Assessment of Ground and Drone Surveys of Large Waterbird Breeding Rookeries: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Ground-Based Surveys
2.3. Drone Surveys
| Development Stage | Characteristics | Age (Days) | Ground Observer View | Drone View |
|---|---|---|---|---|
| Eggs | Whole egg, incubated by adult. | 1–20 |  |  |
| Chicks | Recently hatched (1–5 days old), downy feathers, immobile. | 21–25 |  |  |
| Squirters | Early sheathed feathers starting on wings, still in nest, immobile. | 26–30 |  |  |
| Runners | Development of pin feathers. Mix of down and feathers, walking awkwardly, can leave nest on foot. | 31–35 |  |  |
| Flappers | Nearly fully feathered, cannot fly but flaps between nests. | 36–40 |  |  |
| Flyers | Fully feathered, able to fly and leave nests, still attended by parents at nest. | 41–47 |  |  |
| Fledglings | Independent, does not return to nest but roosts in nearby trees. (Normally not counted by ground observers or drone). | >48 |  | |
2.4. Analysis
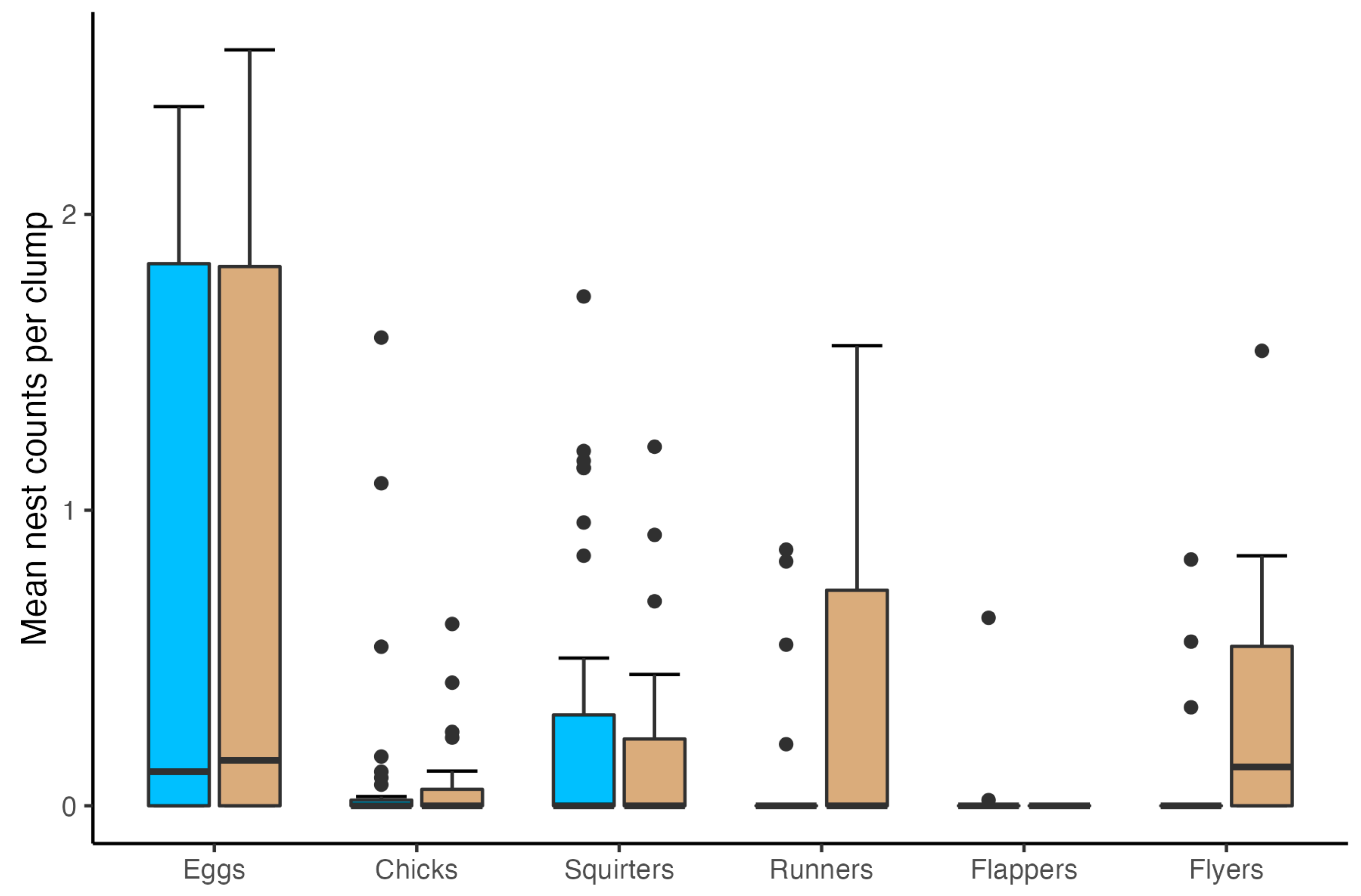
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Old, J.M.; Lin, S.H.; Franklin, M.J.M. Mapping out bare-nosed wombat (Vombatus ursinus) burrows with the use of a drone. BMC Ecol. 2019, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-G.; Yoo, S.H.; Kwon, O. Possibility of applying unmanned aerial vehicle (UAV) and mapping software for the monitoring of waterbirds and their habitats. J. Ecol. Environ. 2017, 41, 21. [Google Scholar] [CrossRef]
- Francis, R.J.; Lyons, M.B.; Kingsford, R.T.; Brandis, K.J. Counting mixed breeding aggregations of animal species using drones: Lessons from waterbirds on semi-automation. Remote Sens. 2020, 12, 1185. [Google Scholar] [CrossRef]
- Inman, V.L.; Kingsford, R.T.; Chase, M.J.; Leggett, K.E. Drone-based effective counting and ageing of hippopotamus (Hippopotamus amphibius) in the Okavango Delta in Botswana. PLoS ONE 2019, 14, e0219652. [Google Scholar] [CrossRef] [PubMed]
- Ezat, M.A.; Fritsch, C.J.; Downs, C.T. Use of an unmanned aerial vehicle (drone) to survey Nile crocodile populations: A case study at Lake Nyamithi, Ndumo game reserve, South Africa. Biol. Conserv. 2018, 223, 76–81. [Google Scholar] [CrossRef]
- Hayes, M.C.; Gray, P.C.; Harris, G.; Sedgwick, W.C.; Crawford, V.D.; Chazal, N.; Crofts, S.; Johnston, D.W. Drones and deep learning produce accurate and efficient monitoring of large-scale seabird colonies. Ornithol. Appl. 2021, 123, duab022. [Google Scholar] [CrossRef]
- Pfeifer, C.; Barbosa, A.; Mustafa, O.; Peter, H.-U.; Rümmler, M.-C.; Brenning, A. Using Fixed-Wing UAV for Detecting and Mapping the Distribution and Abundance of Penguins on the South Shetlands Islands, Antarctica. Drones 2019, 3, 39. [Google Scholar] [CrossRef]
- Kellenberger, B.; Veen, T.; Folmer, E.; Tuia, D. 21 000 birds in 4.5 h: Efficient large-scale seabird detection with machine learning. Remote Sens. Ecol. Conserv. 2021, 7, 445–460. [Google Scholar] [CrossRef]
- Francis, R.; Kingsford, R.; Brandis, K. Using drones and citizen science counts to track colonial waterbird breeding, an indicator for ecosystem health on the Chobe River, Botswana. Glob. Ecol. Conserv. 2022, 38, e02231. [Google Scholar] [CrossRef]
