Environmental Emissions from Chemical Etching Synthesis of Silicon Nanotube for Lithium Ion Battery Applications
Abstract
:1. Introduction
2. Materials and Methods
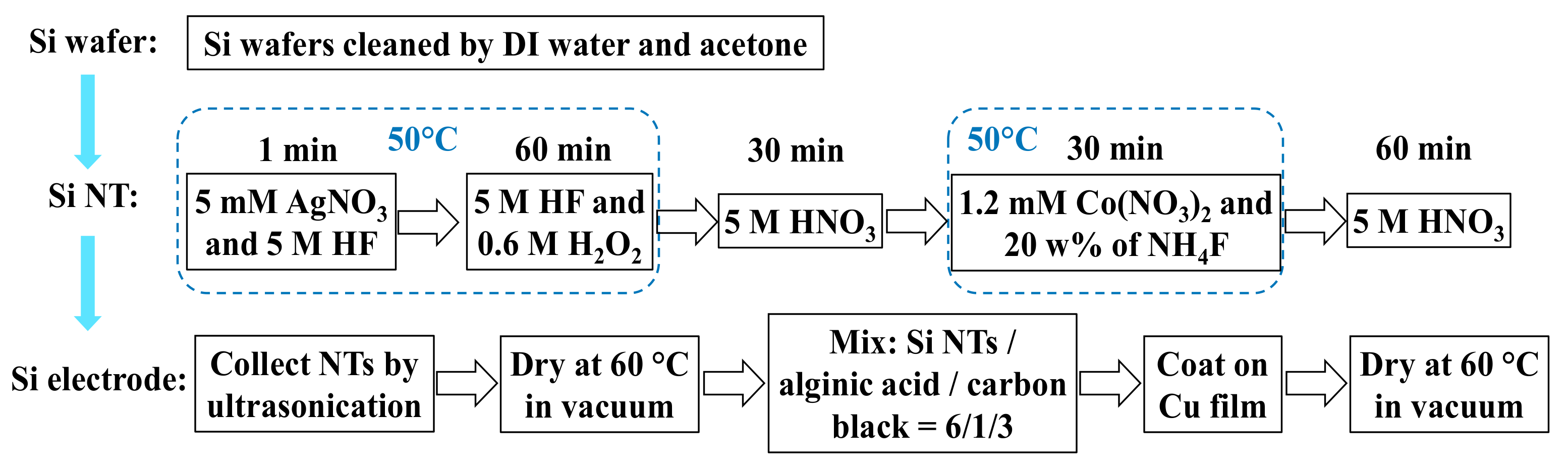
2.1. Synthesis of Silicon Nanotubes
2.2. Silicon Nanotube Battery Fabrication
2.3. Emission Analyses
2.3.1. Aqueous Waste
2.3.2. Gaseous Emissions
2.3.3. Aqueous Nanoparticle Emissions
2.3.4. Gaseous Aerosol Emissions
3. Results and Discussion
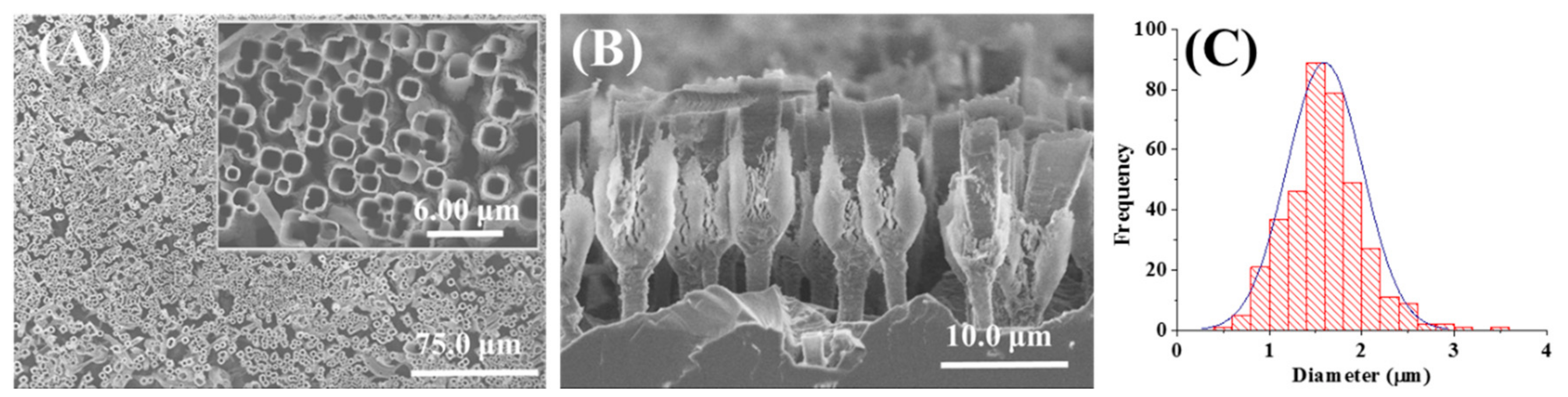
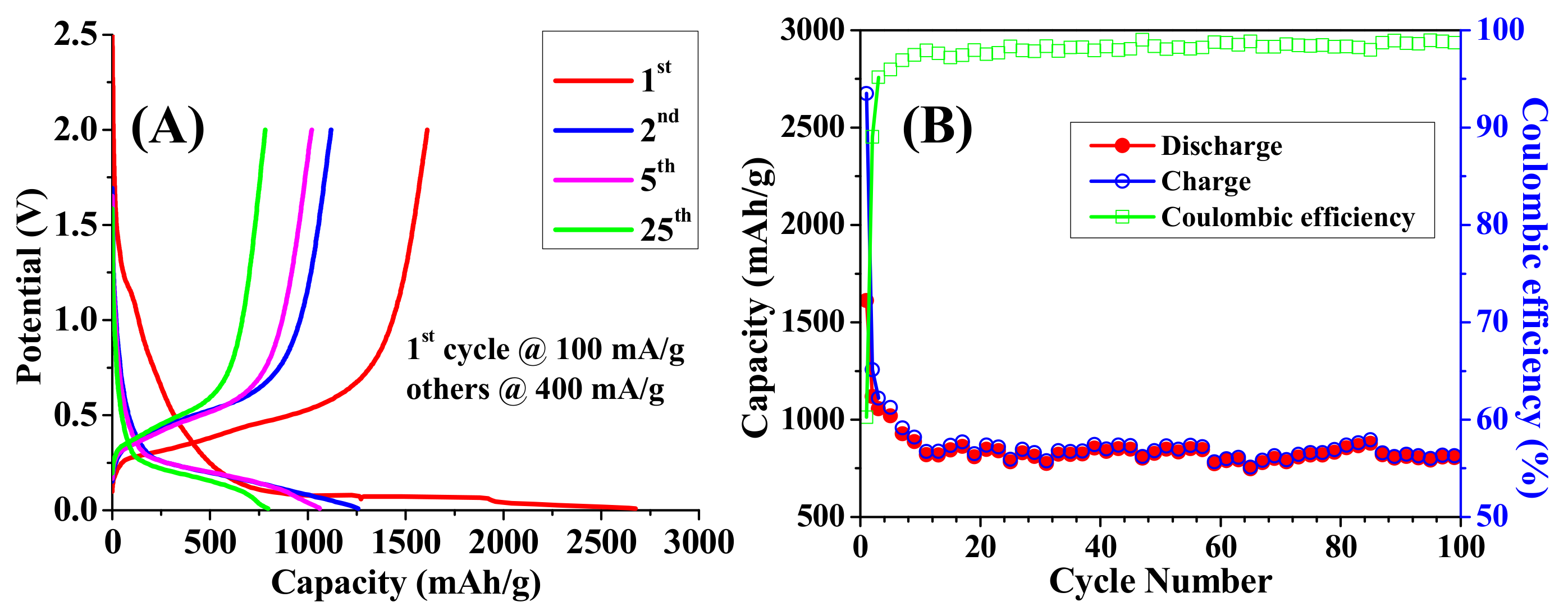
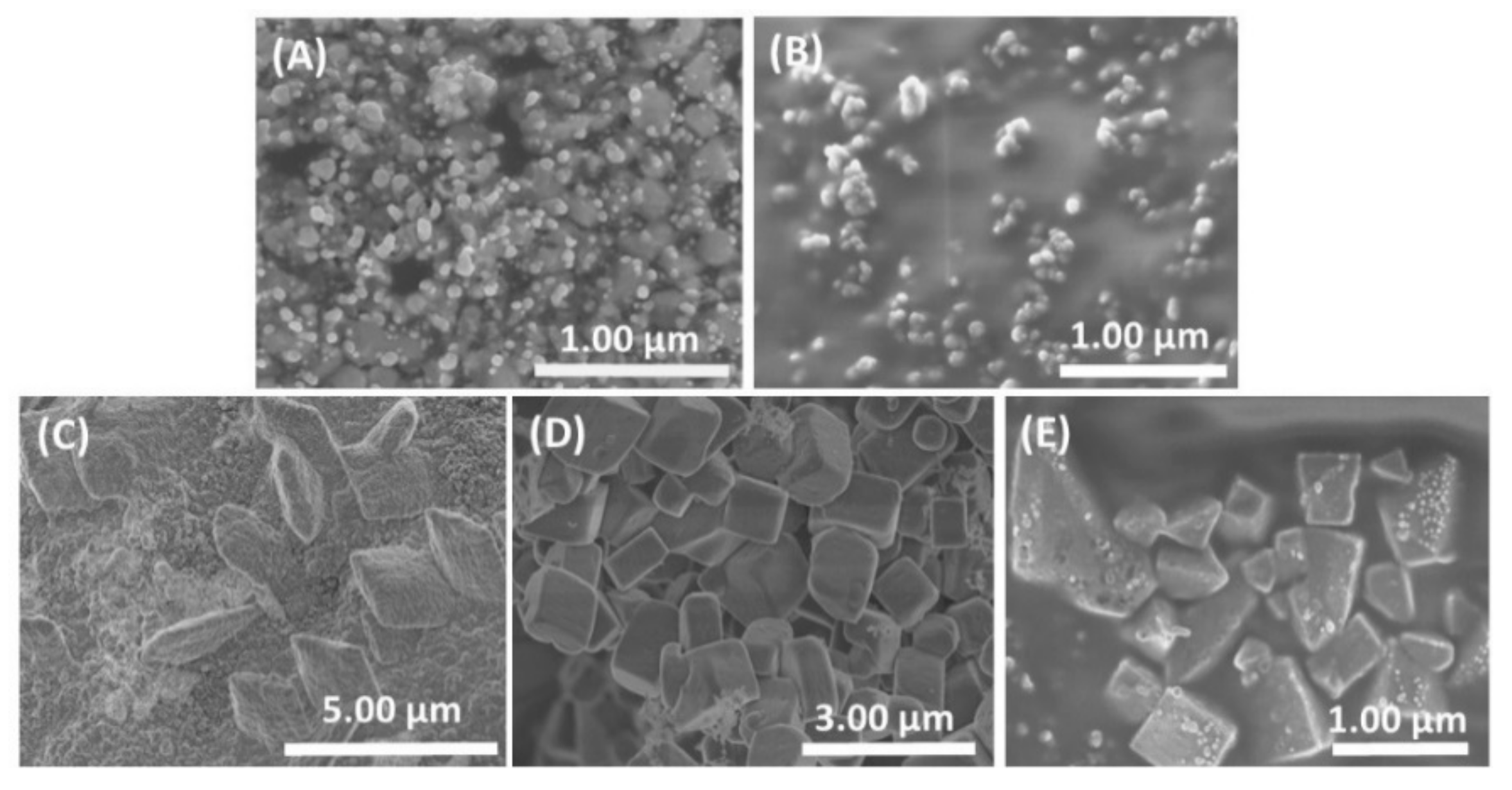
3.1. Morphology and Electrical Performance of SiNTs
3.2. Aqueous Waste
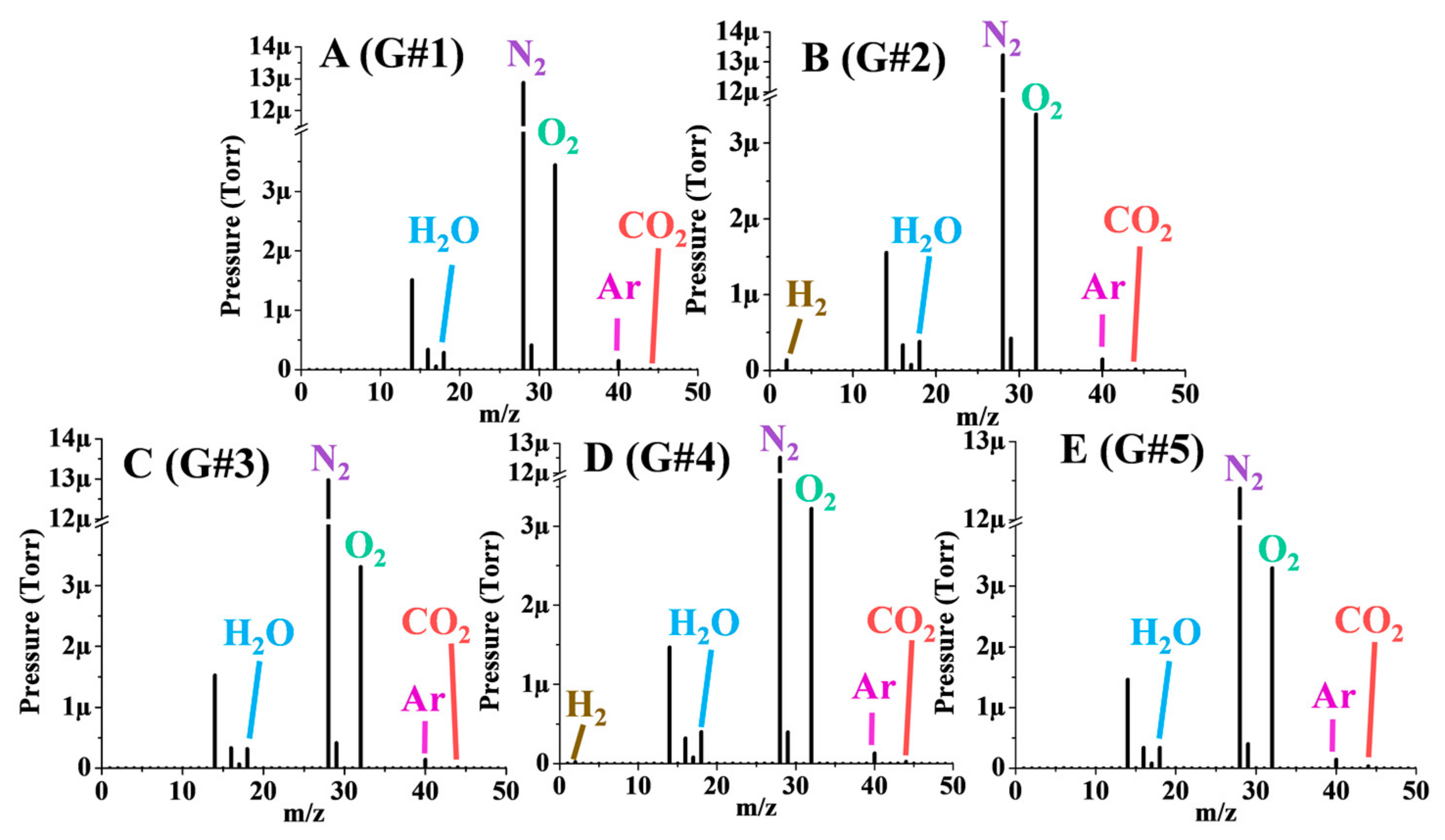
3.3. Gaseous Emissions
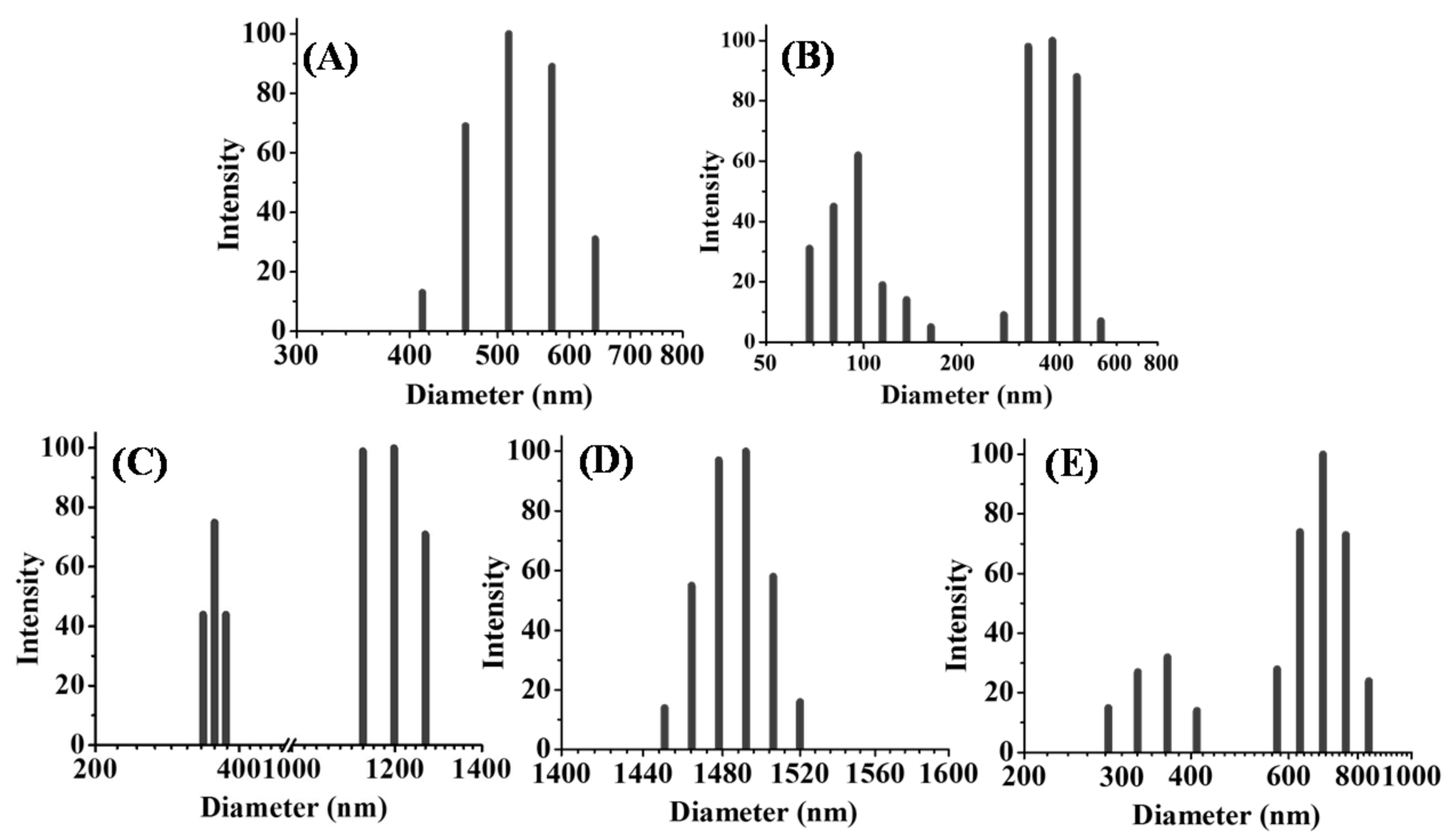
3.4. Aqueous Nanoparticle Emissions
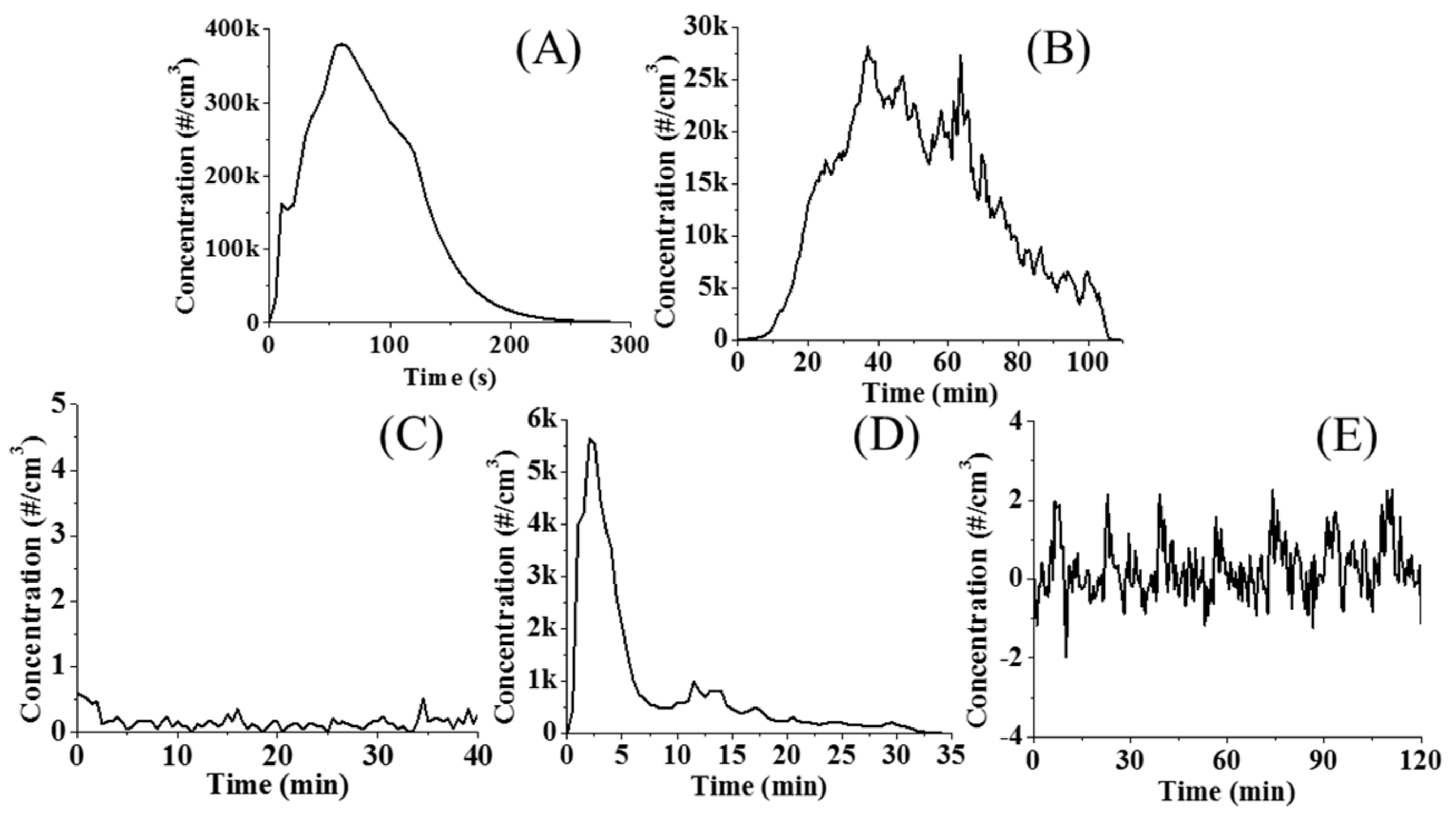
3.5. Gaseous Aerosol Emissions
4. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Tesfaye, A.T.; Gonzalez, R.; Coffer, J.L.; Djenizian, T. Porous silicon nanotube arrays as anode material for li-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 20495–20498. