Vegetation Type Preferences in Red Deer (Cervus elaphus) Determined by Object Detection Models
Abstract
1. Introduction
1.1. Monitoring Possibilities in Population and Conservation Biology
1.2. Ecology and Biology of Red Deer
1.3. Agricultural Damage Associated with Red Deer
- The distribution of red deer in time and space in natural vegetation types and agricultural fields;
- The types of behaviour of red deer using automated object detection from thermal camera footage;
- The vegetation type preference of red deer;
- The behavioural patterns exhibited by red deer in different vegetation types.
2. Materials and Methods
2.1. Image Collection and Study Areas
2.2. Data Collection
2.3. Species Identification
2.4. Behaviour Identification of Red Deer and Fallow Deer
2.5. Data Analysis
2.5.1. Definition and Calculation of Variables
2.5.2. Assignment of Vegetation Type to Populations of Red Deer
2.5.3. Statistical Analysis
3. Results
3.1. Model Performance
3.2. Deer Counts in Lyngby Hede
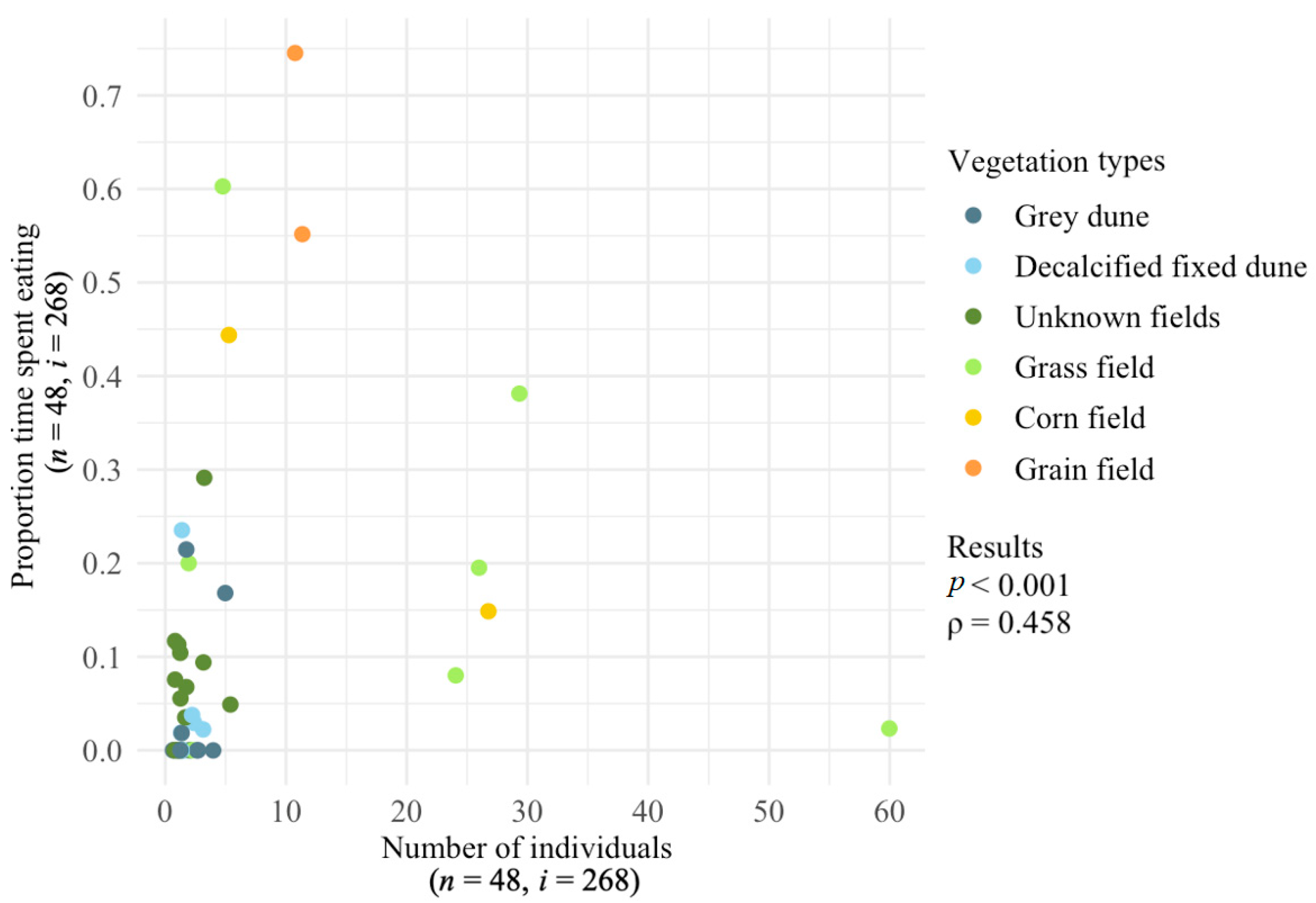
3.3. Proportion of Time Spent Eating
3.4. Proportion of Time Spent Lying
3.5. Distributional Characteristics of Behaviours
3.6. Time of Observation of Populations
4. Discussion
4.1. Methodology and Limitations Using Drone Monitoring
4.2. Foraging Behavioural Patterns in Vegetation Types
4.3. Behavioural Instability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
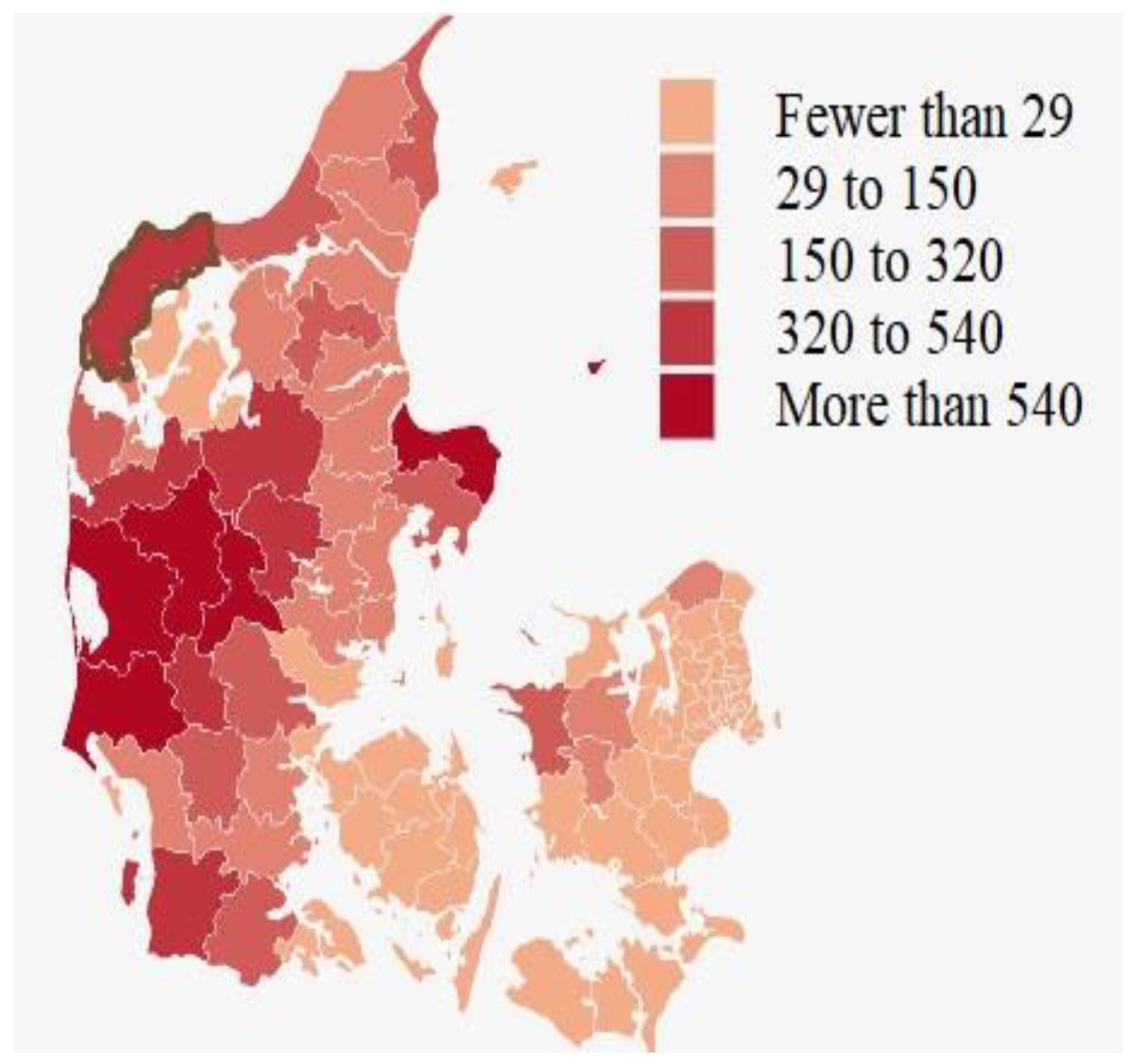
Appendix B

Appendix C. Wilcoxon Ranked Sum Test and Tukey’s Test Results
| Transformation | Comparison | p-Value |
|---|---|---|
| Non-transformed | Grey dune—Decalcified fixed dune | p > 0.05 |
| Grey dune—Unknown fields | p > 0.05 | |
| Grey dune—Grass field | p > 0.05 | |
| Grey dune—Corn field | p > 0.05 | |
| Grey dune—Grain field | p < 0.05 | |
| Decalcified fixed dune—Unknown fields | p > 0.05 | |
| Decalcified fixed dune—Grass field | p < 0.05 | |
| Decalcified fixed dune—Corn field | p < 0.05 | |
| Decalcified fixed dune—Grain field | p < 0.05 | |
| Unknown field—Grass field | p < 0.05 | |
| Unknown field—Corn field | p < 0.05 | |
| Unknown field—Grain field | p < 0.05 | |
| Grass field—Corn field | p > 0.05 | |
| Grass field—Grain field | p > 0.05 | |
| Corn field—Grain field | p > 0.05 | |
| Log-transformed | Grey dune—Decalcified fixed dune | p > 0.05 |
