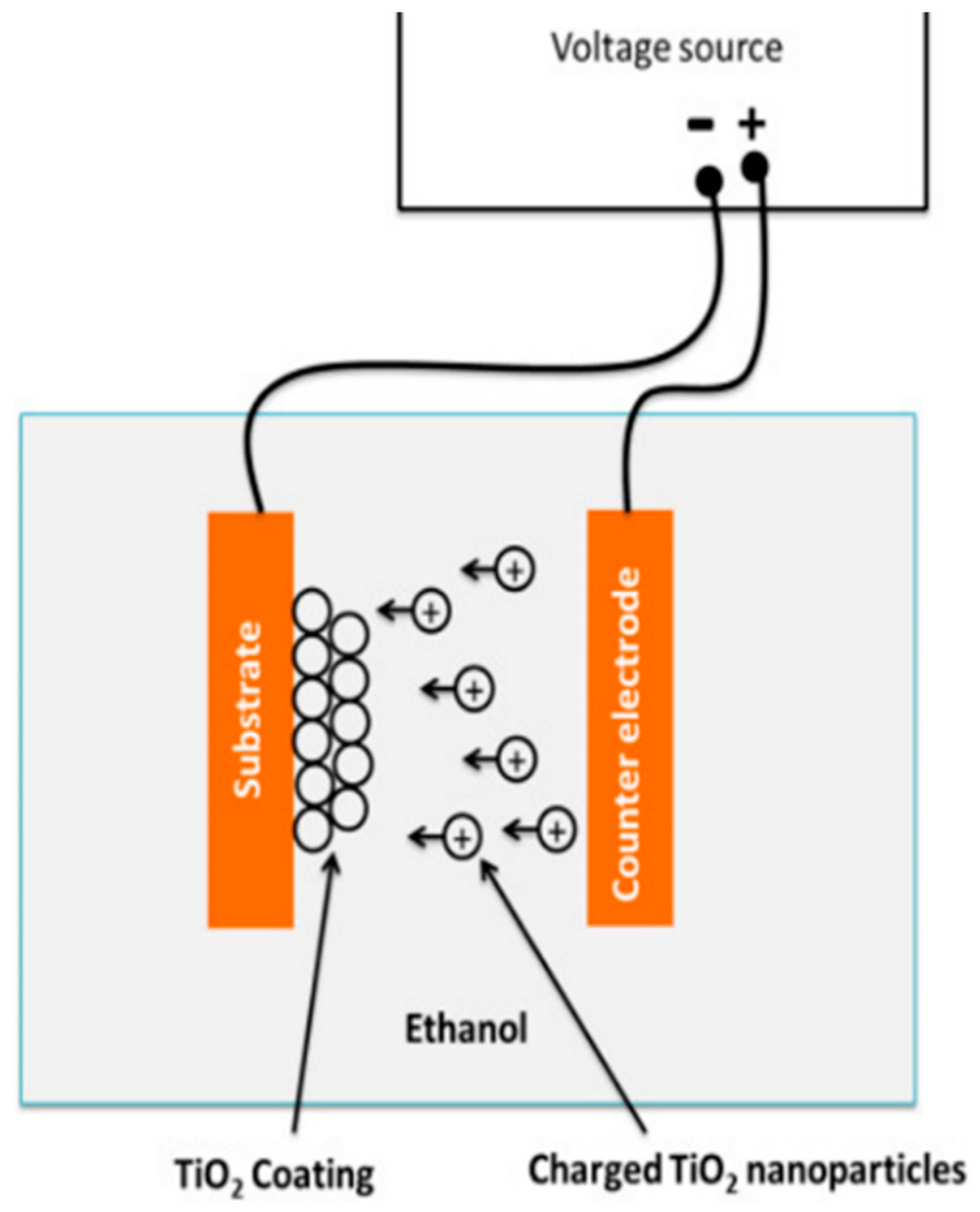
2.4.2. Electrophoretic Deposition (EPD) Coating
- External electric field forces suspended particles in electrolyte toward one electrode called electrophoresis.
- The moving particles gather in one electrode and form a larger coagulated particle.
- The larger particles deposit on the surface of the electrode, which is a to-be-coated substrate.
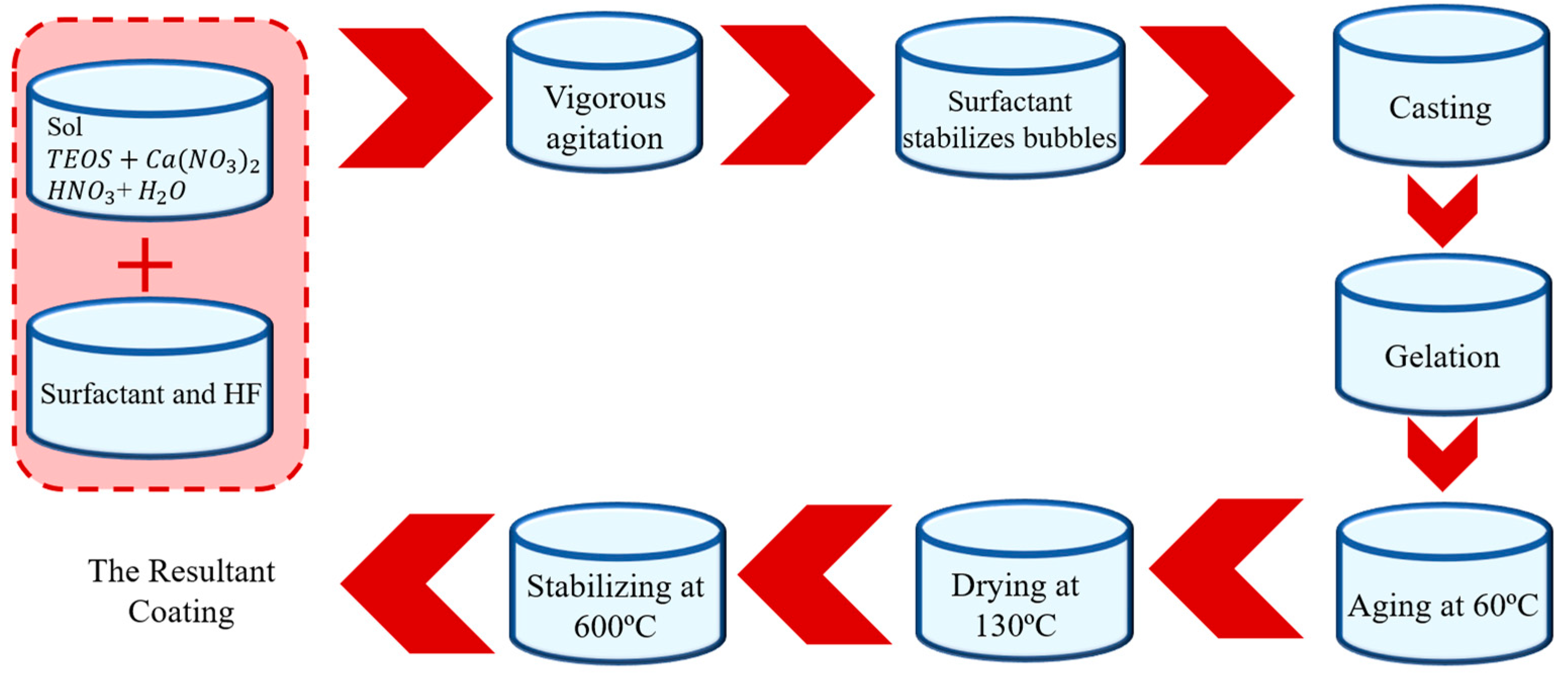
2.5. Sol–gel Coating
2.6. Thermal Spray Coating
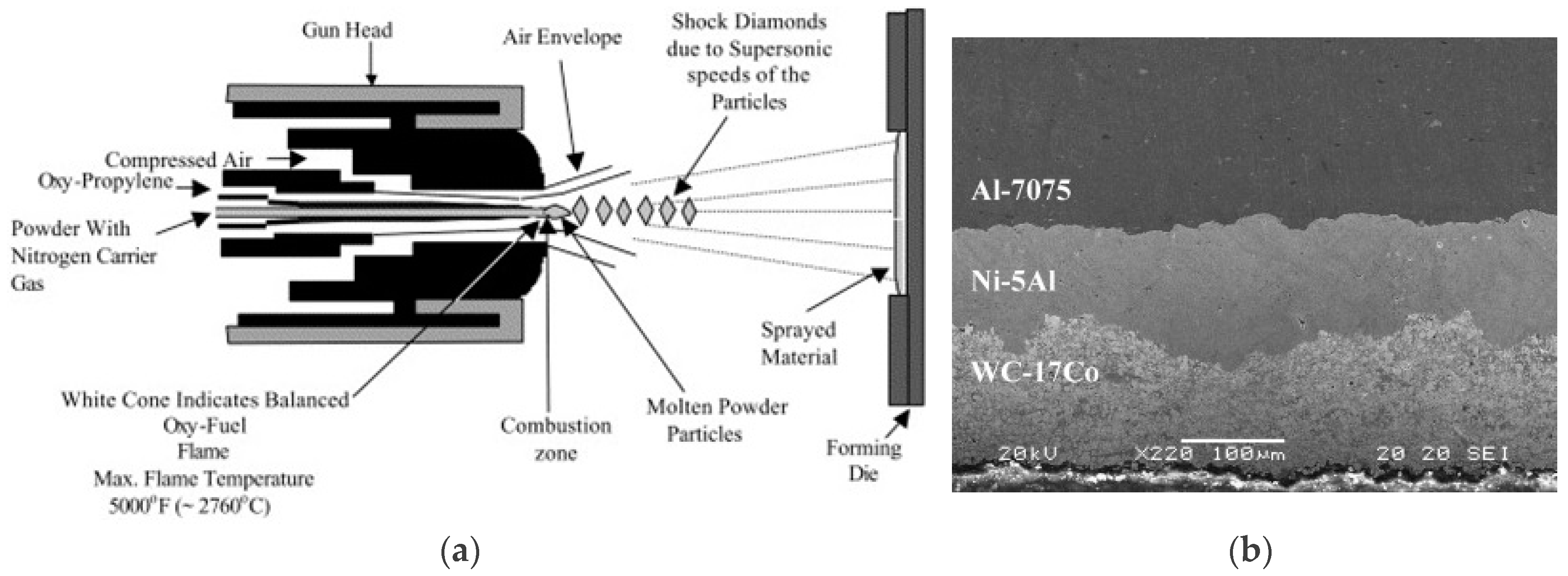
2.6.1. High-Velocity Oxy-Fuel Coating (HVOF)
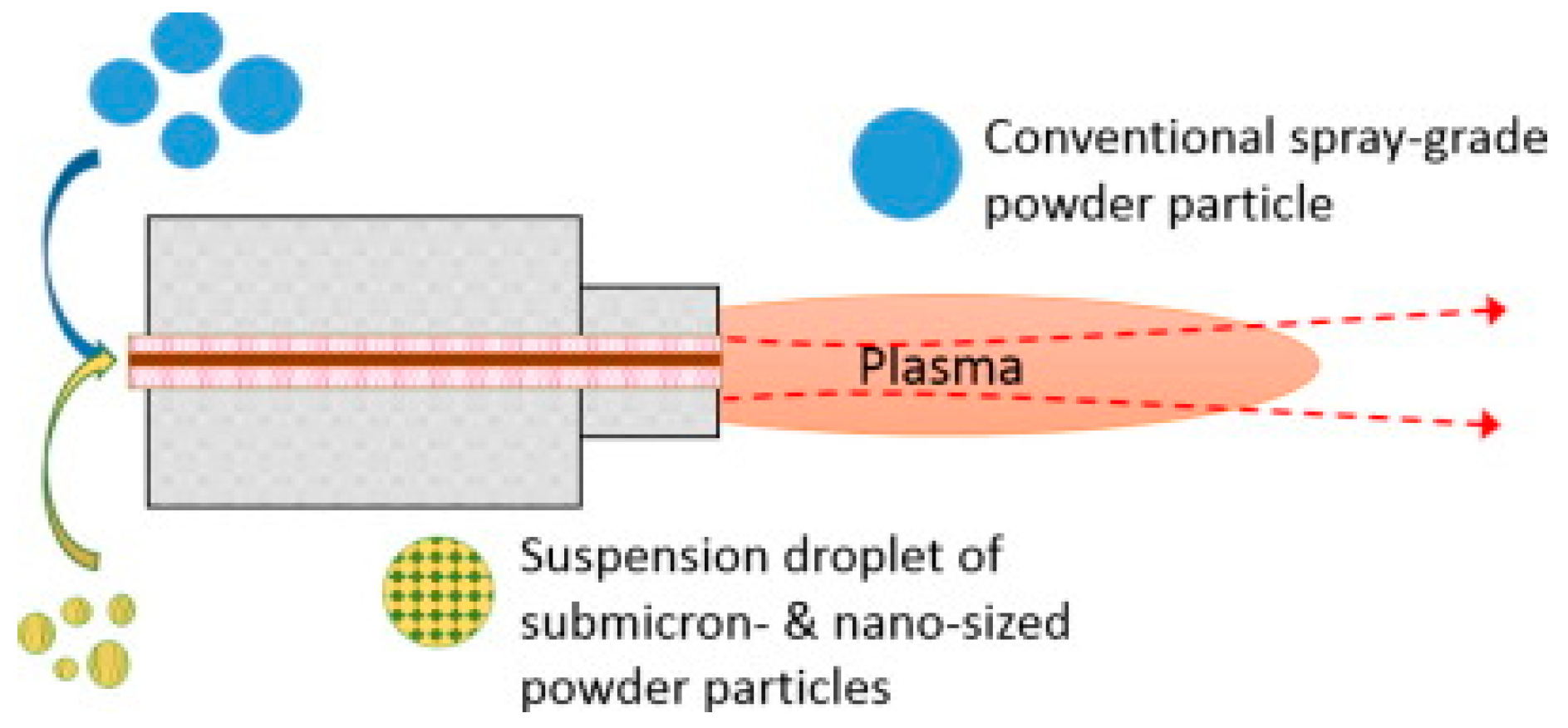
2.6.2. Plasma Spray Coating
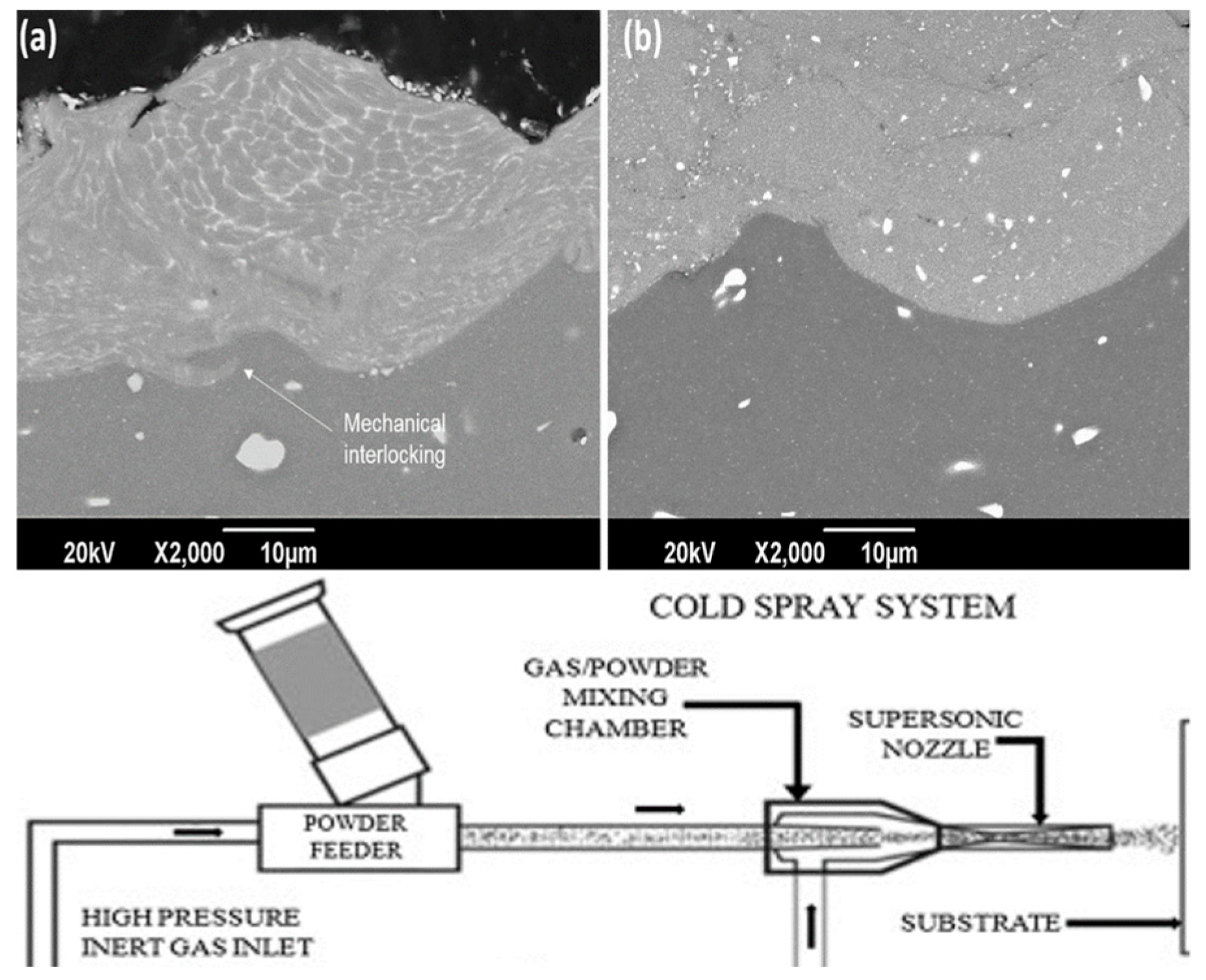
2.6.3. Cold Spray Coating
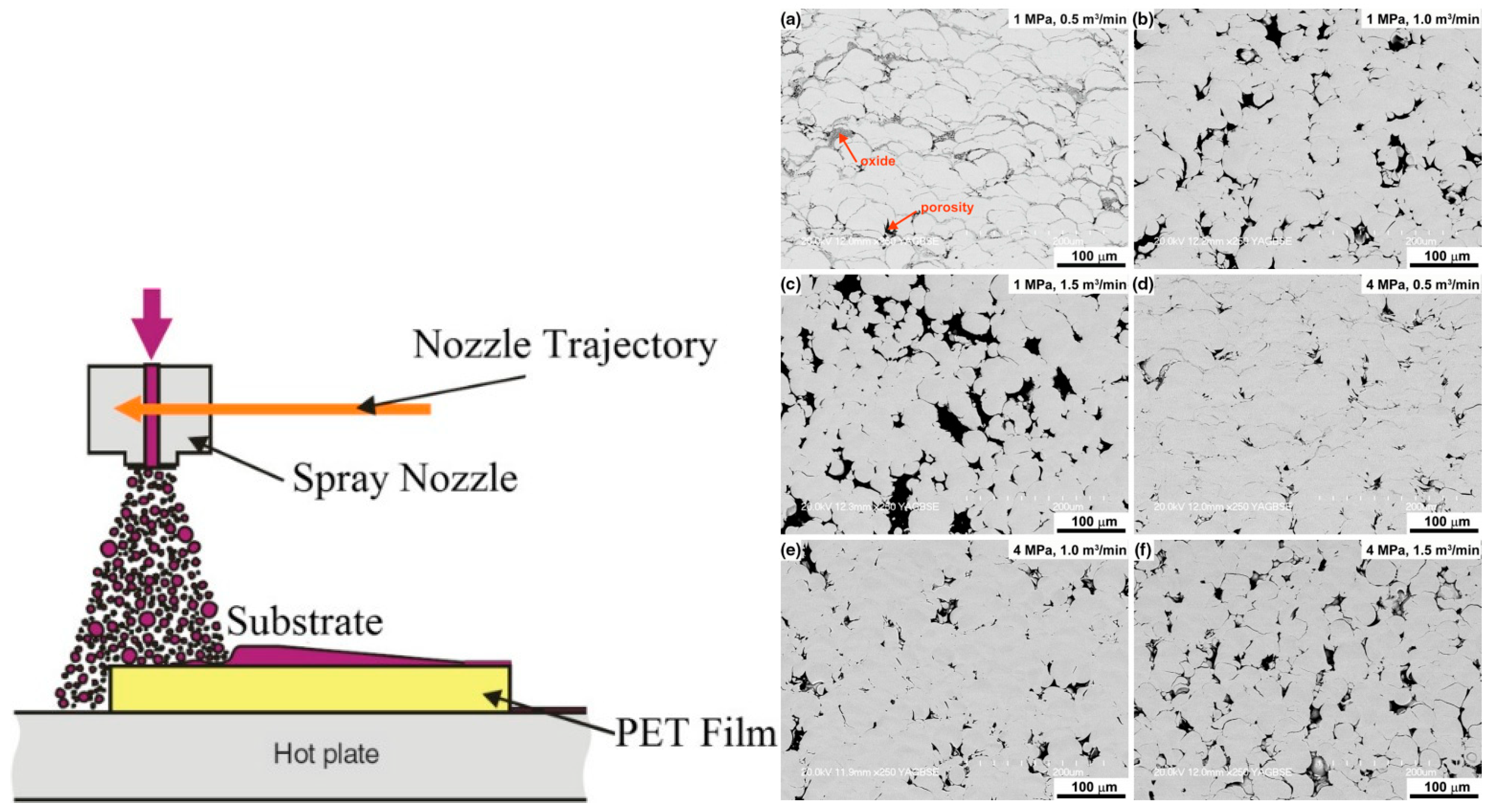
2.6.4. Warm Spray Coating
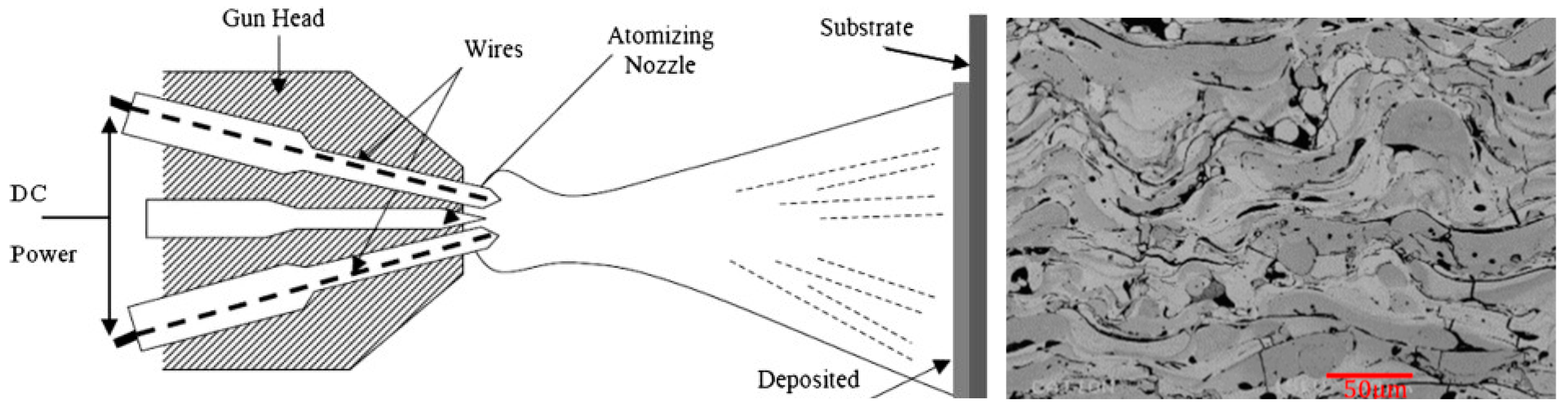
2.6.5. Arc Wire Spray Coating
3. Summary
Funding
Conflicts of Interest
References
- Frommeyer, G.; Wassermann, G. Anomalous properties of in-situ-produced silver-copper composite wires I. Electrical conductivity. Phys. Status Solidi A 1975, 27, 99–105. [Google Scholar] [CrossRef]
- Ibrahim, H.; Jahadakbar, A.; Dehghan, A.; Moghaddam, N.S.; Amerinatanzi, A.; Elahinia, M. In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications. Metals 2018, 8, 164. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Filip, P. Impact of humidity on wear of automotive friction materials. Wear 2017, 376, 717–726. [Google Scholar] [CrossRef]
- Bhushan, B.; Gupta, B.K. Handbook of Tribology: Materials, Coatings, and Surface Treatments; Krieger Pub Co.: Malabar, FL, USA, 1991. [Google Scholar]
- Dehghanghadikolaei, A.; Mohammadian, B.; Namdari, N.; Fotovvati, B. Abrasive Machining Techniques for Biomedical Device Applications. J. Mater. Sci. 2018, 5, 1–11. [Google Scholar]
- Klaassen, C.D.; Watkins, J.B. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 1996; Volume 5. [Google Scholar]
- Thakare, M.; Wharton, J.; Wood, R.; Menger, C. Exposure effects of alkaline drilling fluid on the microscale abrasion–corrosion of WC-based hardmetals. Wear 2007, 263, 125–136. [Google Scholar] [CrossRef]
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective coatings in the gas turbine engine. Surf. Coat. Technol. 1994, 68, 1–9. [Google Scholar] [CrossRef]
- Prengel, H.; Pfouts, W.; Santhanam, A. State of the art in hard coatings for carbide cutting tools. Surf. Coat. Technol. 1998, 102, 183–190. [Google Scholar] [CrossRef]
- De Damborenea, J.; Navas, C.; García, J.; Arenas, M.; Conde, A. Corrosion–erosion of TiN-PVD coatings in collagen and cellulose meat casing. Surf. Coat. Technol. 2007, 201, 5751–5757. [Google Scholar] [CrossRef]
- Mathew, M.; Ariza, E.; Rocha, L.; Fernandes, A.C.; Vaz, F. TiCxOy thin films for decorative applications: Tribocorrosion mechanisms and synergism. Tribol. Int. 2008, 41, 603–615. [Google Scholar] [CrossRef]
- Dahm, K.; Dearnley, P. Abrasion response and abrasion–corrosion interactions for a coated biomedical stainless steel. Wear 2005, 259, 933–942. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Aldrich-Smith, G. Corrosion–wear mechanisms of hard coated austenitic 316L stainless steels. Wear 2004, 256, 491–499. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. Fatigue performance of selective laser melted Ti6Al4V components: State of the art. Mater. Res. Express 2018, 6, 012002. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Namdari, N.; Mohammadian, B.; Fotovvati, B.J.J.O.S.; Research, E. Additive Manufacturing Methods: A Brief Overview. J. Sci. Eng. Res. 2018, 5, 123–131. [Google Scholar]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Luff, P.; White, M. The structure and properties of evaporated polyethylene thin films. Thin Solid Film. 1970, 6, 175–195. [Google Scholar] [CrossRef]