- Lachman, D.; Conway, C.; Vierling, K.; Matthews, T. Drones provide a better method to find nests and estimate nest survival for colonial waterbirds: A demonstration with Western Grebes. Wetl. Ecol. Manag. 2020, 28, 837–845. [Google Scholar] [CrossRef]
- Afán, I.; Máñez, M.; Díaz-Delgado, R. Drone monitoring of breeding waterbird populations: The case of the glossy ibis. Drones 2018, 2, 42. [Google Scholar] [CrossRef]
- Lyons, M.B.; Brandis, K.J.; Murray, N.J.; Wilshire, J.H.; McCann, J.A.; Kingsford, R.T.; Callaghan, C.T. Monitoring large and complex wildlife aggregations with drones. Methods Ecol. Evol. 2019, 10, 1024–1035. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Choi, S.-H.; Banjade, M.; Jin, S.-D.; Park, S.-M.; Kunwar, B.; Oh, H.-S. Insights into the Behavioral Ecology and Niche Separation of Passeriformes through Camera-trap Analysis in the Halla Mountain Wetland of Jeju, Republic of Korea. Preprints 2023. [Google Scholar] [CrossRef]
- Colyn, R.B.; Campbell, A.; Smit-Robinson, H.A. The use of a camera trap and acoustic survey design to ascertain the vocalization and breeding status of the highly elusive White-winged Flufftail, Sarothrura ayresi. Avian Conserv. Ecol. 2020, 15, 12. [Google Scholar] [CrossRef]
- Barr, J.R.; Green, M.C.; DeMaso, S.J.; Hardy, T.B. Drone surveys do not increase colony-wide flight behaviour at waterbird nesting sites, but sensitivity varies among species. Sci. Rep. 2020, 10, 3781. [Google Scholar] [CrossRef]
- Scarton, F.; Valle, R. Drone assessment of habitat selection and breeding success of Gull-billed Tern Gelochelidon nilotica nesting on low-accessibility sites: A case study. Riv. Ital. Di Ornitol. 2020, 90, 69–76. [Google Scholar] [CrossRef]
- Valle, R.G.; Corregidor-Castro, A.; Verza, E.; Scarton, F. Drone monitoring improves nest detection of Squacco Herons, but fails to assess its productivity. Ornis Hung. 2022, 30, 176–187. [Google Scholar] [CrossRef]
- Sikora, A.; Marchowski, D. The use of drones to study the breeding productivity of Whooper Swan Cygnus cygnus. Eur. Zool. J. 2023, 90, 193–200. [Google Scholar] [CrossRef]
- Rančić, K.; Blagojević, B.; Bezdan, A.; Ivošević, B.; Tubić, B.; Vranešević, M.; Pejak, B.; Crnojević, V.; Marko, O. Animal Detection and Counting from UAV Images Using Convolutional Neural Networks. Drones 2023, 7, 179. [Google Scholar] [CrossRef]
- Scarton, F.; Valle, R. Could we assess the hatching success of Pied Avocets (Recurvirostra avosetta Linnaeus, 1758) by drone monitoring? A pilot study. Lav.–Soc. Veneziana Sci. Nat. 2020, 45, 139–142. [Google Scholar]
- Valle, R.G.; Scarton, F. Rapid Assessment of Productivity of Purple Herons Ardea purpurea by Drone Conducted Monitoring. Ardeola 2022, 69, 231–248. [Google Scholar] [CrossRef]
- Scarton, F.; Valle, R.G. Comparison of drone vs. ground survey monitoring of hatching success in the black-headed gull (Chroicocephalus ridibundus). Ornithol. Res. 2022, 30, 271–280. [Google Scholar] [CrossRef]
- Gallego, D.; Sarasola, J.H. Using drones to reduce human disturbance while monitoring breeding status of an endangered raptor. Remote Sens. Ecol. Conserv. 2021, 7, 550–561. [Google Scholar] [CrossRef]