| Grey dune—Unknown field | p > 0.05 | |
| Grey dune—Grass field | p > 0.05 | |
| Grey dune—Corn field | p > 0.05 | |
| Grey dune—Grain field | p > 0.05 | |
| Decalcified fixed dune—Unknown field | p > 0.05 | |
| Decalcified fixed dune—Grass field | p > 0.05 | |
| Decalcified fixed dune—Corn field | p > 0.05 | |
| Decalcified fixed dune—Grain field | p > 0.05 | |
| Unknown field—Grass field | p > 0.05 | |
| Unknown field—Corn field | p > 0.05 | |
| Unknown field—Grain field | p < 0.05 | |
| Grass field—Corn field | p > 0.05 | |
| Grass field—Grain field | p > 0.05 | |
| Corn field—Grain field | p > 0.05 | |
| Arcsin-square-root-transformed | Grey dune—Decalcified fixed dune | p > 0.05 |
| Grey dune—Unknown field | p > 0.05 | |
| Grey dune—Grass field | p < 0.05 | |
| Grey dune—Corn field | p < 0.05 | |
| Grey dune—Grain field | p > 0.05 | |
| Decalcified fixed dune—Unknown field | p > 0.05 | |
| Decalcified fixed dune—Grass field | p < 0.05 | |
| Decalcified fixed dune—Corn field | p < 0.05 | |
| Decalcified fixed dune—Grain field | p < 0.05 | |
| Unknown field—Grass field | p > 0.05 | |
| Unknown field—Corn field | p < 0.05 | |
| Unknown field—Grain field | p < 0.05 | |
| Grass field—Corn field | p > 0.05 | |
| Grass field—Grain field | p > 0.05 | |
| Corn field—Grain field | p > 0.05 |
| Comparison | p-Value |
|---|---|
| Grey dune—Decalcified fixed dune | p > 0.05 |
| Grey dune—Unknown field | p > 0.05 |
| Grey dune—Grass field | p > 0.05 |
| Grey dune—Corn field | p > 0.05 |
| Grey dune—Grain field | p > 0.05 |
| Decalcified fixed dune—Unknown field | p > 0.05 |
| Decalcified fixed dune—Grass field | p > 0.05 |
| Decalcified fixed dune—Corn field | p > 0.05 |
| Decalcified fixed dune—Grain field | p < 0.01 |
| Unknown field—Grass field | p > 0.05 |
| Unknown field—Corn field | p > 0.05 |
| Unknown field—Grain field | p > 0.05 |
| Grass field—Corn field | p > 0.05 |
| Grass field—Grain field | p > 0.05 |
| Corn field—Grain field | p > 0.05 |
| Comparison | p-Value |
|---|---|
| Grey dune—Decalcified fixed dune | p > 0.05 |
| Grey dune—Unknown field | p > 0.05 |
| Grey dune—Grass field | p < 0.01 |
| Grey dune—Corn field | p > 0.05 |
| Grey dune—Grain field | p > 0.05 |
| Decalcified fixed dune—Unknown field | p > 0.05 |
| Decalcified fixed dune—Grass field | p < 0.01 |
| Decalcified fixed dune—Corn field | p > 0.05 |