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Cong, T.N.; Yang, W.; Tan, C.Q.; Li, Y.L.; Ding, Y.L. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 195, 2419–2430. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.L.; Li, J.C.; Xiao, X.C.; Lott, A.; Lu, W.Q.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 375–379. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Hayner, C.M.; Zhao, X.; Kung, H.H. Materials for rechargeable lithium-ion batteries. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Kasavajjula, U.; Wang, C.S.; Appleby, A.J. Nano-and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Tesfaye, A.T.; Gonzalez-Rodriguez, R.; Coffer, J.L.; Djenizian, T. Self-Supported Silicon Nanotube Arrays as an Anode Electrode for Li-Ion Batteries. ECS Trans. 2017, 77, 349–350. [Google Scholar] [CrossRef]
- Epur, R.; Hanumantha, P.J.; Datta, M.K.; Hong, D.; Gattu, B.; Kumta, P.N. A simple and scalable approach to hollow silicon nanotube (h-SiNT) anode architectures of superior electrochemical stability and reversible capacity. J. Mater. Chem. A 2015, 3, 11117–11129. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Xiong, J.; Yang, T.; Qin, Y.; Yan, C. Porous Si nanowires from cheap metallurgical silicon stabilized by a surface oxide layer for lithium ion batteries. Adv. Funct. Mater. 2016, 25, 6701–6709. [Google Scholar] [CrossRef]
- Wang, F.; Gao, X.; Ma, L.; Li, T.; Yuan, C. Sustainability analysis of silicon nanowire fabrication for high performance lithium ion battery anode. Procedia Manuf. 2017, 7, 151–156. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Xie, Y.; Guan, D.; Yuan, C. A multilayered silicon-reduced graphene oxide electrode for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 7855–7862. [Google Scholar] [CrossRef] [PubMed]
- Roiban, L.; Koneti, S.; Wada, T.; Kato, H.; Aires, F.J.C.S.; Curelea, S.; Epicier, T.; Maire, E. Three dimensional analysis of nanoporous silicon particles for li-ion batteries. Mater. Charact. 2017, 124, 165–170. [Google Scholar] [CrossRef]
- Zhang, C.C.; Cheng, H.M.; Liu, X. A convenient way of manufacturing silicon nanotubes on a silicon substrate. Mater. Chem. Phys. 2016, 177, 479–484. [Google Scholar] [CrossRef]