- Takeno, A.; Okui, N.; Kitoh, T.; Muraoka, M.; Umemoto, S.; Sakai, T. Preparation and piezoelectricity of β form poly (vinylidene fluoride) thin film by vapour deposition. Thin Solid Film. 1991, 202, 205–211. [Google Scholar] [CrossRef]
- Annavarapu, R.K.; Kim, S.; Wang, M.; Hart, A.J.; Sojoudi, H. Explaining Evaporation-Triggered Wetting Transition Using Local Force Balance Model and Contact Line-Fraction. Sci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sojoudi, H.; Kim, S.; Zhao, H.; Annavarapu, R.K.; Mariappan, D.; Hart, A.J.; McKinley, G.H.; Gleason, K.K. Stable Wettability Control of Nanoporous Microstructures by iCVD Coating of Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 43287–43299. [Google Scholar] [CrossRef]
- Nemani, S.K.; Sojoudi, H. Barrier Performance of CVD Graphene Films Using a Facile P3HT Thin Film Optical Transmission Test. J. Nanomater. 2018, 2018, 9681432. [Google Scholar] [CrossRef]
- Sojoudi, H.; Nemani, S.K.; Mullin, K.; Wilson, M.G.; Al-Adwani, H.; Lababidi, H.M.; Gleason, K.K. A Micro/Nanoscale Approach for Studying Scale Formation and Developing of Scale-Resistant Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 7330–7337. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Watanabe, T.; Kashima, N.; Nagaya, S.; Muroga, T.; Miyata, S.; Yamada, Y.; Izumi, T.; Shiohara, Y. Development of long YBCO coated conductors by multiple-stage CVD. Phys. C Supercond. Appl. 2006, 445, 515–520. [Google Scholar] [CrossRef]
- Maruyama, T.; Arai, S. Electrochromic properties of niobium oxide thin films prepared by radio-frequency magnetron sputtering method. Appl. Phys. Lett. 1993, 63, 869–870. [Google Scholar] [CrossRef]
- Foster, R.F.; Rebenne, H.E.; LeBlanc, R.E.; White, C.L.; Arora, R. Rotating Susceptor Semiconductor Wafer Processing Cluster Tool Module Useful for Tungsten CVD. U.S. Patent 5,370,739, 6 December 1994. [Google Scholar]
- Gleason, K.K. Overview of Chemically Vapor Deposited (CVD) Polymers. Cvd Polym. Fabr. Org. Surf. Devices 2015, 1–11. [Google Scholar]
- Meyerson, B.S. UHV/CVD growth of Si and Si: Ge alloys: Chemistry, physics, and device applications. Proc. IEEE 1992, 80, 1592–1608. [Google Scholar] [CrossRef]
- Vernardou, D.; Pemble, M.; Sheel, D. Vanadium oxides prepared by liquid injection MOCVD using vanadyl acetylacetonate. Surf. Coat. Technol. 2004, 188, 250–254. [Google Scholar] [CrossRef]
- Li, Y.; Mann, D.; Rolandi, M.; Kim, W.; Ural, A.; Hung, S.; Javey, A.; Cao, J.; Wang, D.; Yenilmez, E. Preferential growth of semiconducting single-walled carbon nanotubes by a plasma enhanced CVD method. Nano Lett. 2004, 4, 317–321. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. Laser-Assisted Coating Techniques and Surface Modifications: A Short Review. Part. Sci. Technol. 2019. submitted for publication. [Google Scholar]
- Fotovvati, B.; Wayne, S.F.; Lewis, G.; Asadi, E. A Review on Melt-Pool Characteristics in Laser Welding of Metals. Adv. Mater. Sci. Eng. 2018, 2018, 4920718. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Matthews, A. Deposition of layered bioceramic hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surf. Coat. Technol. 2000, 125, 407–414. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Li, L.-H.; Narayanan, T.S.; Kim, Y.K.; Kong, Y.-M.; Park, I.S.; Bae, T.S.; Lee, M.H. Deposition of microarc oxidation–polycaprolactone duplex coating to improve the corrosion resistance of magnesium for biodegradable implants. Thin Solid Film. 2014, 562, 561–567. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, D.; Chen, C. Effect of negative voltage on the microstructure, degradability and in vitro bioactivity of microarc oxidized coatings on ZK60 magnesium alloy. Mater. Lett. 2014, 119, 127–130. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Wang, F.; Yu, D.; Zhao, L. The corrosion resistance behavior of Al 2 O 3 coating prepared on NiTi alloy by micro-arc oxidation. J. Alloy. Compd. 2009, 472, 276–280. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Porous TiO2 surface formed on nickel-titanium alloy by plasma electrolytic oxidation: A prospective polymer-free reservoir for drug eluting stent applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 700–708. [Google Scholar] [CrossRef]
- Asri, R.; Harun, W.; Hassan, M.; Ghani, S.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Hwang, I.; Hwang, D.; Ko, Y.; Shin, D. Correlation between current frequency and electrochemical properties of Mg alloy coated by micro arc oxidation. Surf. Coat. Technol. 2012, 206, 3360–3365. [Google Scholar] [CrossRef]
- Radwan, A.B.; Ali, K.; Shakoor, R.; Mohammed, H.; Alsalama, T.; Kahraman, R.; Yusuf, M.M.; Abdullah, A.M.; Montemor, M.F.; Helal, M. Properties Enhancement of Ni-P Electrodeposited Coatings by the Incorporation of Nanoscale Y2O3 Particles. Appl. Surf. Sci. 2018, 457, 956–967. [Google Scholar] [CrossRef]
- Fashu, S.; Mudzingwa, L.; Khan, R.; Tozvireva, M. Electrodeposition of high corrosion resistant Ni–Sn–P alloy coatings from an ionic liquid based on choline chloride. Trans. IMF 2018, 96, 20–26. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Liao, Z.-W.; Fang, C.-X.; Li, H.-X.; Bin, F.; Song, X.; Cao, G.-F.; Kuang, Y.-F. Pulse electroplating of Ni-WP coating and its anti-corrosion performance. Trans. Nonferrous Met. Soc. China 2018, 28, 88–95. [Google Scholar] [CrossRef]
- Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Hydrocarbon versus fluorocarbon in the electrodeposition of superhydrophobic polymer films. Langmuir 2010, 26, 17596–17602. [Google Scholar] [CrossRef] [PubMed]
- Bindra, P.; Gerischer, H.; Kolb, D. Electrolytic deposition of thin metal films on semiconductor substrates. J. Electrochem. Soc. 1977, 124, 1012–1018. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7. [Google Scholar] [CrossRef]
- Minh, N.Q. Solid oxide fuel cell technology—features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Tian, B.; Cheng, Y. Electrolytic deposition of Ni–Co–Al2O3 composite coating on pipe steel for corrosion/erosion resistance in oil sand slurry. Electrochim. Acta 2007, 53, 511–517. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y. Electrolytic deposition of Ni–Co–SiC nano-coating for erosion-enhanced corrosion of carbon steel pipes in oilsand slurry. Surf. Coat. Technol. 2011, 205, 3198–3204. [Google Scholar] [CrossRef]
- Fayomi, O.; Abdulwahab, M. Properties evaluation of ternary surfactant-induced Zn-Ni-Al2O3 films on mild steel by electrolytic chemical deposition. J. Ovonic Res. 2013, 9, 123–132. [Google Scholar]
- Redondo, M.; Breslin, C.B. Polypyrrole electrodeposited on copper from an aqueous phosphate solution: Corrosion protection properties. Corros. Sci. 2007, 49, 1765–1776. [Google Scholar] [CrossRef]
- Reddy, E.L.; Karuppiah, J.; Lee, H.C.; Kim, D.H. Steam reforming of methanol over copper loaded anodized aluminum oxide (AAO) prepared through electrodeposition. J. Power Sources 2014, 268, 88–95. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Yu, J.; Yang, H.; Song, F.; Huang, C. Application of electrophoretic deposition to occlude dentinal tubules in vitro. J. Dent. 2018, 71, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.; Minay, E.J.; Shaffer, M.S. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Dickerson, J.H.; Boccaccini, A.R. Electrophoretic Deposition of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Castro, Y.; Ferrari, B.; Moreno, R.; Durán, A. Corrosion behaviour of silica hybrid coatings produced from basic catalysed particulate sols by dipping and EPD. Surf. Coat. Technol. 2005, 191, 228–235. [Google Scholar] [CrossRef]