| Decalcified fixed dune—Grain field | p > 0.05 |
| Unknown field—Grass field | p < 0.01 |
| Unknown field—Corn field | p > 0.05 |
| Unknown field—Grain field | p > 0.05 |
| Grass field—Corn field | p > 0.05 |
| Grass field—Grain field | p > 0.05 |
| Corn field—Grain field | p > 0.05 |
References
- Larsen, H.L.; Møller-Lassesen, K.; Enevoldsen, E.M.E.; Madsen, S.B.; Obsen, M.T.; Povlsen, P.; Bruhn, D.; Pertoldi, C.; Pagh, S. Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals. Drones 2023, 7, 680. [Google Scholar] [CrossRef]
- Mitchell, C.; Anthony, D.; Fox, J.H.; Clausager, I. Measures of annual breeding success amongst Eurasian Wigeon Anas penelope. Bird Study 2008, 55, 43–51. [Google Scholar] [CrossRef]
- Kahlert, J.; Fox, A.D.; Heldbjerg, H.; Asferg, T.; Sunde, P. Functional Responses of Human Hunters to Their Prey—Why Harvest Statistics may not Always Reflect Changes in Prey Population Abundance. Wildl. Biol. 2015, 21, wlb.00855. [Google Scholar] [CrossRef]
- Chrétien, L.P.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Ito, T.Y.; Miyazaki, A.; Koyama, L.A.; Kamada, K.; Nagamatsu, D. Antler detection from the sky: Deer sex ratio monitoring using drone-mounted thermal infrared sensors. Wildl. Biol. 2022, 2022, e01034. [Google Scholar] [CrossRef]
- Ruette, S.; Stahl, P.; Albaret, M. Applying distance-sampling methods to spotlight counts of red foxes. J. Appl. Ecol. 2003, 40, 32–43. [Google Scholar] [CrossRef]
- Strauß, E.; Grauer, A.; Bartel-Steinbach, M.; Klein, R.; Wenzelides, L.; Greiser, G.; Muchin, A.; Nösel, H.; Winter, A. The German wildlife information system: Population densities and development of European Hare (Lepus europaeus PALLAS) during 2002–2005 in Germany. Eur. J. Wildl. Res. 2008, 54, 142–147. [Google Scholar] [CrossRef]
- Corlatti, L.; Gugiatti, A.; Pedrotti, L. Spring spotlight counts provide reliable indices to track change in population size of mountain-dwelling red deer Cervus elaphus. Wildl. Biol. 2016, 22, wlb.00855. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Israel, M. A UAV-based roe deer fawn detection system. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2011, XXXVIII-1/C22, 51–55. [Google Scholar] [CrossRef]
- Lethbridge, M.; Stead, M.; Wells, C. Estimating kangaroo density by aerial survey: A comparison of thermal cameras with human observers. Wildl. Res. 2019, 46, 639–648. [Google Scholar] [CrossRef]