- Park, M.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 11, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.B.; Lu, Z.D.; Yin, Y.D. Gram-scale synthesis of silica nanotubes with controlled aspect ratios by templating of nickel-hydrazine complex nanorods. Langmuir 2011, 27, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Zhang, Y.H.; Ni, L.F.; You, C.G.; Ye, C.J.; Jiang, R.M.; Liu, L.P.; Liu, J.; Han, C.M. A review of treatment strategies for hydrofluoric acid burns: Current status and future prospects. Burns 2014, 40, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.S.; Brender, J.D.; Sanderson, L.M.; Perrotta, D.M.; Beauchamp, R.A. Acute health effects in a community after a release of hydrofluoric acid. Arch. Environ. Health 1991, 46, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kupc, A.; Bischof, O.; Tritscher, T.; Beeston, M.; Krinke, T.; Wagner, P.E. Laboratory Characterization of a New Nano-Water-Based CPC 3788 and Performance Comparison to an Ultrafine Butanol-Based CPC 3776. Aerosol Sci. Technol. 2013, 47, 183–191. [Google Scholar] [CrossRef]
- Mordas, G.; Manninen, H.E.; Petäjä, T.; Aalto, P.P.; Hämeri, K.; Kulmala, M. On Operation of the Ultra-Fine Water-Based CPC TSI3786 and Comparison with Other TSI Models (TSI 3776, TSI 3772, TSI 3025, TSI 3010, TSI 3007. Aerosol Sci. Technol. 2008, 42, 152–158. [Google Scholar] [CrossRef]
- Yoo, J.K.; Kim, J.; Jung, Y.S.; Kang, K. Scalable fabrication of silicon nanotubes and their application to energy storage. Adv. Mater. 2012, 24, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Gondek, C.; Lippold, M.; Röver, I.; Bohmhammel, K.; Kroke, E. Etching Silicon with HF-H2O2-Based Mixtures: Reactivity Studies and Surface Investigations. J. Phys. Chem. 2014, 118, 2044–2051. [Google Scholar] [CrossRef]
- Feng, T.; Xu, Y.; Zhang, Z.; Mao, S. Fluorinion transfer in silver-assisted chemical etching for silicon nanowires arrays. Appl. Surf. Sci. 2015, 347, 421–427. [Google Scholar] [CrossRef]
- Li, Z.; Li, J. Recent progress in engineering and application of surface plasmon resonance in metal nanostructures. Chin. Sci. Bull. 2011, 56, 2631–2661. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.W. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yao, J.; Liu, Q.; Zhong, Z.; Wang, H. Toluene-assisted synthesis of RHO-type zeolitic imidazolate frameworks: Synthesis and formation mechanism of ZIF-11 and ZIF-12. Dalton Trans. 2013, 42, 16608–16613. [Google Scholar] [CrossRef] [PubMed]
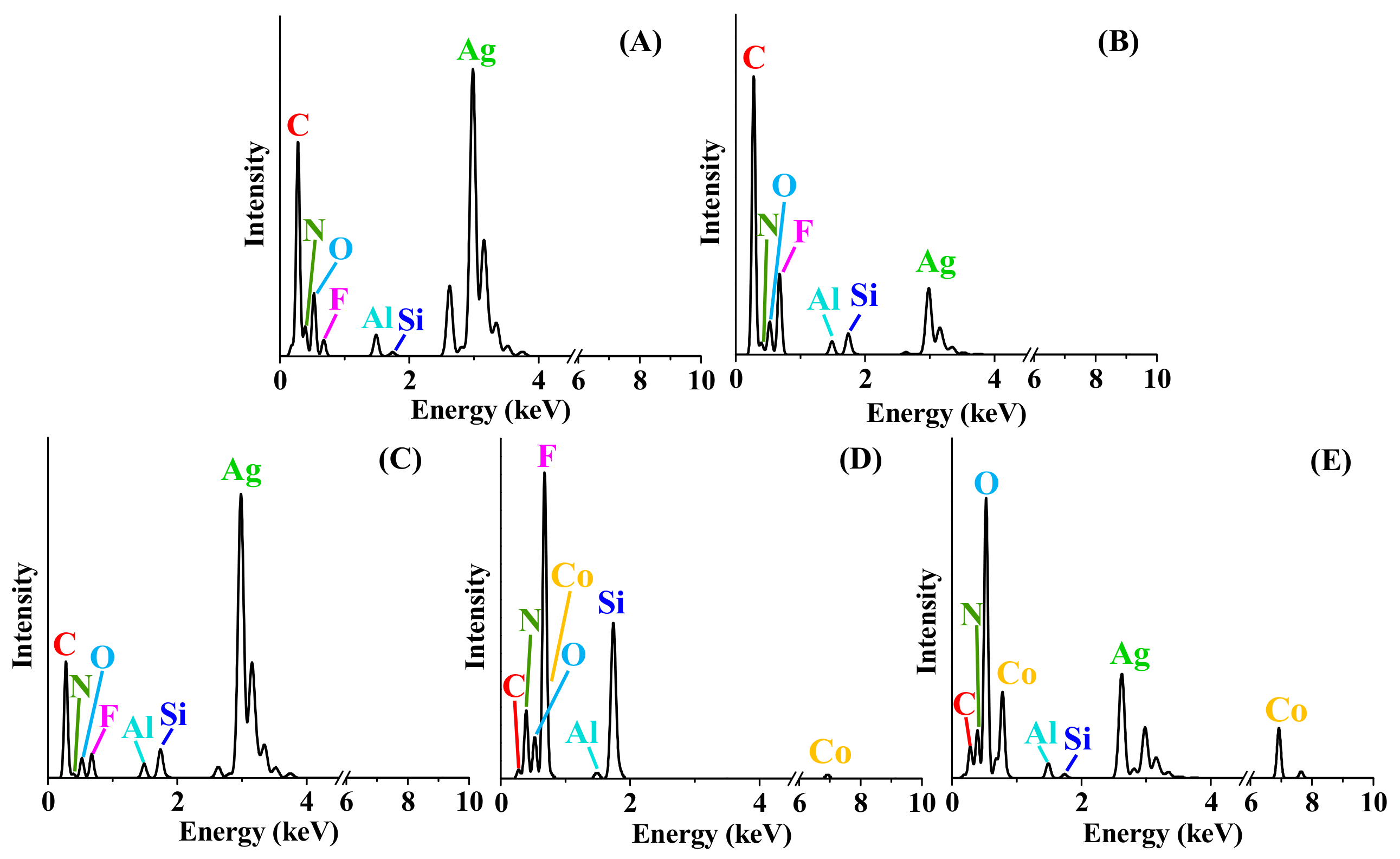
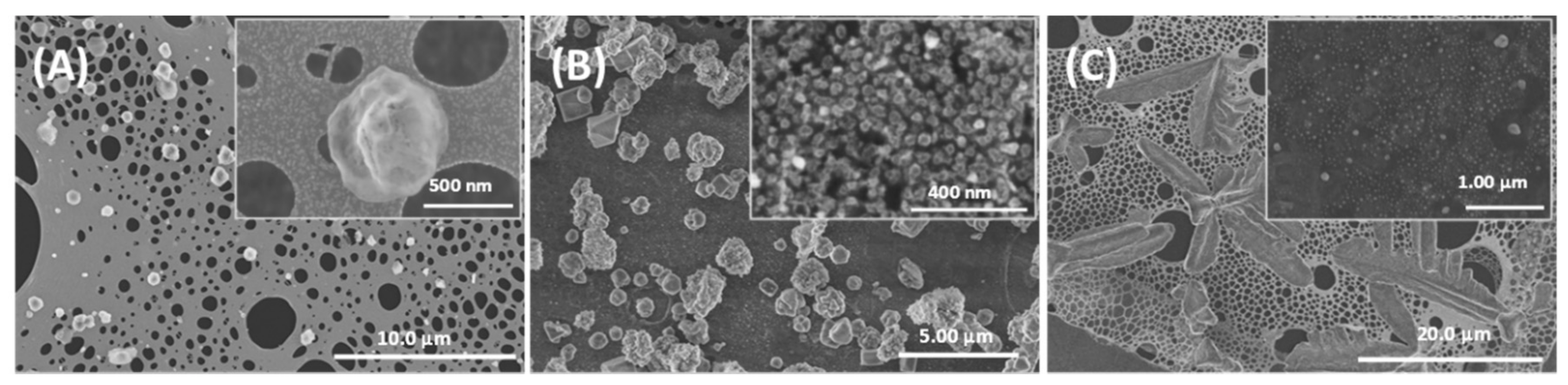
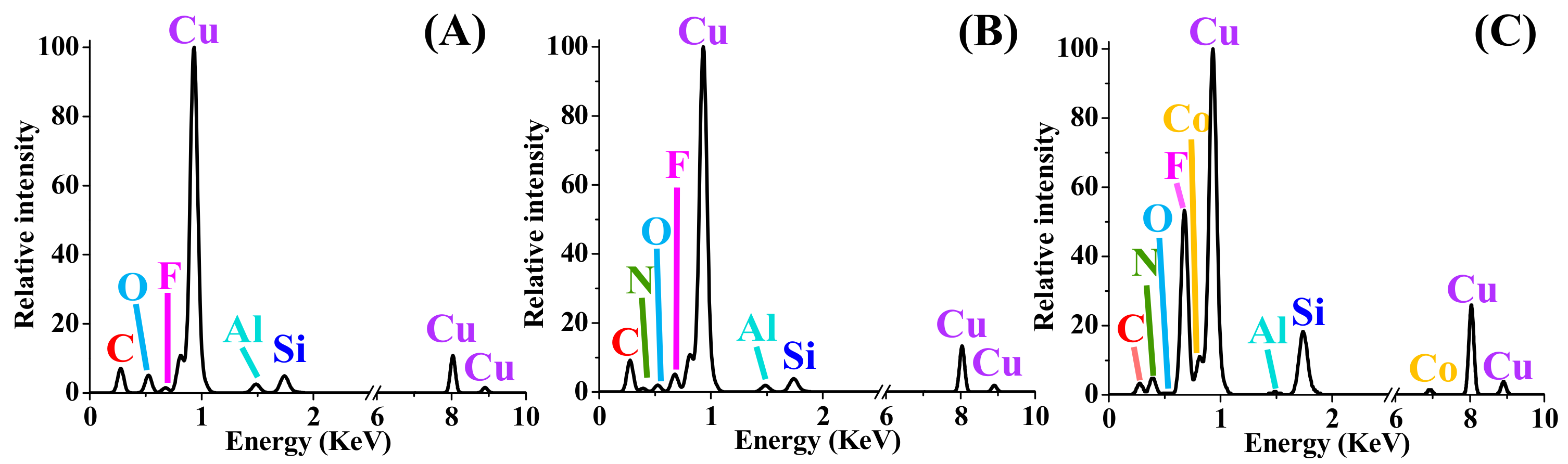
| Ag (mg) | Si (mg) | Co (mg) | |
|---|---|---|---|
| S#1 | 7.861 ± 0.094 | 41.521 ± 0.214 | - |
| S#2 | 0.068 ± 0.003 | 205.787 ± 1.043 | - |
| S#3 | 1.130 ± 0.005 | 3.157 ± 0.028 | - |
| S#4 | 0.319 ± 0.003 | 39.729 ± 0.283 | 1.884 ± 0.027 |
| S#5 | 0.506 ± 0.006 | 13.108 ± 0.040 | 0.841 ± 0.017 |
| Total | 9.885 ± 0.110 | 303.028 ± 1.609 | 2.726 ± 0.045 |
| N2 (%) | O2 (%) | Ar (%) | CO2 (%) | H2O (%) | H2 (%) | |
|---|---|---|---|---|---|---|
| G#1 | 76.92 ± 0.91 | 20.59 ± 0.46 | 0.89 ± 0.01 | 0.03 ± 0.23 | 1.56 ± 0.30 | 0.000 ± 0.000 |
| G#2 | 77.30 ± 2.05 | 19.76 ± 0.34 | 0.84 ± 0.04 | 0.03 ± 0.11 | 1.99 ± 0.48 | 0.070 ± 0.002 |
| G#3 | 77.69 ± 1.20 | 19.83 ± 0.25 | 0.83 ± 0.05 | 0.04 ± 0.08 | 1.61 ± 0.31 | 0.000 ± 0.000 |
| G#4 | 77.07 ± 0.34 | 19.88 ± 0.11 | 0.82 ± 0.01 | 0.04 ± 0.02 | 2.18 ± 0.39 | 0.011 ± 0.000 |