- Gebhardt, F.; Seuss, S.; Turhan, M.; Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Characterization of electrophoretic chitosan coatings on stainless steel. Mater. Lett. 2012, 66, 302–304. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Liang, L.; Hong, H.; Zhang, Z.; Shen, B. Electrochemical behaviour of EPD synthesized graphene coating on titanium alloys for orthopedic implant application. Procedia Cirp 2018, 71, 322–328. [Google Scholar] [CrossRef]
- Fei, J.; Luo, D.; Zhang, C.; Li, H.; Cui, Y.; Huang, J. Friction and wear behavior of SiC particles deposited onto paper-based friction material via electrophoretic deposition. Tribol. Int. 2018, 119, 230–238. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Jaber, N.B.; Lazar, F.S.; Faure, J.; Larbi, A.B.C.; Benhayoune, H. Effect of annealing temperature on the structural and mechanical properties of coatings prepared by electrophoretic deposition of TiO2 nanoparticles. Thin Solid Film. 2017, 638, 201–212. [Google Scholar] [CrossRef]
- Mah, J.C.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008. [Google Scholar] [CrossRef]
- Tracton, A.A. Coatings Materials and Surface Coatings; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Dehghan Ghadikolaei, A.; Vahdati, M. Experimental study on the effect of finishing parameters on surface roughness in magneto-rheological abrasive flow finishing process. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2015, 229, 1517–1524. [Google Scholar] [CrossRef]
- Pope, E.J.; Mackenzie, J. Sol-gel processing of silica: II. The role of the catalyst. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Salvado, I.M.; Ferreira, M. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Faustini, M.; Louis, B.; Albouy, P.A.; Kuemmel, M.; Grosso, D. Preparation of sol− gel films by dip-coating in extreme conditions. J. Phys. Chem. C 2010, 114, 7637–7645. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Dehghanghadikolaei, A.; Namdari, N.; Mohammadian, B.; Ghoreishi, S.R. Deriving one dimensional shallow water equations from mass and momentum balance laws. Int. Res. J. Eng. Technol. 2018, 5, 408–419. [Google Scholar]
- Namdari, N.; Abdi, M.; Chaghomi, H.; Rahmani, F. Numerical Solution for Transient Heat Transfer in Longitudinal Fins. Int. Res. J. Adv. Eng. Sci. 2018, 3, 131–136. [Google Scholar]
- Namdari, N.; Mohammadian, B.; Dehghanghadikolaei, A.; Alidad, S.; Abbasi, M. A Numerical Study on Two-Dimensional Fins with Non-Constant Heat Flux. Int. J. Sci. Eng. Sci. 2018, 2, 12–16. [Google Scholar]
- Namdari, N.; Dehghan, A. Natural Frequencies and Mode Shapes for Vibrations of Rectangular and Circular Membranes: A Numerical Study. Int. Res. J. Adv. Eng. Sci. 2018, 3, 30–34. [Google Scholar]
- Wilkes, G.; Brennan, A.; Huang, H.-H.; Rodrigues, D.; Wang, B. The Synthesis, Structure and Property Behavior of Inorganic-Organic Hybrid Network Materials Prepared by The Sol Gel Process. Mrs Online Proc. Libr. Arch. 1989, 171. [Google Scholar] [CrossRef]
- Noell, J.L.W.; Wilkes, G.L.; Mohanty, D.K.; McGrath, J.E. The preparation and characterization of new polyether ketone-tetraethylorthosilicate hybrid glasses by the sol-gel method. J. Appl. Polym. Sci. 1990, 40, 1177–1194. [Google Scholar] [CrossRef]
- Namdari, N.; Rizvi, R. Damage induced surface texturing of short fiber-PDMS composite materials. In Proceedings of the ANTEC, Orlando, FL, USA, 7–10 May 2018. [Google Scholar]