- Rahman, D.; Setiawan, Y.; Wijayanto, A.; Rahman, A.A.A.F.; Martiyani, T. An experimental approach to exploring the feasibility of unmanned aerial vehicle and thermal imaging in terrestrial and arboreal mammals research. E3S Web Conf. 2020, 211, 02010. [Google Scholar] [CrossRef]
- Dunstan, A.; Robertson, K.; Fitzpatrick, R.; Pickford, J.; Meager, J. Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island. PLoS ONE 2020, 15, e0228524. [Google Scholar] [CrossRef] [PubMed]
- Gallego, D.; Sarasola, J.H. Using drones to reduce human disturbance while monitoring breeding status of an endangered raptor. Remote Sens. Ecol. Conserv. 2021, 7, 550–561. [Google Scholar] [CrossRef]
- Howell, L.; Clulow, J.; Jordan, N.; Beranek, C.; Ryan, S.; Roff, A.; Witt, R. Drone thermal imaging technology provides a cost-effective tool for landscape-scale monitoring of a cryptic forest-dwelling species across all population densities. Wildl. Res. 2021, 49, 66–78. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Povlsen, P.; Linder, A.; Larsen, H.; Durdevic, P.; Arroyo, D.; Bruhn, D.; Pertoldi, C.; Pagh, S. Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark. Drones 2023, 7, 5. [Google Scholar] [CrossRef]
- Povlsen, P.; Bruhn, D.; Pertoldi, C.; Pagh, S. A Novel Scouring Method to Monitor Nocturnal Mammals Using Uncrewed Aerial Vehicles and Thermal Cameras—A Comparison to Line Transect Spotlight Counts. Drones 2023, 7, 661. [Google Scholar] [CrossRef]
- Povlsen, P.; Bruhn, D.; Durdevic, P.; Arroyo, D.O.; Pertoldi, C. Using YOLO Object Detection to Identify Hare and Roe Deer in Thermal Aerial Video Footage—Possible Future Applications in Real-Time Automatic Drone Surveillance and Wildlife Monitoring. Drones 2024, 8, 2. [Google Scholar] [CrossRef]
- Degollada, E.; Amigó, N.; O’Callaghan, S.A.; Varola, M.; Ruggero, K.; Tort, B. A Novel Technique for Photo-Identification of the Fin Whale, Balaenoptera physalus, as Determined by Drone Aerial Images. Drones 2023, 7, 220. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Zhang, H.; Zou, Y. Observing Individuals and Behavior of Hainan Gibbons (Nomascus hainanus) Using Drone Infrared and Visible Image Fusion Technology. Drones 2023, 7, 543. [Google Scholar] [CrossRef]