| G#5 | 76.91 ± 0.72 | 20.51 ± 0.44 | 0.85 ± 0.03 | 0.04 ± 0.06 | 1.69 ± 0.27 | 0.000 ± 0.000 |
| Element | Atom. C (at. %) | ||||
|---|---|---|---|---|---|
| S#1 | S#2 | S#3 | S#4 | S#5 | |
| Al | 0.76 ± 0.1 | 0.48 ± 0.1 | 1.44 ± 0.1 | 0.35 ± 0.1 | 0.69 ± 0.1 |
| C | 14.85 ± 1.9 | 36.93 ± 9.3 | 15.74 ± 1.2 | 1.25 ± 1.2 | 3.01 ± 0.8 |
| Co | - | - | - | 0.99 ± 3.1 | 10.52 ± 1.0 |
| F | 13.08 ± 3.7 | 37.14 ± 15.1 | 30.22 ± 5.4 | 48.46 ± 56.4 | - |
| N | 33.01 ± 5.8 | 10.86 ± 16.7 | 13.38 ± 3.6 | 33.22 ± 10.6 | 24.65 ± 3.3 |
| O | 26.63 ± 4.2 | 12.55 ± 21.7 | 15.06 ± 2.5 | 7.00 ± 3.3 | 54.61 ± 5.6 |
| Si | 0.14 ± 0.0 | 0.73 ± 0.1 | 2.73 ± 0.2 | 8.73 ± 0.7 | 0.18 ± 0.1 |
| Ag | 11.53 ± 2.5 | 1.31 ± 0.3 | 21.43 ± 2.4 | - | 2.00 ± 0.4 |
| Element | Atom. C (at. %) | ||
|---|---|---|---|
| G#1 | G#2 | G#4 | |
| Al | 3.12 ± 0.1 | 1.9 ± 0.1 | 0.33 ± 0.1 |
| C | 16.44 ± 0.9 | 15.48 ± 0.8 | 2.28 ± 0.4 |
| Co | - | - | 1.47 ± 0.2 |
| Cu | 62.12 ± 3.1 | 62.09 ± 3.1 | 46.5 ± 3.1 |
| F | 2.78 ± 0.4 | 6.89 ± 0.6 | 29.17 ± 16.1 |
| N | - | 7.15 ± 0.7 | 13.01 ± 2.0 |
| O | 13.23 ± 0.8 | 3.11 ± 0.3 | 0.47 ± 0.2 |