- Namdari, N.; Mosaddegh, P. Experimental and simulation studies on the mold replicability in the thermoforming process. J. Polym. Eng. 2019. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Davis, J.R. Handbook of Thermal Spray Technology; ASM international: Almere, The Netherlands, 2004. [Google Scholar]
- Kramer, M.S.; Byrnes, L.E.; Holmes, G.L. Method and Apparatus for Application of Thermal Spray Coatings to Engine Blocks. U.S. Patent 5,271,967, 21 December 1993. [Google Scholar]
- Thorpe, M.; Richter, H. A pragmatic analysis and comparison of HVOF processes. J. Therm. Spray Technol. 1992, 1, 161–170. [Google Scholar] [CrossRef]
- Fernández, J.; Gaona, M.; Guilemany, J. Effect of heat treatments on HVOF hydroxyapatite coatings. J. Therm. Spray Technol. 2007, 16, 220–228. [Google Scholar] [CrossRef]
- Scrivani, A.; Bardi, U.; Carrafiello, L.; Lavacchi, A.; Niccolai, F.; Rizzi, G. A comparative study of high velocity oxygen fuel, vacuum plasma spray, and axial plasma spray for the deposition of CoNiCrAlY bond coat alloy. J. Therm. Spray Technol. 2003, 12, 504–507. [Google Scholar] [CrossRef]
- Bolelli, G.; Lusvarghi, L.; Varis, T.; Turunen, E.; Leoni, M.; Scardi, P.; Azanza-Ricardo, C.L.; Barletta, M. Residual stresses in HVOF-sprayed ceramic coatings. Surf. Coat. Technol. 2008, 202, 4810–4819. [Google Scholar] [CrossRef]
- Scrivani, A.; Ianelli, S.; Rossi, A.; Groppetti, R.; Casadei, F.; Rizzi, G. A contribution to the surface analysis and characterisation of HVOF coatings for petrochemical application. Wear 2001, 250, 107–113. [Google Scholar] [CrossRef]
- Lekatou, A.; Regoutas, E.; Karantzalis, A. Corrosion behaviour of cermet-based coatings with a bond coat in 0.5 M H2SO4. Corros. Sci. 2008, 50, 3389–3400. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.; Zhang, H.; Liu, Y.; Xu, S. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Stokes, J.; Looney, L. HVOF system definition to maximise the thickness of formed components. Surf. Coat. Technol. 2001, 148, 18–24. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behaviour of thermally sprayed cermet coatings. Surf. Coat. Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Karthikeyan, J.; Berndt, C.; Tikkanen, J.; Reddy, S.; Herman, H. Plasma spray synthesis of nanomaterial powders and deposits. Mater. Sci. Eng. A 1997, 238, 275–286. [Google Scholar] [CrossRef]
- Bulloch, J.; Callagy, A. An in situ wear-corrosion study on a series of protective coatings in large induced draft fans. Wear 1999, 233, 284–292. [Google Scholar] [CrossRef]
- Knuuttila, J.; Ahmaniemi, S.; Mäntylä, T. Wet abrasion and slurry erosion resistance of thermally sprayed oxide coatings. Wear 1999, 232, 207–212. [Google Scholar] [CrossRef]
- Petrovicova, E.; Schadler, L. Thermal spraying of polymers. Int. Mater. Rev. 2002, 47, 169–190. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Betancourt-Dougherty, L.; Smith, R. Effects of load and sliding speed on the wear behaviour of plasma sprayed TiC NiCrBSi coatings. Wear 1998, 217, 147–154. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Joukar, A.; Mehta, J.; Marks, D.; Goel, V.K. Lumbar-Sacral Destruction Fixation Biomechanics: A Finite Element Study. Spine J. 2017, 17, S335. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Champagne, V.K. The Cold Spray Materials Deposition Process: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Li, C.-J.; Wang, H.-T.; Zhang, Q.; Yang, G.-J.; Li, W.-Y.; Liao, H. Influence of spray materials and their surface oxidation on the critical velocity in cold spraying. J. Therm. Spray Technol. 2010, 19, 95–101. [Google Scholar] [CrossRef]
- Li, W.-Y.; Liao, H.; Li, C.-J.; Bang, H.-S.; Coddet, C. Numerical simulation of deformation behavior of Al particles impacting on Al substrate and effect of surface oxide films on interfacial bonding in cold spraying. Appl. Surf. Sci. 2007, 253, 5084–5091. [Google Scholar] [CrossRef]