- Dyer, F.; Broadhurst, B.; Tschierschke, A.; Higginsson, W.; Giling, D.; Brandis, K.; Thiem, J.; Kerezsy, A.; Lenehan, J.; Thomson, R. Commonwealth Environmental Water Office Monitoring, Evaluation and Research Project: Lachlan River System Selected Area 2021–22 Monitoring and Evaluation Technical Report; Canberra University: Canberra, Australia, 2022. [Google Scholar]
- Wassens, S.; Turner, A.; Spencer, J.; Brandis, K.; Michie, L.; Duncan, M.; Thiem, J.; Thomas, R.; Shuang, X.; Kobayashi, T.; et al. Commonwealth Environmental Water Office Monitoring, Evaluation and Research Program Murrumbidgee River System Technical Report, 2014–2022; Charles Sturt University: Bathurst, Australia, 2022. [Google Scholar]
- Fudala, K.; Bialik, R.J. The use of drone-based aerial photogrammetry in population monitoring of Southern Giant Petrels in ASMA 1, King George Island, maritime Antarctica. Glob. Ecol. Conserv. 2022, 33, e01990. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Prudor, A.; Schull, Q. Flights of drones over sub-Antarctic seabirds show species-and status-specific behavioural and physiological responses. Polar Biol. 2018, 41, 259–266. [Google Scholar] [CrossRef]
- Frederick, P.C.; Collopy, M.W. Nesting success of five ciconiiform species in relation to water conditions in the Florida Everglades. Auk 1989, 106, 625–634. [Google Scholar]
- Marchant, S.; Higgins, P.J. (Eds.) Handbook of Australian, New Zealand and Antarctic Birds Volume 1 Ratites to Ducks; Oxford University Press: Melbourne, Australia, 1990; Volume 1. [Google Scholar]
- Brandis, K.; Kingsford, R.T.; Ren, S.; Ramp, D. Crisis water management and ibis breeding at Narran Lakes in arid Australia. Environ. Manag. 2011, 48, 489–498. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Brandis, K.J.; Lyons, M.B.; Ryall, S.; Kingsford, R.T. A comment on the limitations of UAVS in wildlife research–the example of colonial nesting waterbirds. J. Avian Biol. 2018, 49, e01825. [Google Scholar] [CrossRef]
- Parsons, K.C. Heron nesting at Pea Patch Island, upper Delaware Bay, USA: Abundance and reproductive success. Colon. Waterbirds 1995, 18, 69–78. [Google Scholar] [CrossRef]
- Connor, K.J.; Gabor, S. Breeding waterbird wetland habitat availability and response to water-level management in Saint John River floodplain wetlands, New Brunswick. Hydrobiologia 2006, 567, 169–181. [Google Scholar] [CrossRef]
- Borgmann, K.L. A review of human disturbance impacts on waterbirds. Audubon Calif. 2011, 376, 1–23. [Google Scholar]
- Roshier, D.A.; Robertson, A.I.; Kingsford, R.T. Responses of waterbirds to flooding in an arid region of Australia and implications for conservation. Biol. Conserv. 2002, 106, 399–411. [Google Scholar] [CrossRef]
- Bino, G.; Brandis, K.; Kingsford, R.T.; Porter, J. Waterbird synchrony across Australia’s highly variable dryland rivers—Risks and opportunities for conservation. Biol. Conserv. 2020, 243, 108497. [Google Scholar] [CrossRef]
- Leslie, D.J. Effect of river management on colonially-nesting waterbirds in the Barmah-Millewa Forest, South-Eastern Australia. Regul. Rivers Res. Manag. 2001, 17, 21–36. [Google Scholar] [CrossRef]