- Cheng, M.; Yuan, H.; Wang, Q.; Cai, Z.; Liu, Y.; Zhang, Y. Application of deep learning in sheep behaviors recognition and influence analysis of training data characteristics on the recognition effect. Comput. Electron. Agric. 2022, 198, 107010. [Google Scholar] [CrossRef]
- Moradeyo, O.M.; Olaniyan, A.S.; Ojoawo, A.O.; Olawale, J.A.; Bello, R.W. YOLOv7 Applied to Livestock Image Detection and Segmentation Tasks in Cattle Grazing Behavior, Monitor and Intrusions. J. Appl. Sci. Environ. Manag. 2023, 27, 953–958. [Google Scholar] [CrossRef]
- Hu, T.; Yan, R.; Jiang, C.; Chand, N.V.; Bai, T.; Guo, L.; Qi, J. Grazing Sheep Behaviour Recognition Based on Improved YOLOV5. Sensors 2023, 23, 4752. [Google Scholar] [CrossRef] [PubMed]
- Asferg, T.; Madsen, A.B. Krondyr. In Dansk Pattedyratlas; Baagøe, H., Secher Jensen, T., Eds.; Gyldendal: Copenhagen, Denmark, 2007. [Google Scholar]
- Danmarks Jægerforbund. Bestandsestimater og Monitering af Kronhjorteudbytte 22/23. 2023. Available online: https://www.jaegerforbundet.dk/om-dj/dj-medier/nyhedsarkiv/2023/bestandsestimater-og-tandsnit/ (accessed on 19 September 2024).
- Riis Olesen, C.; Lynge Madsen, T.; Skov-Petersen, H. Brug af GPS til forståelse af krondyrs adfærd og præferencer. Geoforum Perspekt. 2012, 8, 16. [Google Scholar]
- Staalsen, E.V. Jagt- og Vildtforvaltning i Danmark. 2023. Available online: https://fauna.au.dk/jagt-og-vildtforvaltning/vildtudbytte/udbyttet-online-siden-1941 (accessed on 19 September 2024).
- Gebert, C.; Verheyden-Tixier, H. Variations of diet composition of Red Deer (Cervus elaphus L.) in Europe. Mammal Rev. 2001, 31, 189–201. [Google Scholar] [CrossRef]
- Rafn, J.; Frederiksen, C.; Ulstrup, M.; Iacolina, L.; Pertoldi, C. Fødepræference og græsningshøjde på vedplanter om sommeren hos udsatte krondyr og elge i Lille Vildmose. Flora Fauna 2018, 124, 38–41. [Google Scholar]
- Garrett, K.; Beck, M.R.; Froehlich, K.; Fleming, A.; Thompson, B.R.; Stevens, D.R.; Gregorini, P. A comparison of methods for estimating forage intake, digestibility, and fecal output in red deer (Cervus elaphus). J. Anim. Sci. 2020, 98, 1–7. [Google Scholar] [CrossRef]
- Miljø- og Energiministeriet, Skov- og Naturstyrelsen. Rapport Vedrørende Markskader Forårsaget af Kronvildt 1999. Available online: https://mst.dk/media/phgl4x1l/1999_markskaderapport-1999.pdf (accessed on 19 September 2024).