| Si | 5.31 ± 0.2 | 3.38 ± 0.1 | 6.76 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Guan, D.; Wang, F.; Yuan, C. Environmental Emissions from Chemical Etching Synthesis of Silicon Nanotube for Lithium Ion Battery Applications. J. Manuf. Mater. Process. 2018, 2, 11. https://doi.org/10.3390/jmmp2010011
Ma L, Guan D, Wang F, Yuan C. Environmental Emissions from Chemical Etching Synthesis of Silicon Nanotube for Lithium Ion Battery Applications. Journal of Manufacturing and Materials Processing. 2018; 2(1):11. https://doi.org/10.3390/jmmp2010011
Chicago/Turabian StyleMa, Lulu, Dongsheng Guan, Fenfen Wang, and Chris Yuan. 2018. "Environmental Emissions from Chemical Etching Synthesis of Silicon Nanotube for Lithium Ion Battery Applications" Journal of Manufacturing and Materials Processing 2, no. 1: 11. https://doi.org/10.3390/jmmp2010011
APA StyleMa, L., Guan, D., Wang, F., & Yuan, C. (2018). Environmental Emissions from Chemical Etching Synthesis of Silicon Nanotube for Lithium Ion Battery Applications. Journal of Manufacturing and Materials Processing, 2(1), 11. https://doi.org/10.3390/jmmp2010011