- Frixione, M.G.; Salvadeo, C. Drones, gulls and urbanity: Interaction between new technologies and human subsidized species in coastal areas. Drones 2021, 5, 30. [Google Scholar] [CrossRef]
- Orange, J.P.; Bielefeld, R.R.; Cox, W.A.; Sylvia, A.L. Impacts of Drone Flight Altitude on Behaviors and Species Identification of Marsh Birds in Florida. Drones 2023, 7, 584. [Google Scholar] [CrossRef]
- Vas, E.; Lescroël, A.; Duriez, O.; Boguszewski, G.; Grémillet, D. Approaching birds with drones: First experiments and ethical guidelines. Biol. Lett. 2015, 11, 20140754. [Google Scholar] [CrossRef]
- Brisson-Curadeau, É.; Bird, D.; Burke, C.; Fifield, D.; Pace, P.; Sherley, R.; Elliott, K. Seabird species vary in behavioural response to drone census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, G.P.; Rodríguez-Teijeiro, J.D.; Wich, S.A.; Mulero-Pázmány, M. Measuring disturbance at swift breeding colonies due to the visual aspects of a drone: A quasi-experiment study. Curr. Zool. 2021, 67, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.P.; Amano, T.; Fuller, R.A. Drone-induced flight initiation distances for shorebirds in mixed-species flocks. J. Appl. Ecol. 2023, 60, 1816–1827. [Google Scholar] [CrossRef]
- Kuhlmann, K.; Fontaine, A.; Brisson-Curadeau, É.; Bird, D.M.; Elliott, K.H. Miniaturization eliminates detectable impacts of drones on bat activity. Methods Ecol. Evol. 2022, 13, 842–851. [Google Scholar] [CrossRef]
- Valle, R.G.; Scarton, F. Drone-conducted counts as a tool for the rapid assessment of productivity of Sandwich Terns (Thalasseus sandvicensis). J. Ornithol. 2021, 162, 621–628. [Google Scholar] [CrossRef]
- Minias, P.; Kaczmarek, K. Is it always beneficial to breed in the centre? Trade-offs in nest site selection within the colony of a tree-nesting waterbird. J. Ornithol. 2013, 154, 945–953. [Google Scholar] [CrossRef]
- Brzeziński, M.; Chibowski, P.; Gornia, J.; Górecki, G.; Zalewski, A. Spatio-temporal variation in nesting success of colonial waterbirds under the impact of a non-native invasive predator. Oecologia 2018, 188, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, K.B.; Whisson, D.A.; Tan, L.X.; Weston, M.A. Intense predation of non-colonial, ground-nesting bird eggs by corvid and mammalian predators. Wildl. Res. 2015, 42, 518–528. [Google Scholar] [CrossRef]
- Rocke, T.E.; Bollinger, T.K. Avian Botulism; Blackwell Publishing: Oxford, UK, 2007; pp. 377–416. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, R.J.; Brandis, K.J. Assessment of Ground and Drone Surveys of Large Waterbird Breeding Rookeries: A Comparative Study. Drones 2024, 8, 135. https://doi.org/10.3390/drones8040135
Francis RJ, Brandis KJ. Assessment of Ground and Drone Surveys of Large Waterbird Breeding Rookeries: A Comparative Study. Drones. 2024; 8(4):135. https://doi.org/10.3390/drones8040135
Chicago/Turabian StyleFrancis, Roxane J., and Kate J. Brandis. 2024. "Assessment of Ground and Drone Surveys of Large Waterbird Breeding Rookeries: A Comparative Study" Drones 8, no. 4: 135. https://doi.org/10.3390/drones8040135
APA StyleFrancis, R. J., & Brandis, K. J. (2024). Assessment of Ground and Drone Surveys of Large Waterbird Breeding Rookeries: A Comparative Study. Drones, 8(4), 135. https://doi.org/10.3390/drones8040135