- Corgatelli, G.; Mattiello, S.; Columbini, S.; Crovetto, G.M. Impact of red deer (Cervus elaphus) on forage crops in a protected area. Agric. Syst. 2019, 169, 41–48. [Google Scholar] [CrossRef]
- White, C.L.W.; Smart, J.C.R.; Böhm, M.; Langbein, J.; Ward, Y. Economic Impacts of Wild Deer in the East of England; Forestry Commission: Bristol, UK, 2004; pp. 3–18. [Google Scholar]
- Bleier, N.; Lehoczki, R.; Újváry, D.; Szemethy, L.; Csányi, S. Relationships between wild ungulates density and crop damage in Hungary. Mammal Res. 2012, 57, 351–359. [Google Scholar] [CrossRef]
- Jarnemo, A.; Nilsson, L.; Wikenros, C. Home range sizes of red deer in relation to habitat composition: A review and implications for management in Sweden. Eur. J. Wildl. Res. 2023, 69, 92. [Google Scholar] [CrossRef]
- Borkowski, J.; Banul, R.; Jurkiewicz, J.; Hołdynski, C.; Swieczkowska, J.; Nasiadko, M.; Załuski, D. High density of keystone herbivore vs. conservation of natural resources: Factors affecting red deer distribution and impact on vegetation in Słowi ´nski National Park, Poland. For. Ecol. Manag. 2019, 450, 117503. [Google Scholar] [CrossRef]
- Sorensen, A.A.; van Beest, F.M.; Brook, R.K. Quantifying overlap in crop selection patterns among three sympatric ungulates in anagricultural landscape. Basic Appl. Ecol. 2015, 16, 601–609. [Google Scholar] [CrossRef]
- The Danish Environmental Portal. Map of Vegetation Types, 2023. Urn: Dmp:dsgroup:kortlaegning-af-naturtyper. Available online: https://www.miljoeportal.dk/ (accessed on 5 May 2024).
- Terrestrial Cetartiodactyla. Terrestrial Cetartiodactyla. In Handbook of the Mammals of Europe; Corlatti, L., Zachos, F., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Dwyer, B.; Nelson, J.; Hansen, T.; Solawetz, J. Roboflow (Version 1.0). Software. 2024. Available online: https://roboflow.com (accessed on 19 September 2024).
- Jocher, G.; Chaurasia, A.; Qiu, J. Ultralytics YOLOv8. 2023. Available online: https://github.com/ultralytics/ultralytics (accessed on 19 September 2024).
- Pihl, S.; Søgaard, B.; Ejrnæs, R.; Aude, E.; Nielsen, K.E.; Dahl, K.; Laursen, J.S. Habitats and species covered by the EEC Habitats directive. A preliminary assessment of distribution and conservation status in Denmark. NERI Tech. Rep. 2001, 365, 121. [Google Scholar]
- European Environment Agency. Interpretation Manual of European Union Habitats 2013. EUR28. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/temas/espacios-protegidos/doc_manual_intp_habi-tat_ue_tcm30-207191.pdf (accessed on 19 September 2024).
- Agency, D.E.P. Habitatbeskrivelser, årgang 2016 Beskrivelse af Danske Naturtyper Omfattet af Habitatdirektivet (NATURA 2000 Typer) 2016. Available online: https://edit.mst.dk/media/pj3afex3/habitatbeskrivelser-2016-ver-105.pdf (accessed on 19 September 2024).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Duporge, I.; Spiegel, M.P.; Thomson, E.R.; Chapman, T.; Lamberth, C.; Pond, C.; Macdonald, D.W.; Wang, T.; Klinck, H. Determination of optimal flight altitude to minimise acoustic drone disturbance to wildlife using species audiograms. Methods Ecol. Evol. 2021, 12, 2196–2207. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef]
- Danish Meteorological Institute. Sammendrag af Marts 2024. 2024. Available online: https://www.dmi.dk/fileadmin/user_upload/Afrapportering/Maanedssammendrag/Sammendrag_2024_marts.pdf (accessed on 19 September 2024).