- Tsui, Y.; Doyle, C.; Clyne, T. Plasma sprayed hydroxyapatite coatings on titanium substrates Part 1: Mechanical properties and residual stress levels. Biomaterials 1998, 19, 2015–2029. [Google Scholar] [CrossRef]
- Schmidt, T.; Assadi, H.; Gärtner, F.; Richter, H.; Stoltenhoff, T.; Kreye, H.; Klassen, T. From particle acceleration to impact and bonding in cold spraying. J. Therm. Spray Technol. 2009, 18, 794. [Google Scholar] [CrossRef]
- Sabard, A.; de Villiers Lovelock, H.; Hussain, T. Microstructural Evolution in Solution Heat Treatment of Gas-Atomized Al Alloy (7075) Powder for Cold Spray. J. Therm. Spray Technol. 2018, 27, 145–158. [Google Scholar] [CrossRef]
- Dean, S.W.; Potter, J.K.; Yetter, R.A.; Eden, T.J.; Champagne, V.; Trexler, M. Energetic intermetallic materials formed by cold spray. Intermetallics 2013, 43, 121–130. [Google Scholar] [CrossRef]
- Kawakita, J.; Katanoda, H.; Watanabe, M.; Yokoyama, K.; Kuroda, S. Warm Spraying: An improved spray process to deposit novel coatings. Surf. Coat. Technol. 2008, 202, 4369–4373. [Google Scholar] [CrossRef]
- Kawakita, J.; Maruyama, N.; Kuroda, S.; Hiromoto, S.; Yamamoto, A. Fabrication and mechanical properties of composite structure by warm spraying of Zr-base metallic glass. Mater. Trans. 2008, 49, 317–323. [Google Scholar] [CrossRef]
- Tsui, Y.; Doyle, C.; Clyne, T. Plasma sprayed hydroxyapatite coatings on titanium substrates Part 2: Optimisation of coating properties. Biomaterials 1998, 19, 2031–2043. [Google Scholar] [CrossRef]
- Kim, K.; Watanabe, M.; Kawakita, J.; Kuroda, S. Grain refinement in a single titanium powder particle impacted at high velocity. Scr. Mater. 2008, 59, 768–771. [Google Scholar] [CrossRef]
- Chivavibul, P.; Watanabe, M.; Kuroda, S.; Kawakita, J.; Komatsu, M.; Sato, K.; Kitamura, J. Development of WC-Co coatings deposited by warm spray process. J. Therm. Spray Technol. 2008, 17, 750–756. [Google Scholar] [CrossRef]
- Skarvelis, P.; Papadimitriou, G. Plasma transferred arc composite coatings with self lubricating properties, based on Fe and Ti sulfides: Microstructure and tribological behavior. Surf. Coat. Technol. 2009, 203, 1384–1394. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, T.; Nezu, A. Electrode phenomena investigation of wire arc spraying for preparation of Ti-Al intermetallic compounds. Thin Solid Film. 2002, 407, 98–103. [Google Scholar] [CrossRef]
- Gedzevicius, I.; Valiulis, A. Analysis of wire arc spraying process variables on coatings properties. J. Mater. Process. Technol. 2006, 175, 206–211. [Google Scholar] [CrossRef]
- Kooy, N.; Mohamed, K.; Pin, L.T.; Guan, O.S. A review of roll-to-roll nanoimprint lithography. Nanoscale Res. Lett. 2014, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Molak, R.; Araki, H.; Watanabe, M.; Katanoda, H.; Ohno, N.; Kuroda, S. Effects of Spray Parameters and Post-spray Heat Treatment on Microstructure and Mechanical Properties of Warm-Sprayed Ti-6Al-4V Coatings. J. Therm. Spray Technol. 2017, 26, 627–647. [Google Scholar] [CrossRef]
- Champagne, V.K.; Helfritch, D.J. A demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013, 7, 8. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kanbara, T.; Mori, C. Oriented crystalline film of poly (2, 5-thinylen) formed by vacuum deposition and its crystal structure. Comparison with a similarly oriented crystalline film of poly (1, 4-phenylene). Synth. Met. 1990, 38, 399–402. [Google Scholar] [CrossRef]
- Jensen, K.F.; Graves, D. Modeling and analysis of low pressure CVD reactors. J. Electrochem. Soc. 1983, 130, 1950–1957. [Google Scholar] [CrossRef]