- Lande, U.S.; Loe, L.E.; Skjærli, O.J.; Meisingset, E.L.; Mysterud, A. The effect of agricultural land use practice on habitat selection of red deer. Eur. J. Wildl. Res. 2014, 60, 69–76. [Google Scholar] [CrossRef]
- Jayakody, S.; Sibbald, A.M.; Mayes, R.W.; Hooper, R.J.; Gordon, I.J.; Lambin, X. Effects of human disturbance on the diet composition of wild red deer (Cervus elaphus). Eur. J. Wildl. Res. 2011, 57, 939–948. [Google Scholar] [CrossRef]
- Riesch, F.; Wichelhaus, A.; Tonn, B.; Meißner, M.; Rosenthal, G.; Isselstein, J. Grazing by wild red deer can mitigate nutrient enrichment in protected semi-natural open habitats. Oecologia 2022, 199, 471–485. [Google Scholar] [CrossRef]
- Buttenschøn, R.; Gottlieb, L. Heste Naturforvaltningen. 2023. Available online: https://www.researchgate.net/profile/Rita-Buttenschon/publication/371761514_Heste_i_naturforvaltningen/links/6493fdfcc41fb852dd257559/Heste-i-naturforvaltningen.pdf (accessed on 19 September 2024).
- Thomassen, E.E.; Sigsgaard, E.E.; Jensen, M.R.; Olsen, K.; Hansen, M.D.D.; Svenning, J.; Thomsen, P.F. Contrasting seasonal patterns in diet and dung-associated invertebrates of feral cattle and horses in a rewilding area. Mol. Ecol. 2023, 32, 2071–2091. [Google Scholar] [CrossRef] [PubMed]
- Scasta, J.D.; Beck, J.L.; Angwin, C.J. Meta-Analysis of Diet Composition and Potential Conflict of Wild Horses with Livestock and Wild Ungulates on Western Rangelands of North America. Rangel. Ecol. Ecol. Ecol. Manag. 2016, 69, 310. [Google Scholar] [CrossRef]
- Rowe, Z.W.; Robins, J.H.; Rands, S.A. Red deer Cervus elaphus blink more in larger groups. Ecol. Evol. 2023, 13, e9908. [Google Scholar] [CrossRef] [PubMed]
- Lagory, K.E. Habitat, Group Size, and the Behaviour of White-Tailed Deer. Behaviour 1986, 98, 168–179. [Google Scholar] [CrossRef]
- AlKhaddar, R.; Singh, R.K.; Dutta, S.; Kumari, M. Statistical Parameters of Hydrometeorological Variables: Standard Deviation, SNR, Skewness and Kurtosis; Lecture Notes in Civil Engineering; Springer Singapore Pte. Limited: Singapore, 2020; Volume 39, pp. 59–70. [Google Scholar]
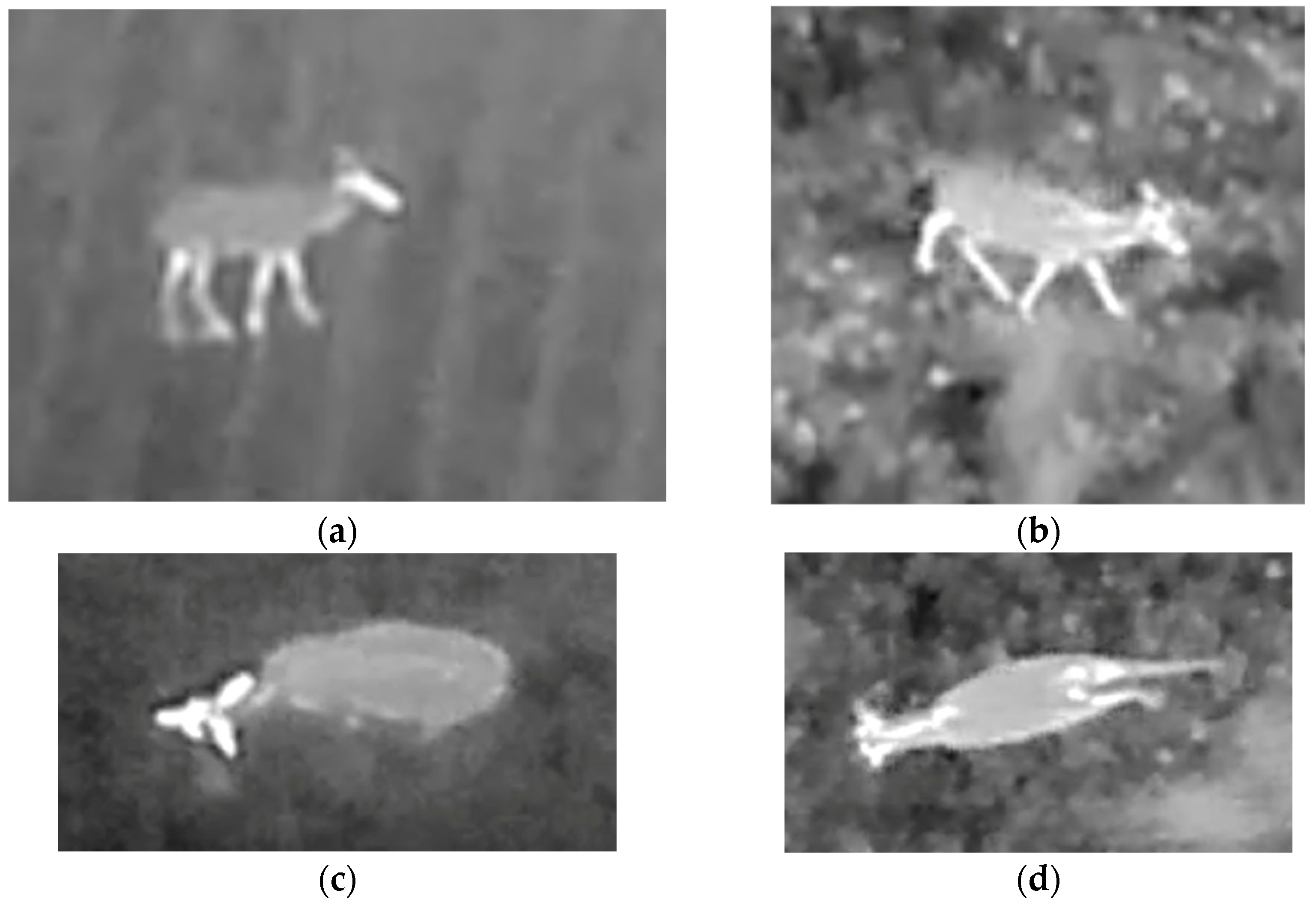





| Behaviour | Description |
|---|---|
| Eating | Animals standing with their head lowered towards the ground |
| Standing | Animals standing up and surveying their surroundings |
| Lying | Animals lying down |
| Locomotion | Animals being in motion, either running or walking |
| Heat spots | Spots on the ground with elevated temperatures caused by deer lying |
| Multiple together | Multiple animals being close in proximity hindering separation |
| Other | E.g., scratching itself or instances where behaviour was inconclusive |
| Class | Images | Instances | mAP50 | mAP50-95 |
|---|---|---|---|---|
| All | 169 | 1042 | 0.704 | 0.448 |
| Eating | 169 | 231 | 0.85 | 0.67 |
| Heatspot | 169 | 104 | 0.434 | 0.169 |
| Horse | 169 | 12 | 0.948 | 0.475 |
| Locomotion | 169 | 62 | 0.502 | 0.315 |
| Lying | 169 | 289 | 0.77 | 0.421 |
| Multiple-together | 169 | 55 | 0.671 | 0.502 |
| Other | 169 | 120 | 0.567 | 0.353 |
| Sheep | 169 | 71 | 0.968 | 0.715 |
| Standing | 169 | 98 | 0.626 | 0.416 |
| Natural Vegetation Types | Fields | |
|---|---|---|
| Skewness | 1.32 | 1.11 |
| Kurtosis | 0.29 | −0.18 |
| MAD | 0.01 | 0.16 |
| Natural Vegetation Types | Fields | |
|---|---|---|
| Skewness | 0.14 | 0.49 |
| Kurtosis | −1.85 | −1.39 |
| MAD | 0.55 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fugl, A.; Jensen, L.L.; Korsgaard, A.H.; Pertoldi, C.; Pagh, S. Vegetation Type Preferences in Red Deer (Cervus elaphus) Determined by Object Detection Models. Drones 2024, 8, 522. https://doi.org/10.3390/drones8100522
Fugl A, Jensen LL, Korsgaard AH, Pertoldi C, Pagh S. Vegetation Type Preferences in Red Deer (Cervus elaphus) Determined by Object Detection Models. Drones. 2024; 8(10):522. https://doi.org/10.3390/drones8100522
Chicago/Turabian StyleFugl, Annika, Lasse Lange Jensen, Andreas Hein Korsgaard, Cino Pertoldi, and Sussie Pagh. 2024. "Vegetation Type Preferences in Red Deer (Cervus elaphus) Determined by Object Detection Models" Drones 8, no. 10: 522. https://doi.org/10.3390/drones8100522
APA StyleFugl, A., Jensen, L. L., Korsgaard, A. H., Pertoldi, C., & Pagh, S. (2024). Vegetation Type Preferences in Red Deer (Cervus elaphus) Determined by Object Detection Models. Drones, 8(10), 522. https://doi.org/10.3390/drones8100522