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.S.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing polymer surfaces via vapor deposition. Mater. Today 2010, 13, 26–33. [Google Scholar] [CrossRef]
- Von Fieandt, L.; Larsson, T.; Lindahl, E.; Bäcke, O.; Boman, M. Chemical vapor deposition of TiN on transition metal substrates. Surf. Coat. Technol. 2018, 334, 373–383. [Google Scholar] [CrossRef]
- Meyer, N.; Rivera, L.R.; Ellis, T.; Qi, J.; Ryan, M.P.; Boccaccini, A.R. Bioactive and Antibacterial Coatings Based on Zein/Bioactive Glass Composites by Electrophoretic Deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, R.; Zhang, X. Synthesis and characterization of sol–gel hydroxyapatite coatings deposited on porous NiTi alloys. J. Alloy. Compd. 2011, 509, 4643–4648. [Google Scholar] [CrossRef]
- Nascimento, M.P.; Souz, R.C.; Miguel, I.M.; Pigatin, W.L.; Voorwald, H. Effects of tungsten carbide thermal spray coating by HP/HVOF and hard chromium electroplating on AISI 4340 high strength steel. Surf. Coat. Technol. 2001, 138, 113–124. [Google Scholar] [CrossRef]
- Kim, K.; Kuroda, S.; Watanabe, M. Microstructural development and deposition behavior of titanium powder particles in warm spraying process: From single splat to coating. J. Therm. Spray Technol. 2010, 19, 1244–1254. [Google Scholar] [CrossRef]
| Property | ELD | EPD |
|---|---|---|
| Coating elements | Ions | Solid particles |
| Surface charge | Medium | High |
| Preferred electrolyte | Water | Organic |
| Ionic electrolytic strength | High | Low |
| Electrolytic conductivity | High | Low |
| Approximate rate of deposition | 0.1 | 1000 |
| Coating Composition | Corrosion Rate (mm/y) | ||
|---|---|---|---|
| 0.1M NaOH | 0.1M H2SO4 | Sea Water | |
| WCCr3C2Ni | 0.38 | 0.15 | |
| Cr3C2NiCr | 0.17 | 0.077 | |
| WCCo | 0.76 | ||
| WCCoCr | 0.32 | ||
| Cr2O3Al2O3TiO2 | 3.2 | 3.6 | |
| Cr2O3 | 7.6 | 1.5 | |
| Deposition Process | Source | Feedstock Material | Substrate Material | Coating Thickness (µm) | Reference |
|---|---|---|---|---|---|
| PVD | Physical | TiCxOy-ZrCxOy, TiN, PE, PVDF, PTh | AISI M2 steel, SS, glass, Si, potassium bromide(KBr)-carbon-Au-Al, Ag-Au-Cu-Al | 1.2–6.3, 5, 0.2, 0.2, 0.1, 0.1 | [10,11,17,18,118] |
| CVD | Chemical | Niobium oxide(Nb2O5), W-TiN-WSi2-Ta2O5-Cu-SiO2, polycrystalline Si- Si3N4-SiO2, PTFE, Ni3Ti | Glass, Si, Si, Kleenex, Ni-Co-Fe | 0.05–0.2, -, 0.2–0.6, 0.04–0.1–16 | [24,25,119,120,121] |
| MAO | Electrochemical | Hydroxyapatite (HA)/TiO2, PCL duplex, HA- HA/ZrO2 | Ti-6Al-4V, Mg, NiT | 10–20, 2–3, 7 | [32,34,37] |
| ELD | Electrochemical | Ni-Co-Al2O3, Ni-Co-SiC, Zn-Ni-Al2O3, PPy | Steel, carbon steel, mild steel, Cu | 50–200, 10–70, -, - | [48,49,50,51] |
| EPD | Electrochemical | bioactive glass (45S5 BG)-Cu-doped BG, SiO2, chitosan, grapheme, SiC | AISI 316L SS, AISI 304 SS, AISI 316 L SS, Ti-6Al-4V alloy (TC4), Aramid-carbon-cellulose fibers composite | -, 7, 1–6, -, - | [122,56,57,58,59] |
| Sol–gel | Physical | TiCl4-(tetraethyl orthosilicate) TEOS- (methyltriethyl orthosilicate)MTEOS, HA, PDMS, Polycarbonate | Si, NiTi, stainless steel | 0.01–1, 1–4 | [68,123,62,74], |
| HVOF | Thermal | HA, CoNiCrAlY, WC | Ti-6Al-4V, Inconel 738 metal, AISI 4340 SS | 70, -, 100 | [83,84,124] |
| Plasma spray | Thermal | Al2O3-ZrO2- yttria stabilized zirconia (YSZ), Metco 447- Alumina/Titania 87/13- Nicrome 80/20- Hastalloy G30, TiC-NiCrBSi | SS, steel, AISI 4140 steel | -, 0.5–1, - | [91,92,96] |
| Cold spray | Physical | HA, AA7075, Ni/Al, mixed Ni/Al/MoO3, and Ni-clad Al | Ti-6Al-4V, Al 6061-T6, Al 6061 | 100–1000, 40–300, - | [103,105,106] |
| Warm spray | Physical | Zr-Cu12.3Ni7.6-Al3.5, Ti, Ti, WC-Co | 316L SS, steel, steel, carbon steel | 400–1000, -, 400, 300 | [108,110,125,111] |
| Arc wire spray | Thermal | MoS2-TiC-Fe, Ti/Al | Carbon steel, SUS 304 | 1000, - | [112,113] |
| Deposition Process | Advantages | Disadvantages | Reference |
|---|---|---|---|
| PVD | Corrosion and wear resistance/thin film deposition is possible/adjustable mechanical, corrosion and aesthetic properties | Requires a high vacuum/corrosion resistance is affected by abrasion/degradation control is challenging for polymer deposition applications | [9,17,18] |
| CVD | Corrosion and wear resistance/deposition of various types of materials with different microstructures/works with low and atmospheric pressures | Requires ultra-high vacuum/requires heat resistant substrates/small amount of coating materials waste | [24,25,30,31] |
| MAO | High corrosion resistance and hardness/porous structure for biomedical applications/different scales of porosity through the thickness/ | Mostly applicable to valve metals | [33,35] |
| ELD | Decorative and low-corrosion/wear applications/high-temperature applications | Works for conductive substrates | [46,47] |
| EPD | Various kinds of selective, graded material, and porous structure depositions/biomedical applications/wear resistant | Works for conductive substrates | [53,54,59] |
| Sol–gel | Cost effective/biomedical applications/providing corrosion and ion release protection/multilayered (thick) coating/high adhesion/ability to coat complex geometries/flexibility in the composition/no need of conductive substrates | Thickness control/slow rate of coating cycle/possibility of coating failure during heat treatment on multilayered coating structures | [66,67] |
| HVOF | High density of coating layer and well substrate adherence/works for non-conductive substrates/corrosion and wear resistance | Requires a small range of powder size (5–60 µm) with a narrow size distribution/numerous process variable to change the coating structure/requires a heat source | [83,84,85,86,87,88] |
| Plasma spray | High corrosion and wear resistance/high substrate adherence/surface modification of engineering polymers, rubbers, metals, and fibers/anti-stick coatings | A low-temperature process that is mostly used for materials that cannot perform reactions in atmospheric pressure to modify the surface of the substrate/requires a heat source | [92,93] |
| Cold spray | Simple and cheap method compared to the other thermal spray methods | Limited operation range/mostly used for soft and hard metal substrates/low efficiency and reliability due to low temperatures/not useful extremely harsh environments | [102,103] |
| Warm spray | Applicable to materials with sensitivity to oxidization at high temperatures or heat sensitive materials | Impurity complications/not useful extremely harsh environments | [108,109] |
| Arc wire spray | Internal surface coatings such as engine blocks/wear and corrosion resistant | Limited to conductive wires and materials as the coating layer | [79,113] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. https://doi.org/10.3390/jmmp3010028
Fotovvati B, Namdari N, Dehghanghadikolaei A. On Coating Techniques for Surface Protection: A Review. Journal of Manufacturing and Materials Processing. 2019; 3(1):28. https://doi.org/10.3390/jmmp3010028
Chicago/Turabian StyleFotovvati, Behzad, Navid Namdari, and Amir Dehghanghadikolaei. 2019. "On Coating Techniques for Surface Protection: A Review" Journal of Manufacturing and Materials Processing 3, no. 1: 28. https://doi.org/10.3390/jmmp3010028
APA StyleFotovvati, B., Namdari, N., & Dehghanghadikolaei, A. (2019). On Coating Techniques for Surface Protection: A Review. Journal of Manufacturing and Materials Processing, 3(1), 28. https://doi.org/10.3390/jmmp3010028






